Abstract
Objective
To assess the efficacy of biodegradable temporising matrix (BTM) in complex wound reconstruction.
Methods
The authors conducted a systematic review and meta‐analysis as per the preferred reporting items for systematic review and meta‐analysis (PRISMA) guidelines following a literature search assessing BTM in complex wound reconstruction. The primary outcome measures included the proportion of BTM integration as well as integration time. Secondary outcomes included graft take over BTM, infection rate and other complications as well as scar outcome.
Results
Twenty six studies met the inclusion criteria with a total of 1153 complex wounds. The mean proportional integration was 92.7% at (95% confidence intervals [CI] 88.57, 96.87, p < 0.001) with a mean integration time of 34.05 days (95% CI 33.33, 34.79, p < 0.001). The infection rate was low at 12.6% with an untransformed proportion metric assessment (0.126, 0.08–0.168, p < 0.001) at the site of BTM application. Favourable scar outcomes were reported using the matching assessment using photographs with scars (MAPS) and patient and observer scar assessment scales (POSAS).
Conclusion
BTM offers a robust dermal template in reconstruction of complex wounds. The authors recommend for randomised controlled trials to enhance the current evidence base.
Keywords: biodegradable temporising matrix, complex wound, dermal substitute
1. INTRODUCTION
Biodegradable temporising matrix (BTM) is a synthetic dermal substitute constituted by a polyurethane foam utilised to reconstruct complex wounds. It possesses a fenestrated polyurethane seal acting as a pseudo‐epidermis which allows egress of fluid 1 but concomitantly minimises fluid losses and scar contraction. 2 It has been used in complex wounds in many different settings for reconstruction including burns, 3 necrotising fasciitis, 4 bone denuded of periosteum, 5 tendon without paratenon 6 and pressure ulcers. 7
BTM is used as a two staged reconstructive technique after initiating angiogenesis with subsequent application of a skin graft once the dermal component has biodegraded. 2 This is primarily by hydrolysis 2 with no toxic substance generation. 1 The initial phase involves a thorough debridement of the wound bed and a template cut to fit the defect that can be affixed with either suture material or staples. The sealing membrane is faced externally and removed at the second stage of delamination at which point the neodermis can be refreshed and a skin graft applied. 8 , 9
BTM's increasing role as part of the surgeon's armamentarium has led to numerous reports within the literature depicting its outcomes. 1 , 2 , 5 , 7 , 10 , 11 , 12
The authors' aim to perform a comprehensive systematic review and meta‐analysis of the literature with an outcomes synthesis to enhance the current evidence base for this dermal substitute.
2. METHODS
This systematic review and meta‐analysis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement standards. 13
2.1. Eligibility criteria
All studies including randomised, non‐randomised trials, observational studies and case series with at least five patients were included assessing the efficacy of using BTM in complex wound reconstruction. A complex wound was defined as one that had exposed critical structures like bone or tendon, those caused by necrotising infections or deep burns and wound beds where split thickness grafts were not usually considered. More specifically Ferreira, 14 depicted a complex wound as one that came under the following categories, which, the authors adopted:
Extensive loss of the integument in acute or chronic wounds
Infection, as a complication in chronic wounds
Necrosis and compromised viability of surrounding tissue or signs of circulation impairment
Systematic pathologies that impair normal wound healing (e.g., diabetes, vasculitis or immune suppression).
In accordance with the aforementioned criteria, wounds included as part of the review consisted of deep burns, full‐thickness wounds in the lower extremity, diabetic ulcers, pressure ulcers, chronic venous ulcers, as well as wounds following extensive necrotic processes caused by infection and those of ischaemic aetiology. Studies with no adult patients, those not reported in English, animal studies as well as those with fewer than five patients were all excluded. There was no restriction on patient comorbidities, wound aetiology or type of exposed structure on which BTM templates were applied over. A summation of the inclusion and exclusion criteria is given below.
2.2. Inclusion criteria
BTM application in any complex wound reconstruction: full thickness or deep burns, complex lower/upper limb wounds with exposed muscle/bone/tendon, chronic wounds, pressure ulcers as well as diabetic wounds and those post debridement of infective and ischaemic aetiologies
Randomised or non‐randomised trials or any observational/cohort study/case series
Minimum patient number of five
2.3. Exclusion criteria
Studies with no adult patients
Studies with less than five patients
Animal/in‐vitro/in‐vivo studies
Individual case reports
Letters to the editor
Abstracts
Unpublished data
Review articles
Book chapters
2.4. Outcome measures
The primary outcome measures included proportion of BTM integration as well as BTM integration time. BTM integration was reported as a percentage proportion of the wound surface area overall to which it was implanted to as well as the proportion of devices integrated relative to the total patient population within the different studies. Integration is routinely assessed clinically by checking for blanching on digital pressure as well as observing a uniform pink colouration. 10 The secondary outcomes included the percentage graft take over BTM, infection rate as well as other miscellaneous device related complications and scar appearance using matching assessment using photographs with scars (MAPS) and patient and observer scar assessment scale (POSAS) scores.
2.5. Literature search strategy
Two authors (GL and SR) independently searched the electronic databases including Google Scholar, Pubmed, MEDLINE, Embase, CINAHL, and the Cochrane Central Register of Controlled Trials (CENTRAL). The last search was performed on 9th of July 2024. The search terminologies included “biodegradable temporising matrix” or “BTM,” “Novosorb,” “complex wounds,” “burns,” “tendon,” “bone,” “diabetic foot ulcers,” “pressure ulcers,” “chronic wounds,” and “necrotising infection.” These search terms were chosen to comprehensively cover all relevant conditions and applications for BTM in wound care and reconstruction. The bibliographic lists of relevant articles were also screened to maximise search retrieval.
2.6. Selection of studies
The titles and abstracts of the studies retrieved from the literature were independently assessed by two authors GL and SR. All articles that met the eligibility criteria were selected and the full texts of the articles were reviewed. Any discrepancy in selection was discussed with third author NF.
2.7. Data extraction and management
An electronic data extraction spreadsheet in line with the Cochrane's data collection form for intervention was created. A pilot test was performed with the spreadsheet extracting data from articles selected and adjusted accordingly.
2.8. Data synthesis
The authors conducted a meta‐analysis for outcomes reported by at least three studies. The mean was used for measurement of continuous data and the untransformed proportion (PR) metric for all dichotomous data in a single group. The odds ratio (OR) was used as a summary measure for dichotomous variables in two groups. OpenMetaAnalyst software was instigated for data synthesis. The outcomes were reported in forests plot with 95% confidence interval (CIs).
The heterogeneity was assessed using the Cochran Q test (χ 2), which measures the degree of variation in the effect estimates from the different studies. The inconsistency was further quantified by calculating I 2, and this was interpreted as follows: 0%–25% (low heterogeneity), 25%–75% (moderate heterogeneity) and 75%–100% (considerable heterogeneity), indicating the proportion of variation across studies. For outcomes where the heterogeneity was high this was circumvented by adaptation of a random effects model or an inverse variance function. These statistical functions are conducted to account for scenarios when it is elevated and equates for any discrepancies in outcomes in the overall effect estimate.
