Abstract
The recovery of the parameters of the kinetic properties of carnitine palmitoyltransferase (CPT) I in liver mitochondria of starved rats was studied after re-feeding animals for various periods of time. There were no significant changes either in the activity of the enzyme at high palmitoyl-CoA concentrations or in the affinity of the enzyme for palmitoyl-CoA, or in the sensitivity of CPT I to malonyl-CoA inhibition after 3 h or 6 h re-feeding. After 24 h re-feeding, both the affinity of the enzyme for palmitoyl-CoA and the activity of the enzyme were still not significantly different from those for the enzyme in mitochondria from 24 h-starved animals. By contrast, the sensitivity of CPT I to malonyl-CoA inhibition was largely, but not fully, restored to that observed in mitochondria from fed rats.
Full text
PDF
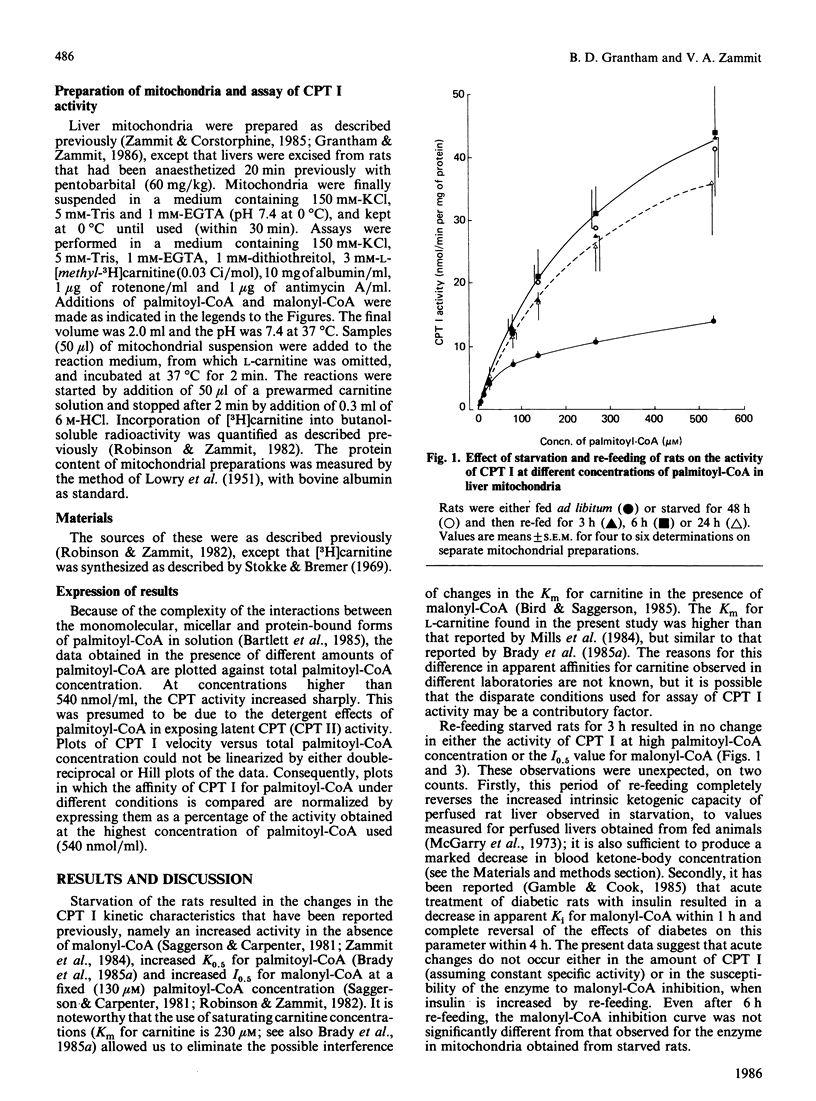
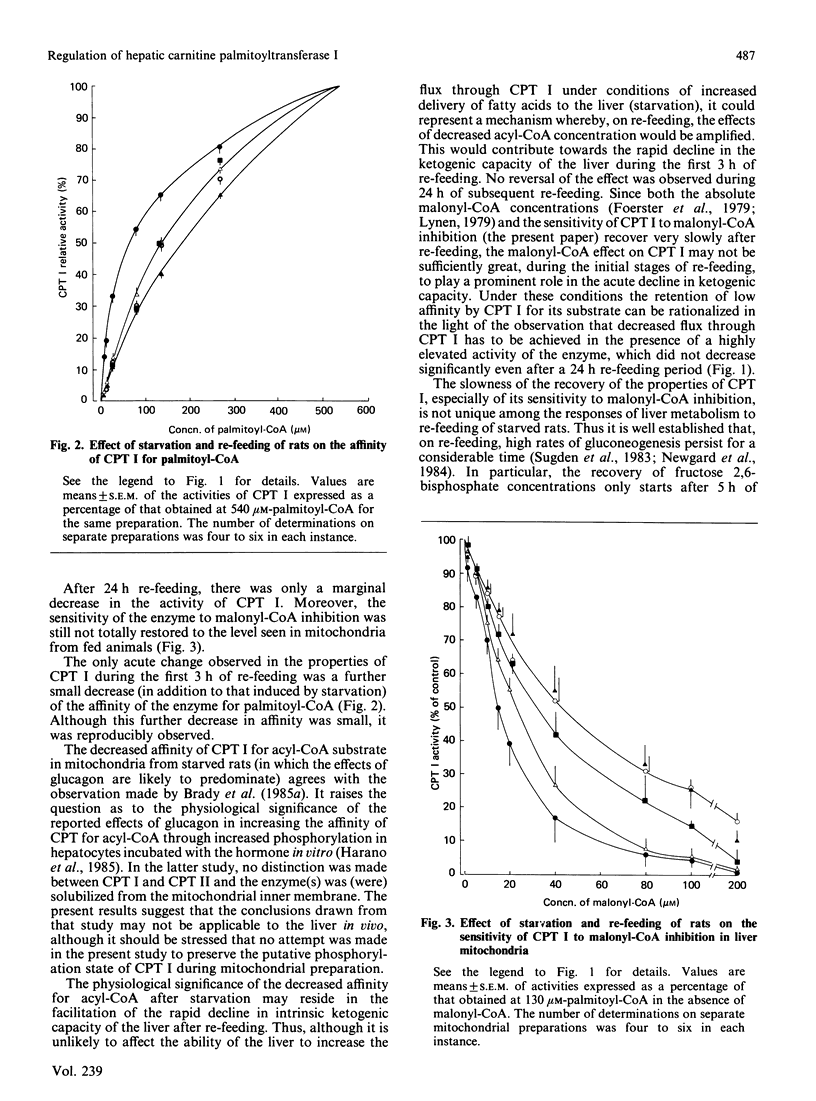

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Williamson D. H. The utilization of ketone bodies by the interscapular brown adipose tissue of the rat. Biochim Biophys Acta. 1981 Oct 23;666(1):127–132. doi: 10.1016/0005-2760(81)90098-9. [DOI] [PubMed] [Google Scholar]
- Bartlett K., Bartlett P., Bartlett N., Sherratt H. S. Kinetics of enzymes requiring long-chain acyl-CoA esters as substrates: effects of substrate binding to albumin. Biochem J. 1985 Jul 15;229(2):559–560. doi: 10.1042/bj2290559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird M. I., Saggerson E. D. Interacting effects of L-carnitine and malonyl-CoA on rat liver carnitine palmitoyltransferase. Biochem J. 1985 Aug 15;230(1):161–167. doi: 10.1042/bj2300161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L. J., Hoppel C. L., Brady P. S. Hepatic mitochondrial inner-membrane properties, beta-oxidation and carnitine palmitoyltransferases A and B. Effects of genetic obesity and starvation. Biochem J. 1986 Jan 15;233(2):427–433. doi: 10.1042/bj2330427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L. J., Silverstein L. J., Hoppel C. L., Brady P. S. Hepatic mitochondrial inner membrane properties and carnitine palmitoyltransferase A and B. Effect of diabetes and starvation. Biochem J. 1985 Dec 1;232(2):445–450. doi: 10.1042/bj2320445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A. Differences in the sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem. 1984 Oct 10;259(19):12030–12033. [PubMed] [Google Scholar]
