Abstract
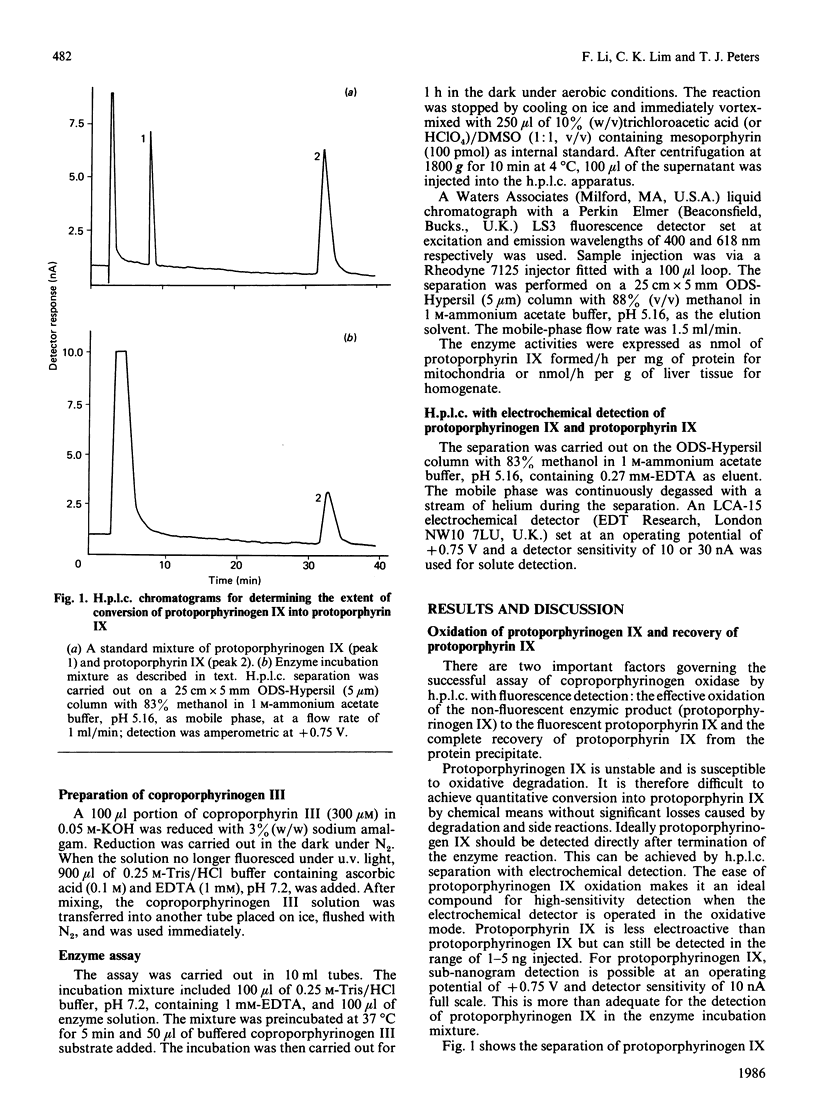
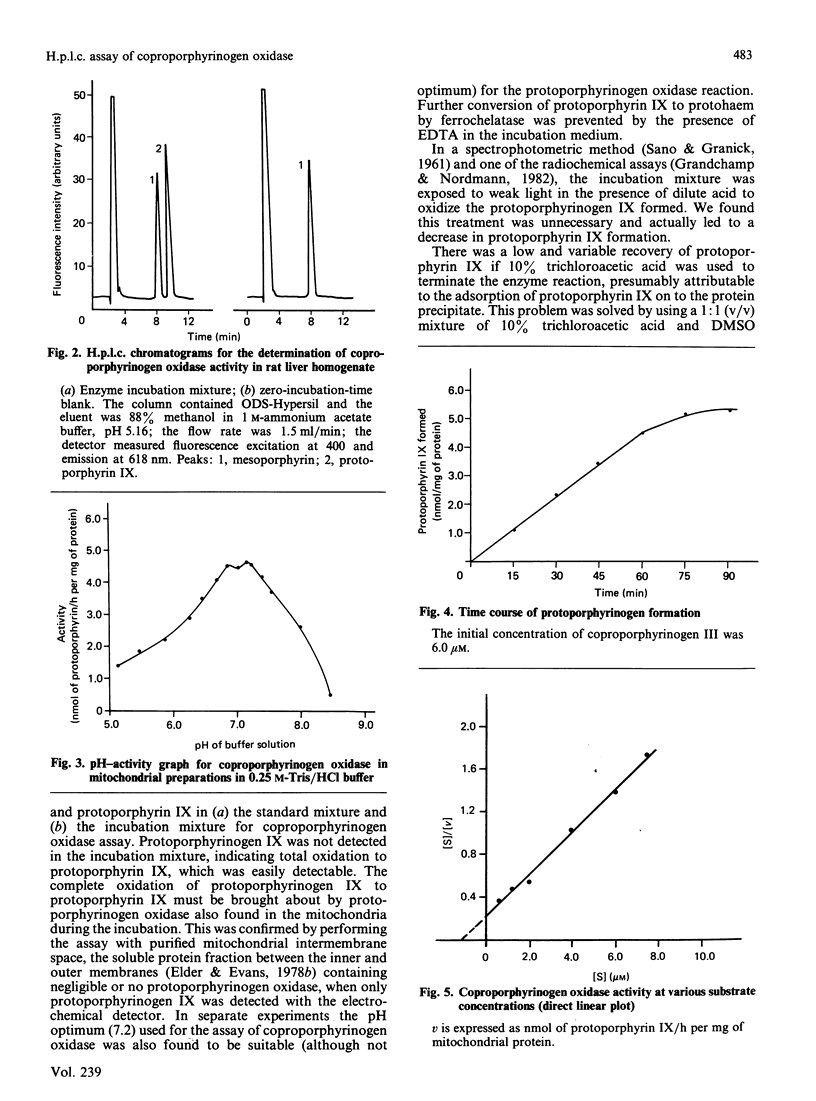
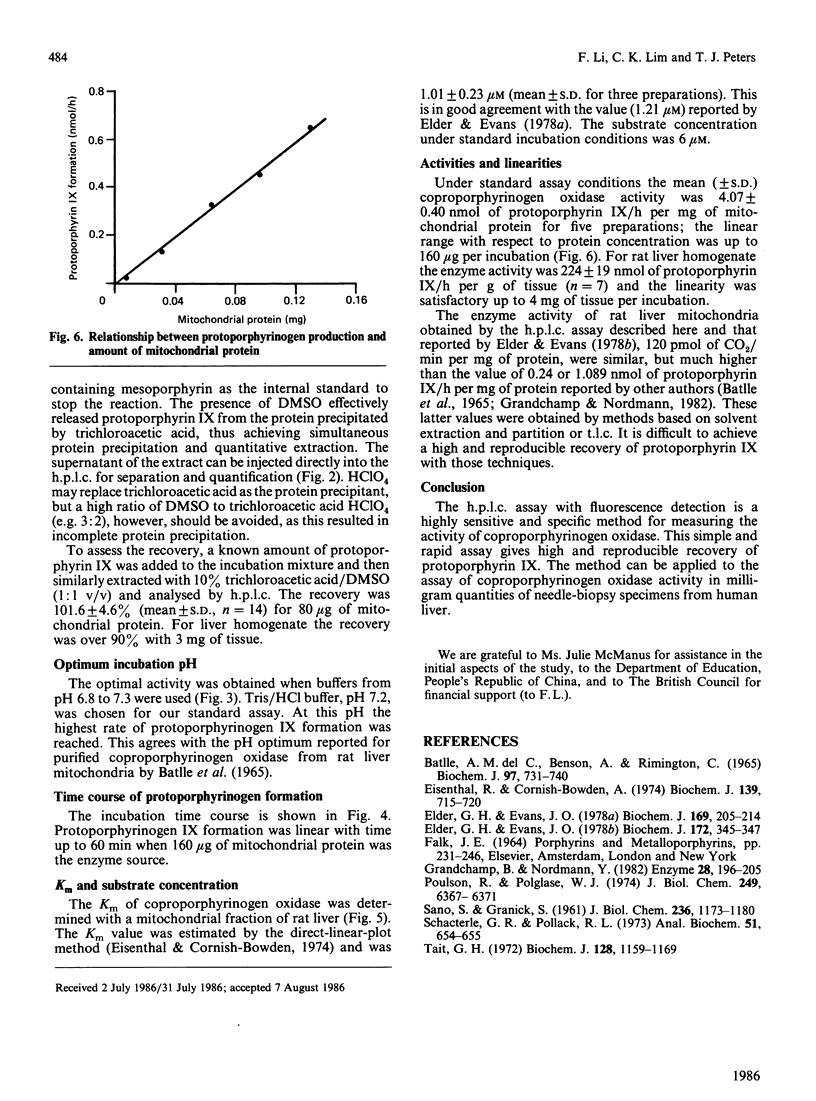
An h.p.l.c. method was developed for the assay of coproporphyrinogen oxidase activity in rat liver. The protoporphyrinogen IX formed is completely oxidized to protoporphyrin IX for separation and quantification by reversed-phase chromatography with mesoporphyrin as the internal standard. The Km of coproporphrinogen oxidase is 1.01 +/- 0.23 microM. The activities are 4.07 +/- 0.40 nmol of protoporphyrin IX/h per mg of mitochondrial protein and 224 +/- 19 nmol of protoporphyrin IX/h per g of liver tissue homogenate. The method is sensitive enough for measuring enzyme activity in small amounts of human tissue from needle biopsy.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O. A radiochemical method for the measurement of coproporphyrinogen oxidase and the utilization of substrates other than coproporphyrinogen III by the enzyme from rat liver. Biochem J. 1978 Jan 1;169(1):205–214. doi: 10.1042/bj1690205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O. Evidence that the coproporphyrinogen oxidase activity of rat liver is situated in the intermembrane space of mitochondria. Biochem J. 1978 May 15;172(2):345–347. doi: 10.1042/bj1720345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., Nordmann Y. Coproporphyrinogen III oxidase assay. Enzyme. 1982;28(2-3):196–205. doi: 10.1159/000459102. [DOI] [PubMed] [Google Scholar]
- Poulson R., Polglase W. J. Aerobic and anaerobic coproporphyrinogenase activities in extracts from Saccharomyces cerevisiae. J Biol Chem. 1974 Oct 25;249(20):6367–6371. [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Tait G. H. Coproporphyrinogenase activities in extracts of Rhodopseudomonas spheroides and Chromatium strain D. Biochem J. 1972 Aug;128(5):1159–1169. doi: 10.1042/bj1281159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Batlle A. M., Benson A., Rimington C. Purification and properties of coproporphyrinogenase. Biochem J. 1965 Dec;97(3):731–740. doi: 10.1042/bj0970731. [DOI] [PMC free article] [PubMed] [Google Scholar]