2.9. Sensitivity analyses
A sensitivity analysis was performed to assess the robustness of using BTM in wounds inherently more predisposed to infection despite thorough debridement. This included diabetic foot wounds, those produced from necrotising fasciitis as well as chronic wounds, pressure ulcers and any other previous infections or those with ischaemia. Diabetic patients in particular often suffer from neuropathy and can develop more profound infections before they are identified. 15 Endothelial dysfunction compromising vascularity as well as hyperglycaemia inhibiting a chemotaxic response all enhance the risk of infection in diabetic cohorts. 15 In wounds with pre‐existing necrotising fasciitis that have been debrided, there has still been a reluctance with the application of dermal substitutes for reconstruction due to the risk of infection. 4 , 16 The authors therefore conducted a sensitivity analysis to evaluate the infection rate when using BTM in diabetic wounds post debridement, those with a background of necrotising fasciitis as well as any chronic wounds or pressure ulcers and those with any previous infections or ischaemia. This would enable an assessment of the robustness of BTM in withstanding wound beds where the risk of infection is higher. The authors determined wounds pre‐disposed to infection as those which were at increased risk and defined them in accordance with the below criteria:
Diabetic wounds
Chronic wounds
Previous infection
Pressure ulcers
Post debridement of necrotising fasciitis
Ischaemic injury
2.10. Methodological quality and risk of bias
The methodological quality and risk of bias was evaluated using the Newcastle Ottawa scale for all observational studies. 17 This uses a star scoring system with a maximum total score of nine for each study. The score is calculated by awarding stars based on specific criteria within the three domains, with up to four stars for selection, two for comparability, and three for exposure. In each domain there are clear criteria for each star. A study with more stars is considered to have met more of the criteria for good methodological quality.
3. RESULTS
3.1. Literature search results
One hundred and eighty nine articles were identified with 26 meeting the inclusion criteria (Figure 1).
FIGURE 1.
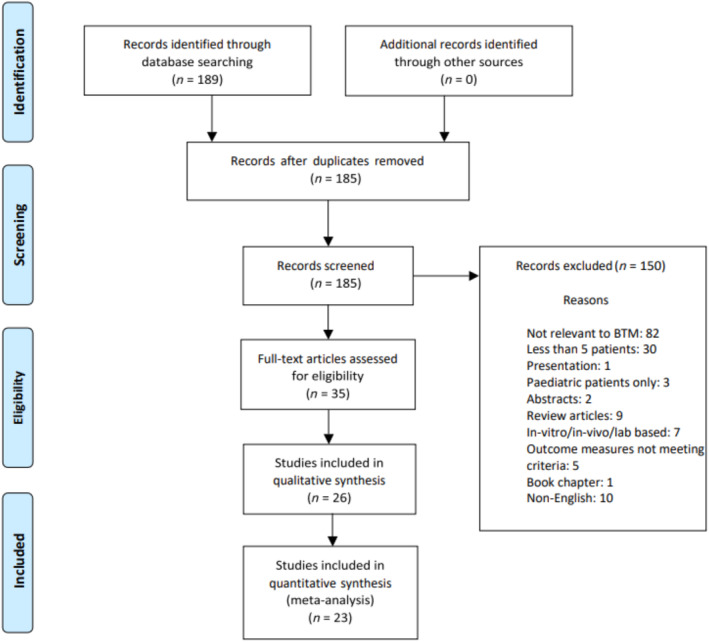
Preferred reporting items for systematic review and meta‐analysis (PRISMA) flow chart illustrating article screening and selection.
3.2. Primary outcomes
3.2.1. Proportion of BTM integration
Nine studies in total homogenously reported on the percentage of BTM integration during complex wound reconstruction with a mean rate of 92.7% take. This was the proportion of BTM integration as a percentage of the wound surface area overall to which BTM was implanted to and incorporated 502 wounds in total as shown in Figure 2. This was the point at which the BTM template had fully healed and successfully integrated into the wound bed and is normally assessed clinically for blanching on digital pressure as well as observing for a uniform pink colouration. 10 Heterogeneity was considerable but this was circumvented with adaptation of a random effects model. All studies reported good percentage integration rates on average. Wagstaff 16 had a 100% BTM integration rate over flap donor sites and although Lo; 88.6%, 2 as well as Greenwood; 88.4% 1 reported slightly lower percentage integration rates, wound aetiology was different. Both these studies reported on burn wounds with Lo 2 reporting a sizeable average area of 2137.8 cm2 to which BTM was applied. Wagstaff 4 had a comparable average integration percentage of 99.8% to their previous 2015 study 16 as included within this analysis. All wounds again were flap donor sites. The overall average integration percentage for the nine studies incorporating the different wound types was very high at 92.7% during the analysis which also accounted for devices that had failed. Failures of BTM integration as part of the data synthesis are summarised below in table format (Table 1).
FIGURE 2.
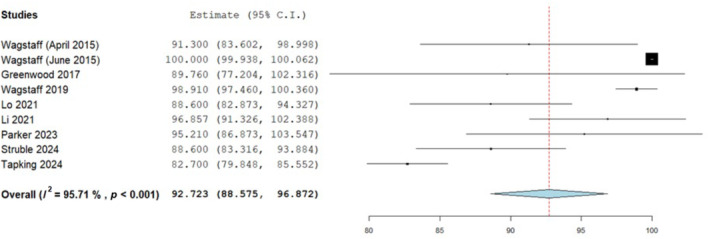
Percentage proportion of biodegradable temporising matrix (BTM) integration overall with a mean analysis; 92.7 (88.57, 96.87) standard error 2.117, p < 0.001.
TABLE 1.
Summary of BTM failures as average percentage in relation to wound area and whether replaced.
| Study | Number (n) | Average BTM failure/% (percentage of BTM in relation to wound area) | Replaced/not replaced |
|---|---|---|---|
| Wagstaff April 2015 16 | 10 | 8.7% | Not replaced |
| Wagstaff June 2015 27 | 10 | Nil | N/A |
| Greenwood 2016 3 | 5 | 13.9% | Replaced in 2 cases |
| Wagstaff 2019 27 | 7 | 0.14% | Not replaced |
| Lo 2022 2 | 26 | 11.4% | Replaced in 2 patients |
| Li 2021 5 | 35 | 3.1% | Not replaced |
| Parker 2023 43 | 24 | 2.4% | Not replaced |
| Struble 2024 25 | 86 | 11.4% | Replaced in 4 cases |
| Tapking 2024 44 | 300 | 6.6% | Not reported |
Abbreviations: BTM, biodegradable temporising matrix; N/A, non‐applicable.
Eight other studies also reported on BTM integration but did so as the proportion of devices integrated in relation to the total population of patients within the study. Schlottman 7 recorded a high rate of BTM take amongst their patient cohort at 75% as did Austin 18 with a 97.8% success rate whilst Kuang 19 had complete wound healing in all 14 cases of BTM application with all devices successfully integrated. Wu 20 identified an integration success rate of 60.8% in patients. Chen 21 reported good BTM take in 33 out of 37 patients to which it was applied (89.2%) and Devine 22 identified 10 in 12 cases where BTM had integrated. Guerriero 23 had success with BTM in 15 out of 23 cases (65.3%) where it was implanted, these were all diabetic patients however. Kidd 24 reported successful integration in 70.3% of cases.
3.3. BTM integration time
A cumulative synthesis of BTM integration time prior to skin graft application was calculated from 16 studies with a total of 709 wounds reporting this homogenously (Figure 3). The mean length of time was 34.05 days at a 95% confidence interval (33.326, 34.785), with an inverse variance function to account for considerable heterogeneity. This was the time taken for the BTM template to fully integrate into the host wound bed prior to second stage skin graft application. The mean integration times ranged from 27.7 25 to 83 days. 26 A further analysis was conducted to compare integration times dependant on the wound bed type to evaluate any differences. Six studies homogenously reported the integration time of BTM applied to exposed bone comparing it against other wound bed types (Figure 4) which included tendon as well as neurovascular structures, fascia, fat, muscle, perichondrium, granulation tissue, submandibular gland, testicle and cartilage.
FIGURE 3.
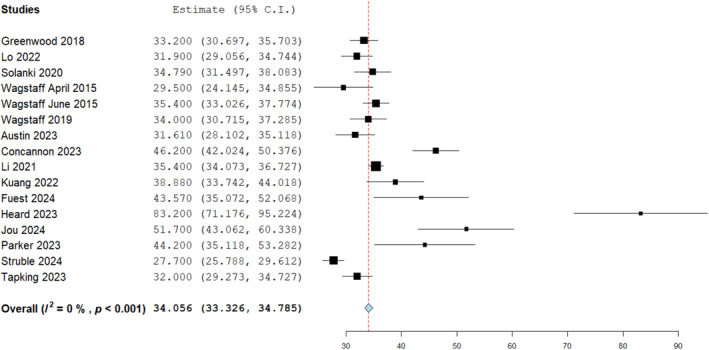
Biodegradable temporising matrix (BTM) integration (days) as analysed in 16 studies at 95% confidence intervals, mean time: 34.05 days (33.326, 34.785), standard error 0.372, p < 0.001.