- Foster D. W. Banting lecture 1984. From glycogen to ketones--and back. Diabetes. 1984 Dec;33(12):1188–1199. doi: 10.2337/diab.33.12.1188. [DOI] [PubMed] [Google Scholar]
- Gamble M. S., Cook G. A. Alteration of the apparent Ki of carnitine palmitoyltransferase for malonyl-CoA by the diabetic state and reversal by insulin. J Biol Chem. 1985 Aug 15;260(17):9516–9519. [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Binding of [14C]malonyl-CoA to rat liver mitochondria after blocking of the active site of carnitine palmitoyltransferase I. Displacement of low-affinity binding by palmitoyl-CoA. Biochem J. 1986 Jan 15;233(2):589–593. doi: 10.1042/bj2330589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo. J Biol Chem. 1972 Nov 25;247(22):7325–7331. [PubMed] [Google Scholar]
- Harano Y., Kashiwagi A., Kojima H., Suzuki M., Hashimoto T., Shigeta Y. Phosphorylation of carnitine palmitoyltransferase and activation by glucagon in isolated rat hepatocytes. FEBS Lett. 1985 Sep 2;188(2):267–272. doi: 10.1016/0014-5793(85)80385-9. [DOI] [PubMed] [Google Scholar]
- Holness M. J., French T. J., Sugden M. C. Hepatic glycogen synthesis on carbohydrate re-feeding after starvation. A regulatory role for pyruvate dehydrogenase in liver and extrahepatic tissues. Biochem J. 1986 Apr 15;235(2):441–445. doi: 10.1042/bj2350441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M., Newgard C. B., Foster D. W., McGarry J. D. Time course and significance of changes in hepatic fructose-2,6-bisphosphate levels during refeeding of fasted rats. J Clin Invest. 1984 Sep;74(3):1108–1111. doi: 10.1172/JCI111479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem J. 1984 Apr 15;219(2):601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard C. B., Moore S. V., Foster D. W., McGarry J. D. Efficient hepatic glycogen synthesis in refeeding rats requires continued carbon flow through the gluconeogenic pathway. J Biol Chem. 1984 Jun 10;259(11):6958–6963. [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Bird M. I., Carpenter C. A., Winter K. A., Wright J. J. Cycloheximide blocks changes in rat liver carnitine palmitoyltransferase 1 activity in