FIGURE 4.
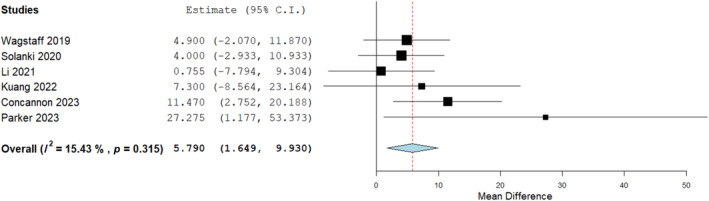
Mean difference analysis comparing biodegradable temporising matrix (BTM) integration time (days) on bone versus other wound bed types (neurovascular structures, fascia, fat, muscle, perichondrium, granulation, submandibular gland, testicle and cartilage). Mean difference: 5.790 (1.649, 9.930) p = 0.006.
A significant difference was seen on mean difference analysis with a slower rate of integration for BTM on bone compared to other wound bed types. Heterogeneity was neglible on the Cochrane Q‐test giving further consistency to the outcome and suggesting BTM should be applied for longer in cases where its applied directly on to bone. A mean metric analysis for BTM integration time applied to bone overall found it to be an average of 40.7 days (Figure 5).
FIGURE 5.
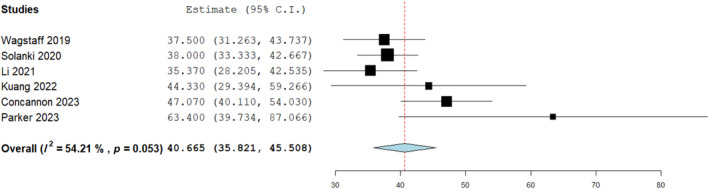
Mean analysis for biodegradable temporising matrix (BTM) integration time on bone (days): 40.665 (35.821, 45.508), standard error 2.47, p < 0.001.
BTM integration time on bone was also compared to tendons in 4 studies and homogenously reported. Results were found to be comparable with no significant difference seen in terms of integration times in both wound bed types (Figure 6).
FIGURE 6.
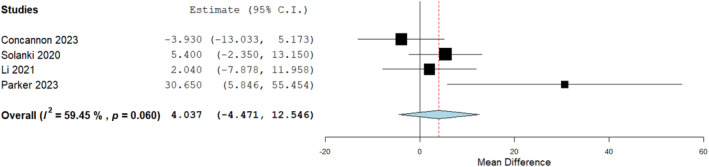
Mean difference analysis comparing biodegradable temporising matrix (BTM) integration time (days) on bone to tendon. No significant difference seen: 4.037 (−4.471, 12.546), p = 0.352.
A further sensitivity analysis was conducted to evaluate integration time for BTM on tendon compared to other tissues types as demonstrated in Figure 7 below with no significant differences seen. An overall analysis for time to integration on tendons was deduced at 37.4 from 4 studies (Figure 8).
FIGURE 7.
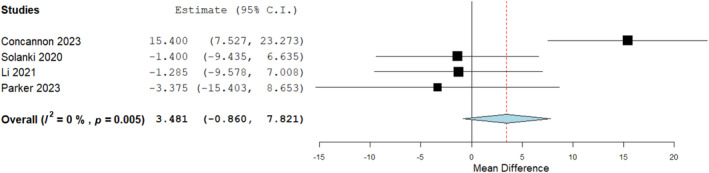
Mean difference assessment of biodegradable temporising matrix (BTM) integration time on tendon versus other soft tissues (neurovascular structures, fascia, fat, muscle, perichondrium, granulation, submandibular gland, testicle and cartilage). Mean difference 3.481 (−0.860, 7.821), p = 0.116.
FIGURE 8.
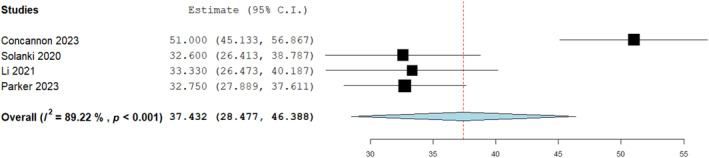
Mean assessment of average integration time for biodegradable temporising matrix (BTM) over tendons(days); 37.432 (28.477, 46.388), Standard error 4.569, p < 0.001.
3.4. Secondary outcomes
3.4.1. Graft take
A high percentage graft take over BTM of 98.9% was reported by nine studies with a total of 511 wounds (Figure 9). This was the proportion of take in relation to the wound surface area overall which was covered by BTM and the point at which the skin graft had successfully healed. All these wounds which were grafted had assumed successful BTM integration in the first stage and explains the high success rate. Where the BTM was not believed to have integrated, it was not grafted in those instances. All included studies within the analysis reported it homogenously with take rates seen ranging from averages of 70% 22 to 100% 4 , 27 across a variety of different wounds reconstructed. The analysis was conducted taking into account instances of graft failure too and the outcomes are summarised below in Table 2 in relation to these.
FIGURE 9.
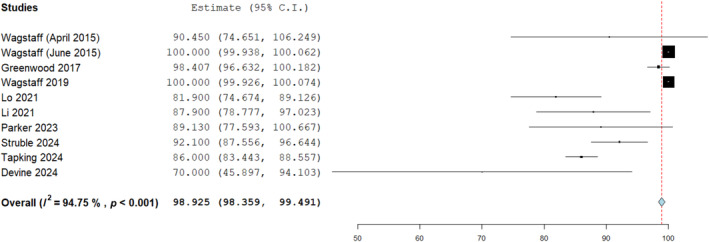
Split thickness skin graft (SSG) take over biodegradable temporising matrix (BTM) template as analysed in nine studies, mean percentage: 98.925% (95% confidence intervals: 98.359, 99.491), standard error 0.289, p = 0.001.
TABLE 2.
Summary of average graft failure rates as percentage of area reconstructed with BTM.
| Study | Number (n) | Average graft failure/% (percentage of graft failure in relation to wound area covered by BTM) | Regrafted Yes/No |
|---|---|---|---|
| Wagstaff April 2015 16 | 10 | 9.6% | Yes: 1 case |
| Wagstaff June 2015 27 | 10 | Nil | N/A |
| Greenwood 2016 9 | 5 | 0.4% | Yes: 2 cases |
| Wagstaff 2019 4 | 7 | 0% | N/A |
| Lo 2022 2 | 26 | 0.44% | N/R |
| Li 2021 5 | 32 | 8.9% | Yes: 1 case |
| Parker 2023 43 | 23 | 10.9% | Yes: 1 case |
| Struble 2024 25 | 55 | 0.14% | N/R |
| Tapking 2024 44 | 300 | 0.05% | N/R |
| Devine 2024 22 | 9 | 6.6% | No |
Abbreviations: BTM, biodegradable temporising matrix; N/A, non‐applicable; NR; not reported.
3.5. Infection rate and other complications
Twenty one studies with a total of 577 wounds reported on the infection rate when applying BTM for complex wound reconstruction and an amalgamated incidence of 12.6% was reported in the outcome synthesis shown in Figure 10. Infection was defined as the presence of one or more; erythema, pain, purulence, swelling or confirmation with positive microbial swabs.
FIGURE 10.
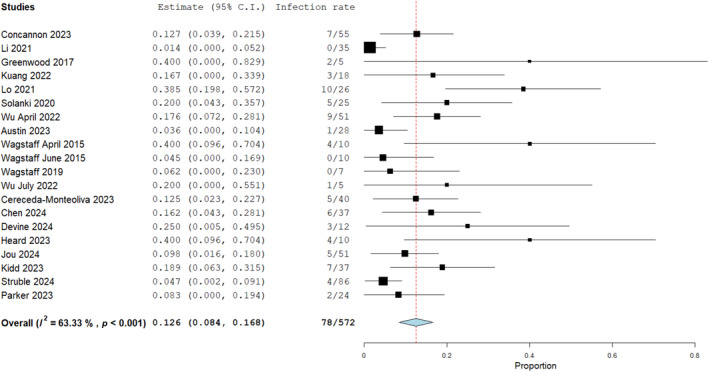
Infection rate for biodegradable temporising matrix (BTM) in complex wound reconstruction, 12.6% with an untransformed proportion metric (0.126, 0.084, 0.168), Heterogeneity, I 2 = 63.33%, p < 0.001. Standard error 0.021, p = 0.001.
A further sensitivity analysis was conducted to assess wounds in the general population to high risk wounds predisposed to infection (necrotising fasciitis aetiology post debridement, diabetic foot wounds, pressure ulcers, any chronic wound, previous infections or wounds of ischemic aetiology). Overall, five studies reported on these two groups of patients and a comparable infection rate was identified on odds ratio assessment with no significant difference observed (Figure 11). This emphasised the robustness of BTM in hostile wound beds.
FIGURE 11.
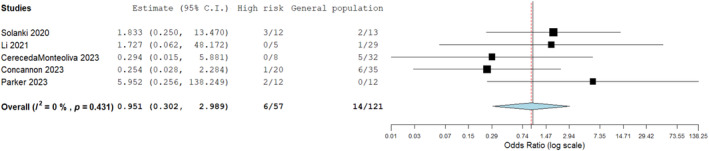
Sensitivity analysis of infection rate comparing high risk wounds (necrotising fasciitis post debridement, diabetic wounds, pressure ulcers, previous infection and any chronic or ischaemic wounds) versus those in the general population. No significant difference seen; odds ratio estimate 0.951 (0.302, 2.989), p = 0.931.
The overall infection rate for BTM in complex wound reconstruction has shown to be fairly low (12.6%, Figure 10) and in comparison to other complications that could be related to the BTM implant, such as haematomas, non‐adherence as well as adhesions necessitating tenolysis, the infection rate occurred at a significantly lower incidence to these (Figure 12). This was observed on odds ratio assessment (Figure 12) where the infection rate was compared to the incidence of these other complications.
FIGURE 12.
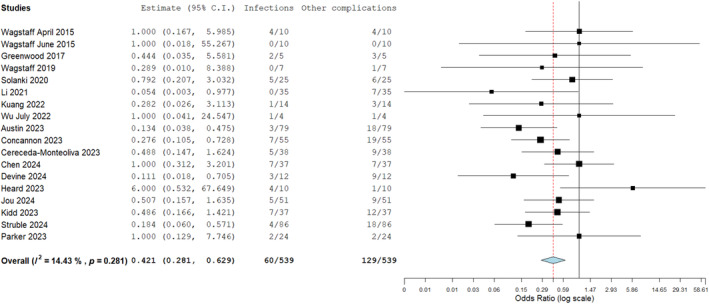
Odds ratio analysis of infection rate compared to other device related complications in biodegradable temporising matrix (BTM) application. The infection rate was significantly lower on assessment: 0.421 (0.281, 0.629, p = 0.281).
Haematomas were reported by numerous studies although of a low incidence. Austin 18 identified two in 79 wounds which were adequately drained allowing the BTM to successfully integrate. Li 5 only identified one in 35 wounds in their case series due to the patient having a fall and Concannon 10 shared a similar incidence with one report in 70 wounds. In Greenwood's 1 case series of five patients, five different sites of haematoma developed in one patient who was heparinised for dialysis following a burn injury whereas on the other hand in the study by Wu, 28 there were no reports and two in 51 within their other study in April 2022. 20 Li 5 demonstrated two cases of failed BTM integration in 35 wounds with Concannon 10 reporting 4 in 50 cases. Concannon 10 also had two cases needing tenolysis in 21 wound beds where the templates were used to reconstruct defects with exposed tendon. Lo 2 reported 254 adverse events overall in a 30 patient cohort. These were categorised into 5.2% severe, 53.5% moderate, and 41.3% mild, with the predominant complication being infection related. This involved re‐application, removal of BTM as well as antibiotic therapy. The authors excluded it from a quantitative comparison with the infection rate as it was not clear what percentage were device related. Four patients died of causes unrelated to BTM, and one patient required surgical release for a contracture. Solanki 11 reported non‐adherence and lack of vascularisation in 5 cases and Wu 20 identified two cases of dehiscence. Concannon 10 reported 11 (39%) complications for BTM applied directly over 28 wounds with bone only and 6 in 9 (67%) over wounds with tendon only. These all included a range of infection, BTM and graft loss as well as haematoma. Solanki 11 reported 2 incidences in 7 wounds (29%) with bone only with non‐adherence as well as lack of vascularisation. Five of the 9 wounds (56%) where BTM was applied to tendons developed complications all of which were infection related and other wound bed types reported a rate of 4 in 7 (57%). 5 , 11 This included non‐adherence as well as lack of vascularisation. Li 5 reported 2 complications in 8 wounds (25%) with bone only. One was an infection and one suffered a breakdown. 5 One wound in 6 (17%) with tendon only sustained a graft loss. 5 The incidence was also fairly low in other wound bed types with 3 in 13 cases (23%) as reported by Li. 5 Kuang 19 had two cases of wound break down (14%) with both cases having BTM applied over granulation tissue. There were four cases of infection reported, 3 of these were over granulation tissue and only one over bone. 19 The current paucity of evidence in relation to lack of detailed descriptions of wound bed types in the review studies and their reported complications limits a thorough assessment of how wound bed type can influence complications with BTM. A summary of all the studies and different complications is given in Tables 3 and 4 below.
TABLE 3.
Summary of complications recorded in each study.