starvation. Biochem J. 1984 Nov 15;224(1):201–206. doi: 10.1042/bj2240201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of fasting, adrenalectomy and streptozotocin-diabetes on sensitivity of hepatic carnitine acyltransferase to malonyl CoA. FEBS Lett. 1981 Jul 6;129(2):225–228. doi: 10.1016/0014-5793(81)80170-6. [DOI] [PubMed] [Google Scholar]
- Stokke O., Bremer J. A simple method for preparation of methyl-labelled (-) carnitine. Biochim Biophys Acta. 1970 Dec 15;218(3):552–554. doi: 10.1016/0005-2760(70)90021-4. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Watts D. I., Palmer T. N., Myles D. D. Direction of carbon flux in starvation and after refeeding: in vitro and in vivo effects of 3-mercaptopicolinate. Biochem Int. 1983 Sep;7(3):329–337. [PubMed] [Google Scholar]
- Sugden M. C., Watts D. L., Marshall C. E. Lipogenesis in response to an oral glucose load in fed and starved rats. Biosci Rep. 1981 Jun;1(6):469–476. doi: 10.1007/BF01121580. [DOI] [PubMed] [Google Scholar]
- Zammit V. A. Carnitine acyltransferases in the physiological setting: the liver. Biochem Soc Trans. 1986 Aug;14(4):676–679. doi: 10.1042/bst0140676. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G. Altered release of carnitine palmitoyltransferase activity by digitonin from liver mitochondria of rats in different physiological states. Biochem J. 1985 Sep 1;230(2):389–394. doi: 10.1042/bj2300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G., Gray S. R. Changes in the ability of malonyl-CoA to inhibit carnitine palmitoyltransferase I activity and to bind to rat liver mitochondria during incubation in vitro. Differences in binding at 0 degree C and 37 degrees C with a fixed concentration of malonyl-CoA. Biochem J. 1984 Sep 1;222(2):335–342. doi: 10.1042/bj2220335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Regulation of hepatic fatty acid metabolism. The activities of mitochondrial and microsomal acyl-CoA:sn-glycerol 3-phosphate O-acyltransferase and the concentrations of malonyl-CoA, non-esterified and esterified carnitine, glycerol 3-phosphate, ketone bodies and long-chain acyl-CoA esters in livers of fed or starved pregnant, lactating and weaned rats. Biochem J. 1981 Jul 15;198(1):75–83. doi: 10.1042/bj1980075. [DOI] [PMC free article] [PubMed] [Google Scholar]