| Study and year | Wounds (n) | Number of complications | Complications |
|---|---|---|---|
| Wagstaff et al., April 2015, 16 | 10 | 7 | Infection under BTM–2 |
| Serous collection under BTM–2 | |||
| BTM failure–1 | |||
| Necrotic wound bed–1 | |||
| Infected donor site | |||
| Wagstaff et al., June 2015, 27 | 10 | 4 | Unrelated death–3 |
| Lost to follow up–1 | |||
| Greenwood et al., 2017 1 | 5 | 12 | Infection under BTM–2 |
| Contracture–3 | |||
| Haematoma under BTM–1 | |||
| BTM failure on shoulder–1 | |||
| Olecranon pressure ulcer–1 | |||
| Faecal contamination–1 | |||
| Necrotic wound bed–1 | |||
| Graft failure over malleoli–1 | |||
| Wagstaff et al., 2019, 4 | 7 | Not reported | Not reported |
| Solanki et al., 2020 11 | 25 | 11 | Infection–5 |
| Non‐adherence–3 | |||
| Incomplete vascularization–3 | |||
| Li et al., 2021, 5 | 35 | 6 | Infection under BTM–1 |
| Graft failure–2 | |||
| Haematoma–1 | |||
| Abdominal sinus–1 | |||
| Wound breakdown under graft–1 | |||
| Lo et al., 2021, 2 | 100 | 254 | Not detailed |
| Kuang et al., 2022, 19 | 18 | Not reported | Infection–22% 4 |
| Schlottmann et al., 2022, 7 | 27 | Not reported | Not reported |
| Wu et al., April 2022, 20 | 51 | 15 | Infection or cellulitis–9 |
| Dehiscence–2 | |||
| Haematoma or seroma–2 | |||
| Other–2 | |||
| Wu et al., July 2022, 28 | 5 | 1 | Infection–1 |
| Austin et al., 2023, 18 | 79 | Not reported | Infection–3 |
| Haematoma–2 | |||
| Cereceda‐monteoliva et al., 2023, 12 | 40 | 7 | Infection–5 |
| Non adherence–5 | |||
| Haematoma–1 | |||
| Delayed healing–1 | |||
| Osteomyelitis–1 | |||
| Seroma–1 | |||
| Concannon et al., 2023, 10 | 70 | 26 | Wound infection–7 |
| BTM loss–9 | |||
| Skin graft loss–7 | |||
| Amputation–2 | |||
| Tendon adhesions–2 | |||
| Haematoma–1 | |||
|
Tapking et al (REF) Feb 2024 |
300 | Not reported | Not reported |
| Struble et al., (REF), 2024 | 86 | 15 | Cellulitis or infection–4 |
| Haematoma or collection–7 | |||
| Early delamination–4 | |||
| Parker et al., (REF), July 2023 | 24 | 9 | Wound break down–2 |
| Overgranulation–1 | |||
| Graft loss–4 | |||
| Columella misalignment–1 | |||
| Necrotic wound base–1 | |||
| Meagher et al., (REF), March 2024 | 22 | Not reported | Not reported |
| Lo et al., (REF) May 2023 | 16 | Not reported | Not reported |
| Kidd et al., (REF), August 2023 | 37 | 19 | Total BTM loss–9 |
| Partial BTM loss–2 | |||
| Cellulitis–5 | |||
| Deep infection–2 | |||
| Haematoma–1 | |||
| Jou et al., (REF) May 2024 | 51 | 14 | Infection–5 |
| Harmatoma or sermoma–5 | |||
| Total BTM loss–3 | |||
| Partial BTM loss–1 | |||
| Heard et al., (REF), 2023 | 10 | 5 | Infection–4 |
| Haematoma–1 | |||
| Guerriero et al., Feb 2023 | 23 | 7 | Infection–3 |
| Minor amputation–3 | |||
| Major amputation–1 | |||
| Fuest et al., (REF), May 2023 | 27 | Not reported | Not reported |
| Devine et al., (REF), Feb 2024 | 12 | 9 | Infection–3 |
| Haematoma–3 | |||
| Failure of integration–2 | |||
| Delayed wound break down–1 | |||
| Chen et al., (REF), May 2024 | 37 | 10 | Infection–6 |
| BTM poor take–4 |
Abbreviation: BTM, biodegradable temporising matrix.
TABLE 4.
Summary of demographic characteristics as well as wound aetiology, wound acuity, structures in wound base, anatomical location, area of wound, follow up periods for all included studies and outcomes analysed.
| Study and year | Mean/median Age/Range (yrs) | Patients (n) | Male/Female (n) | Wounds (n) | Wound aetiology (n) | Acute/Chronic | Wound base (n) | Wound location (n) | Average Surface area of BTM cm2/%TBSA | Follow up period (months) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wagstaff et al., April 2015, 16 | 62.2 (mean) | 10 | Not reported | 10 | Alt flap–3 | 10:0 | Not reported | Upper limb–4 | Not reported | 12 months | Integration (91.0%) |
| Fibular osseous flap–3 radial/ulnar forearm flap–4 | Lower limb–6 | ||||||||||
| (46–76) | |||||||||||
| Integration time (29.5 days) | |||||||||||
| SSG take (90.5%) | |||||||||||
| Infection rate (40.0%) | |||||||||||
| Wagstaff et al., June 2015, 27 | 58.5 (mean) | 10 | Not reported | 10 | Fibular osseous flap–2 | 10:0 | Not reported | Upper limb–7 | Not reported | 2.9–14.1 months | Integration (100%) |
| Lower limb–2 | |||||||||||
| Radial forearm flap–7 | |||||||||||
| (48–68) | Integration time (35.4 days) | ||||||||||
| SSG take (100%) | |||||||||||
| Infection rate (4.5%) | |||||||||||
| Greenwood et al., 2017 1 | 42.2 (mean) | 5 | 5 males | 5 | Burns–5 | 5:0 | Extensive wound with burn eschar excision | Axilla–1 | 23% | 12 months | Integration (89.8%) |
| Upper limb–5 | |||||||||||
| (18–70) | |||||||||||
| Trunk–3 | Integration time (33.2 days) | ||||||||||
| Lower limb–3 | |||||||||||
| SSG take (98.4%) | |||||||||||
| Infection rate (40.0%) | |||||||||||
| Wagstaff et al., 2019, 4 | 51.6 (mean) | 7 | 4 males | 7 | Post‐debridement of necrotising fasciitis–7 | 7:0 | Gland–1 | Neck–2 | 8.57% | 1–20 months (range) | Integration (98.1%) |
| 3 females | Muscle–1 | Chest–2 | |||||||||
| Blood vessels–2 | Lower limb–3 | Integration time (34.0 days) | |||||||||
| Exposed bone denuded of periosteum–2 | |||||||||||
| SSG take (100%) | |||||||||||
| Exposed joints–1 | |||||||||||
| Infection rate (6.2%) | |||||||||||
| Solanki et al., 2020 11 | 50 (median) | 25 | 19 males | 25 | Acute full thickness burn–3 | 20:5 | Exposed bone–8 exposed tendon–11 wound temporalisation with SSG–3 Desirable/aesthetic reconstruction–5 | Head–2 | Not reported | 3 months (median) | Integration time (37.8 days) |
| 6 females | Neck–2 | ||||||||||
| (15–86) | Burn scar/contracture release–5 | Upper limb–9 | |||||||||
| Trunk–3 | |||||||||||
| Lower limb–9 | 1–7 months (range) | Infection rate (20.0%) | |||||||||
| Necrotising soft tissue infection–7 | |||||||||||
| Tumour excision–3 | |||||||||||
| Traumatic loss–7 | |||||||||||
| Li et al., 2021, 5 |
73.7 (mean) (47–95) |
27 | 19 males | 35 | Pressure ulcer–2 | 16:11 | Muscle–10 | Head and neck–12 | Not reported | 3–18 months (range) | Integration (96.9%) |
| 8 females | |||||||||||
| Failed SSG–5 | Bone–7 | Breast–1 | |||||||||
| Wound breakdown–4 | Tendon–10 | Upper limb–7 | Integration time (35.4 days) | ||||||||
| Fat–7 | Abdomen–1 | ||||||||||
| Surgical wounds–12 | Paratenon–2 | Lower limb–14 | |||||||||
| Trauma–4 | Perichondrium–3 | ||||||||||
| Periosteum–2 | SSG take (87.9%) | ||||||||||
| Infection rate (1.4%) | |||||||||||
| Lo et al., 2021, 2 |
45.2 (mean) (18–70) |
26 |
22 males 4 females |
100 | Burn–26 | 26:0 | Not reported | Chest–23 | 2137.8 cm2 | 12 months | Integration (88.6%) |
| Abdomen–13 | |||||||||||
| Back–2 | Integration time (31.9 days) | ||||||||||
| Upper limb–34 | |||||||||||
| Lower limb–28 | |||||||||||
| SSG take (81.9%) | |||||||||||
| Infection rate (38.5%) | |||||||||||
| Kuang et al., 2022, 19 |
56 (median) (31–86) |
18 |
16 males 2 females |
18 |
Debridement–9 Amputation–8 Ulcer–1 |
17:1 |
Granulation tissue–12 Bone–3 Fascia/peroneal retinaculum/plantar fascia–3 |
Foot–18 | Not reported | 3–18 months (range) | Intergration time (38.8 days) |
| Infection rate (7.1%) | |||||||||||
| Schlottmann et al., 2022, 7 |
50.8 (median) (15–82) |
20 |
12 males 8 females |
27 |
Pressure ulcer–2 Neurofibromatosis–1 Necrotising fasciitis–1 Burn–7 Chronic ulcer–2 Malignancy resection–2 Unstable scar–2 Full thickness soft tissue defect–3 |
14:6 | Not reported | Head–3 | Not reported | Not reported | |
| Neck–2 | |||||||||||
| Upper limb–6 | |||||||||||
| Trunk–4 | |||||||||||
| Lower limb–11 | |||||||||||
| Genital–1 | |||||||||||
| Wu et al., April 2022, 20 |
48.2 (mean) (18–93) |
51 | 33 males | 51 | Burn–7 | Not categorised | Not reported | Head and neck–5 | 110 cm2 | 6.4 months (median) | Infection rate (17.6%) |
| 18 females | Trauma–24 | ||||||||||
| Surgical wound/pressure ulcer–10 | Upper limb–24 | ||||||||||
| Trunk–1 | |||||||||||
| Osteomyelitis–2 | Lower limb–21 | ||||||||||
| Compartment syndrome–2 | |||||||||||
| Skin Malignancy–2 | |||||||||||
| Others–4 | |||||||||||
| Wu et al., July 2022, 28 | 66 (mean) | 5 | 3 males | 5 | Burn–2 | Not categorised | Not reported | Head and neck–5 | 213 cm2 |
15.3 (median) Range: 3.8–26.8 |
Infection rate (20.0%) |
| 2 females | Trauma–1 | ||||||||||
| Surgical wound or pressure ulcer–1 | |||||||||||
| Skin Malignancy–1 | |||||||||||
| Austin et al., 2023, 18 | 46.6 (mean) | 28 | 16 males | 79 | Burns–20 | 23:5 | Not reported | Not reported | 1227 cm2 | Not reported | Integration time (31.6 days) |
| 12 females | Complex wound–5 | ||||||||||
| Trauma–3 | |||||||||||
| Infection rate (3.8%) | |||||||||||
| Cereceda‐monteoliva et al., 2023, 12 |
60 (mean) (5–91) |
38 | 26 males | 40 | Skin cancer–19 | 35:3 | Exposed tendon–14 | Scalp–6 | 1.29% | 3.4 months (mean) | Infection rate (13.2%) |
| 12 females | Burns–12 | Exposed bone–8 | Face–2 | ||||||||
| Scar revision–3 | Exposed tendon and bone–10 exposed tendon and bone plus failed SSG–2 | Upper limb–8 | |||||||||
| Infection–3 | Trunk–2 | ||||||||||
| Lower limb–22 (including 1 perineal burn) | |||||||||||
| Trauma–3 | |||||||||||
| Failed skin graft–2 | |||||||||||
| Pressure area–2 | |||||||||||
| Delayed wound healing–1 | |||||||||||
| Overlying umbilicus–1 | |||||||||||
| Concannon et al., 2023, 10 |
55 (mean) (17–94) |
55 | 41 males | 70 |
Burns–15 Trauma–19 Infection–10 Ischaemic–7 Oncological defect reconstruction–4 |
55:0 |
Exposed bone devoid of periosteum–42 Exposed tendon–21 Bone and tendon–7 |
Scalp–8 Neck–2 Upper limb–11 Trunk–5 Lower limb–31 |
Not reported |
18 months (mean) 3–72 months (range) |
Integration time (46.2 days) |
| 14 females | |||||||||||
| Infection rate (12.7%) | |||||||||||
| Tapking et al., Feb 2024, 44 | 54.2 (mean) | 300 | 199 males | 300 |
Burns–179 Trauma–59 Chronic wound–39 Infection–14 Malignancy–6 Not mentioned–3 |
39:261 |
Subcutis–144 Tendon–42 Joint capsule–46 Bone–29 Not mentioned–49 |
Head and neck–8 Trunk–47 Upper limb–70 Lower limb–141 Not mentioned–34 |
4.1 | Not reported | Integration (82.7%) |
| 101 females | |||||||||||
| Integration time (32 days) | |||||||||||
| SSG take (86%) | |||||||||||
| Struble et al., 2024, 25 |
16.9 (mean) (0.3–81.4) |
54 | 28 males | 86 |
Trauma–31 Infection–16 Malignancy–10 Burns–9 Flap donor site–8 Chronic wound–6 Vasopressor necrosis–4 Flap loss–4 Fasciotomy site–1 |
80:6 |
Muscle–41 Tendon–33 Bone–19 Joints–11 Nerve–7 Blood vessel–6 |
Upper limb–45 | 60 cm2 | Not reported | Integration (88.6%) |
| 23 females | |||||||||||
| Lower limb–41 | |||||||||||
| 3 transgender (FtM) | Integration time (27.7 days) | ||||||||||
| SSG take (92.1%) | |||||||||||
| Infection rate (4.7%) | |||||||||||
| Parker et al., 2023, 43 |
57 (mean) (19–89) |
23 | 14 male | 24 | Infection–8 | 21:3 | Muscle–8 | Lower limb–10 | <1% | Not reported | Integration (95.2%) |
| 9 female | Malignancy–6 | Bone–5 | Head and neck–6 | ||||||||
| Burns–4 | Fascia–5 | Integration time (44.2 days) | |||||||||
| Torso–3 | |||||||||||
| Chronic wound–3 | Tendon–4 |
Groin/genitals–3 Upper limb–1 |
|||||||||
| Free flap failure–2 | Testicle–3 | ||||||||||
| Surgical wound dehiscence–1 | SSG take (86.1%) | ||||||||||
| Fat and cartilage–1 | |||||||||||
| Infection rate (8.3%) | |||||||||||
| Meagher et al., 2024, 6 | (30–95) | 22 | 16 male | 22 | Peripheral vascular disease–5 | 18:4 | Not reported | Lower limb–21 | Not reported | Not reported | None |
| 6 female | |||||||||||
| Chest–1= | |||||||||||
| Necrotising fasciitis–4 | |||||||||||
| Pressure ulcers–4 | |||||||||||
| Haematoma–3 | |||||||||||
| Crush–2 | |||||||||||
| Malignancy–1 | |||||||||||
| Vasculitis–1 | |||||||||||
| Lo et al., 2023, 29 | 49.1 (mean) | 15 | 13 male | 16 | Burns–13 | 15:1 | Subcutaneous fat–25 | Upper limb–11 | 25.2% | 56 | None |
| 2 female | Necrotising fasciitis–2 | ||||||||||
| Fascia–11 | Lower limb–17 | ||||||||||
| Bone–2 | |||||||||||
| Secondary burn reconstruction–1 | Mixed wound bed–1 | Trunk–4 | |||||||||
| Head and neck–1 | |||||||||||
| Multiple area–6 | |||||||||||
| Kidd et al., 2023, 24 |
52 (mean) (14–93) |
37 | 24 male | 37 | Trauma–19 | 28:9 | Muscle–17 | Lower limb–21 | 1% (0.5%–15%) | Not reported | Integration time (53 days) |
| 13 female | Chrnoic wounds–9 | Tendon–12 | Upper limb–7 | ||||||||
| Infection–6 | Bone–6 | Torso–6 | Infection rate (18.9%) | ||||||||
| Malignancy–3 | Head and neck–3 | ||||||||||
| Jou et al., 2024, 45 |
44.3 (mean) (13–97) |
51 | 39 male | 51 | Trauma–30 | 51:0 | Tendon–27 | Upper limb–51 | 162.5 cm2 | 2–40 | Integration time (51.7 days) |
| 12 female | Burns–12 | Bone–24 | |||||||||
| Infection–8 | |||||||||||
| Iatrogenic–1 | Infection rate (9.8%) | ||||||||||
| Heard et al., 2023, 26 |
24.1 (mean) (10–41) |
10 | 7 male | 10 | Burns–10 | 10:0 | Not reported | Massive burns (mean 80.8% TBSA) | Not reported | Not reported | Integration time (83.2 days) |
| 3 female | |||||||||||
| Infection rate (40.0%) | |||||||||||
| Guerriero et al., 2023, 23 | 67.3 (mean) | 22 | 19 male | 23 | Diabetic foot wounds–22 | 22:0 | Not reported | Foot–22 | Not reported | 5 months | Infection rate (13.0%) |
| 3 female | |||||||||||
| Fuest et al., 2023, 46 |
51 (mean) (12–77) |
27 | 16 male | 27 | Trauma–20 | 27:0 | Not reported | Hand–27 | Not reported | Not reported | Integration time (43.6 days) |
| 11 female | Infection–4 | ||||||||||
| Malignancy–1 | |||||||||||
| Dupuyten's–1 | SSG take (96%) | ||||||||||
| Dermatomyositis–1 | |||||||||||
| Devine et al., 2024, 22 | 70 (mean) | 12 | 8 male | 12 | Malignancy–12 | 12:0 | Not reported | Head and neck–8 | Not reported | Not reported | Integration time (49 days) |
| 4 female | |||||||||||
| Lower limb–3 | SSG take (70.0%) | ||||||||||
| Genirourinary–1 | Infection rate (25%) | ||||||||||
| Chen et al., 2024, 21 | 51.8 (mean) | 37 | 22 male | 37 | Trauma–25 | 37:0 | Not reported | Lower limb–19 | 50.6 cm2 | 7.0 months | Integration time (36.9 days) |
| 15 female | Necrotising fasciitis–6 | Foot–10 | |||||||||
| (18–86) | Hand–4 | ||||||||||
| Burn–4 | |||||||||||
| Other–2 | Upper limb–3 | Infection rate (2.7%) | |||||||||
| Trunk–1 |
Abbreviation: BTM, biodegradable temporising matrix; SSG, split thickness skin graft: TBSA, total body surface area.
3.6. Scar appearance
Scar appearance was reported by numerous studies using both POSAS and MAPS assessment scales with Greenwood 1 reporting good objective outcomes. The average MAPS score was 3 with a mean observer score of 18. Lo 2 reported a significant improvement in the Vancouver scar scale with a decrease in the score from 5.6 at 3 months (95% CI 4.7, 6.6) to 3.0 at 12 months (95% CI 2.6, 3.5). A significant proportion (54.1%) of patients had normal pigmentation at the 12 month point with 36.7% also having normal vascularity and 82.8% demonstrating normal or supple pliability as well. Li 5 also reported good outcomes with POSAS observer scale showing consistently low scores with a mean overall patient score of 5.67 ± 2.82 out of 10 being observed. On this scale one represents normal skin and 10 being very different to normal skin. A mean overall observer score also from an independent plastic surgeon was 3.63 ± 2.04. In addition Li 5 reported average sensory restoration of 5.86 ± 2.72 out of 10 in their series. Wagstaff 16 reported low MAPS and POSAS scores both indicating good scar characteristics. Chen 21 used the Manchester scar scale for assessment which consisted of evaluating for colour, shine, contour, distortion, texture and overall rating with the Visual Analogue scale. Patients who underwent BTM application without grafting achieved better scar outcomes compared to those who had skin graft only. Lo 29 used the POSAS assessment scale to evaluate scar outcomes. Low to indeterminate scores were achieved for many domains including vascularity, pigmentation, thickness, relief pliability and surface area on both observer as well as patient assessments. Struble 25 briefly discussed long term scar outcomes although they didn't use any formal assessment tools but reported scars to be more softer and supple after BTM than directly going for a skin graft during reconstruction.
3.7. Methodological quality assessment
Methodological quality was assessed using the Newcastle‐Ottawa scale in which studies were scored across three domains using an advocated star system (Table 5). The star system uses predefined guidelines to score the selection of study groups, comparability of the groups, and the assessment of outcome. 17 All studies included in the meta‐analysis were awarded three or four stars for selection, indicating that the studies appropriately represented their respective populations. However, comparability was limited due to the inherent nature of most of studies and the absence of a control arm in the majority of articles. All studies received scores of two or three stars for the assessment of outcomes, demonstrating that the outcomes were measured with appropriate rigour.
TABLE 5.
Newcastle‐Ottawa assessment for methodological quality with good scores for selection and outcome domains but poor for comparability.
| Study | Selection | Comparability | Outcome |
|---|---|---|---|
| Wagstaff et al., 2015 16 | **** | ** | |
| Wagstaff et al., 2015 27 | **** | ** | |
| Greenwood et al., 2016 3 | *** | ** | |
| Wagstaff et al., 2019 4 | **** | ** | |
| Solanki et al., 2020 11 | **** | ** | |
| Lo et al., 2021 2 | **** | *** | |
| Li et al., 2021 5 | **** | ** | |
| Wu et al., 2022 (April) 20 | **** | * | *** |
| Wu et al., 2022 (July) 28 | **** | * | *** |
| Kuang et al., 2022 19 | **** | ** | |
| Schlottman et al., 2022 7 | **** | ** | |
| Austin et al., 2023 18 | **** | *** | |
| Concannon et al., 2023 10 | **** | *** | |
| Cereceda‐Monteoliva et al., 2023 12 | **** | ** | |
| Chen et al., 2024 21 | *** | *** | |
| Devine et al., 2024 22 | *** | *** | |
| Fuest et al., 2024 46 | ** | ** | |
| Guerriero 2023 23 | ** | * | |
| Heard et al., 2023 26 | *** | ** | |
| Jou et al., 2024 45 | ** | *** | |
| Kidd et al., 2023 24 | ** | ** | |
| Lo et al., 2023 29 | ** | *** | |
| Meagher et al., 2024 6 | * | * | |
| Parker et al., 2023 43 | **** | *** | |
| Struble et al., 2024 25 | **** | *** | |
| Tapking 2024 44 | **** | *** |
Note: The Newcastle‐Ottawa Scale awards stars for study quality across three domains: Selection, Comparability, and Outcome/Exposure. More stars indicate higher quality.
4. DISCUSSION
The authors report a systematic review and meta‐analysis of the literature on the application of BTM for complex wound reconstruction with an outcome synthesis. Previously, Ferreira, 14 depicted a complex wound as one which fell into the following catergories; extensive loss of the integument in acute or chronic wounds, infection as a complication in chronic wounds, necrosis and compromised viability of surrounding tissue or signs of circulation impairment as well as systematic pathologies that impair normal wound healing (e.g., diabetes, vasculitis or immune suppression). The authors abided to these criteria upon selection of wounds as part of this review for applying BTM with defects involving exposed bone, deep to full thickness burns post excision, wounds with exposed muscle, tendon, chronic wounds such as ulcers and wounds post debridement from infective as well as ischaemic aetiologies.
BTM offer an alternative to other complex therapies in soft tissue reconstruction such as free tissue transfer or locoregional flaps. These techniques are routinely instigated for large defects with non graftable wound beds or those where skin grafts would produce sub‐optimal outcomes due to large volumes of tissue loss. The use of BTM for any complex wound routinely involves a two stage process for usage with the initial phase normally necessitating a thorough debridement with all burn wounds undergoing excision and a template cut to fit the defect that can be affixed with either suture material or staples. The sealing membrane is faced externally and removed at the second stage of delamination at which point the neodermis can be refreshed and a skin graft applied. 8 , 9
Twenty one studies met the inclusion criteria for quantitative assessment overall and a high percentage of BTM take (92.7%) was evidenced across all wound types with an average of 34 days to integrate prior to the second stage of skin graft application. Secondary outcomes including the percentage graft take over integrated BTM was promising at 98.9%. The infection rate was low (12.6%) with a further sensitivity analysis comparing high risk wounds to those in the general population demonstrating the rate to be comparable on odds ratio assessment evidencing the dermal template's resistance to infection (Figure 11). This included defects post debridement for necrotising fasciitis, diabetic foot wounds, any chronic wound, pressure ulcers as well as those with previous or current infections and ones of an ischaemic aetiology. The rate of infection was also found to be significantly lower compared to miscellaneous device related adverse effects (Figure 12) some of which included, haematomas, non‐adherence as well as adhesions necessitating tenolysis. Scar appearance was encouraging with improvements in both MAPS and POSAS assessment scales as reported by Greenwood, Li and Wagstaff. 1 , 5 , 27 Lo 2 identified an improvement in the Vancouver scar scale with a decrease in score at a 12‐month period. A direct comparison of BTM application over bone compared to tendon showed no significant difference in terms of the integration time needed however the integration period was significantly longer for bone compared to other tissue wound beds. This would suggest that clinicians need to leave the device on for longer when using it to reconstruct bony defects. The authors found a comparable integration time for BTM in tendon reconstruction compared to general soft tissues on mean difference assessment with no significant difference seen. In the context of all quantified outcomes within the review heterogeneity was moderate to high for the majority of variables including integration time. This was circumvented by adaptation of a random effects model or an inverse variance function. These are statistical functions conducted to account for scenarios when heterogeneity is elevated and equates for any discrepancies in outcomes in the overall effect estimate.
In a recent systematic review by Grande, 30 they also identified a high integration rate of BTM with over 84% of wounds experiencing greater than 95% integration with a mean take of 95%. Similarly, the mean rate of skin graft survival over implanted BTM was 95%. These findings are comparable to the results of the current meta‐analysis. Grande 30 also identified the time for BTM integration as 36.7 days which parallels to the author's finding of 34 days. The results of the current review identified a comparable infection rate between high risk wounds and those in the general population and while time to implantation can be prolonged with infection, 30 results for skin graft survival have shown to be equivocal by Grande. 30
BTM's versatility in complex wound reconstruction has seen it to successfully integrate despite patients with multiple co‐morbidities. 5 This offers a simplistic option in patients who are not suitable for more complex reconstructive techniques such as free tissue transfer. In addition, numerous cases have shown successful re‐epithelialisation without second stage skin graft application as well. 5 , 19 BTM has drawn comparable outcomes to other dermal substitutes in the literature including integra, matriderm as well as glyaderm in burn injuries. 2 Near equivocal device integration and split thickness skin graft take rates have been reported as well as the duration to graft. 2 , 31 , 32 , 33 , 34 Although in some animal studies on direct comparison, BTM has demonstrated greater neovascularisation at similar time periods on histological assessment 35 and an increased wound regeneration capacity. 36 This would explain BTMs ability to form vascularised neo‐dermal layers in complex avascular wound beds. The original work of Yannas and Burke 37 on requirements of dermal matrices already indicated that there is an optimal porosity. BTM's porosity is optimised to enhance cell migration and survival, as demonstrated in their research. It's more porous than collagen based dermal templates enabling increased cell migration and survival. 2 The additional potential for sensory restoration using BTM can enhance the quality of life in patients with lower limb defects. In the case series by Li, 5 partial sensory restoration was reported in most of the reconstructed wounds which is encouraging.
Integra which consists of bovine collagen and cross linked glycosaminoglycans has reported graft take rates between 90% and 93% 38 which although comparable appears inferior to that of BTM as evidenced from the authors' analysis (98.9%). This in addition to other studies having reported even lower graft take rates over Integra. 31 , 39 The comparison is similar for MatriDerm, another bovine derived dermal substitute with graft take rates of 83.4% 32 as well as 73.4%. 34 Cheshire 35 directly compared BTM and Integra in mice models with BTM demonstrating a more extensive vascular network which could explain its superior wound healing. MatriDerm and Integra have inherent limitations as well given their animal origin precluding their usage in certain religious groups. 40 BTM is purely synthetic and therefore mitigates this problem. Reported infection rates for Integra have been referenced as high as 19.3% 41 in contrast to BTM (12.6%) from the authors' quantification. Matriderm demonstrates a more comparable rate of 11.8%. 42 Both Integra and BTM are two stage devices and are seen to have comparable matrix integration rates with reports for Integra ranging at 95%–100% 31 , 33 and BTM identified as 92.7% from the current meta‐analysis.
The authors report a systematic review and meta‐analysis on the use of BTM in complex wound reconstruction. It collates 26 studies that have met the inclusion criteria for analysis. The limitations of this review include all studies being of an observational design with low comparability scores on methodological quality assessment, however there was a large number of wounds. Overall, 1153 wounds in 26 studies assessed the effectiveness of BTM in a variety of wound aetiologies with good scores for the selection and exposure domains on Newcastle‐Ottawa assessment although comparability was very poor (Table 5). Many variables were not consistently reported by a lot of the articles which limited the number of studies that were amenable to meta‐analysis. For instance, the proportion of BTM integration was reported heterogeneously with some reports recording it as the proportion of integration relative to the area implanted whereas others reported it as a success rate relative to the number of patients within which it was used. The meta‐analysis did not include any randomsied control trials either which inherently limits it. Heterogeneity also varied from moderate to considerable but the authors adapted random effects models and inverse variance functions to circumvent this. To further enhance the evidence base, the authors recommend for randomised controlled trials directly comparing BTM with other dermal substitutes in complex wound reconstruction. In addition, sub cohort analyses assessing the effectiveness of BTM in different wound types is recommended to evaluate the differences in time to integration as well as complication rates. This current paucity in evidence would be helpful in guiding clinicians when using the device in different wound environments and with varying aetiologies.
5. CONCLUSIONS
This systematic review and meta‐analysis has comprehensively examined the existing literature on the efficacy and versatility of BTM in complex wound reconstruction. It has shown a high rate of take across a variety of wounds and shown promising results including secondary skin graft integration and low infection rates. These outcomes have been strengthened by positive scar outcome and the potential sensory restoration advantages of BTM. The authors recommend further randomised control trials directly comparing BTM with other dermal substitutes.
FUNDING INFORMATION
No funding was received for this work.
CONFLICT OF INTEREST STATEMENT
The author(s) have no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Lane G, Fitzpatrick NJ, Kastritsi O, et al. Biodegradable Temporising matrix in the reconstruction of complex wounds: A systematic review and meta‐analysis. Int Wound J. 2024;21(10):e70025. doi: 10.1111/iwj.70025
George Lane and Niall Fitzpatrick contributed equally to this study and are equal first authors. They both take responsibility for this manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Greenwood JE. The evolution of acute burn care‐retiring the split skin graft. Ann R Coll Surg Engl. 2017;99(6):432‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lo CH, Brown JN, Dantzer EJG, et al. Wound healing and dermal regeneration in severe burn patients treated with NovoSorb® biodegradable Temporising matrix: a prospective clinical study. Burns. 2022;48(3):529‐538. [DOI] [PubMed] [Google Scholar]
- 3. Greenwood JE, Schmitt BJ, Wagstaff MJD. Experience with a synthetic bilayer biodegradable Temporising matrix in significant burn injury. Burns Open. 2018;2(1):17‐34. [Google Scholar]
- 4. Wagstaff MJD, Salna IM, Caplash Y, Greenwood JE. Biodegradable Temporising matrix (BTM) for the reconstruction of defects following serial debridement for necrotising fasciitis: a case series. Burns Open. 2019;3(1):12‐30. [Google Scholar]
- 5. Li H, Lim P, Stanley E, et al. Experience with NovoSorb® biodegradable Temporising matrix in reconstruction of complex wounds. ANZ J Surg. 2021;91(9):1744‐1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meagher H, Holmes T, Hanson C, et al. Application of Novosorb biodegradable temporising matrix in wounds of different aetiologies: a case series. J Wound Care. 2024;33(Sup3):S51‐S58. [DOI] [PubMed] [Google Scholar]
- 7. Schlottmann F, Obed D, Bingöl AS, März V, Vogt PM, Krezdorn N. Treatment of complex wounds with NovoSorb® biodegradable Temporising matrix (BTM)—a retrospective analysis of clinical outcomes. J Pers Med. 2022;12(12):2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Damkat‐Thomas L, Greenwood JE, Wagstaff MJD. A synthetic biodegradable Temporising matrix in Degloving lower extremity trauma reconstruction: a case report. Plast Reconstr Surg Glob Open. 2019;7(4):e2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenwood JE, Wagstaff MJD, Rooke M, Caplash Y. Reconstruction of extensive Calvarial exposure after major burn injury in 2 stages using a biodegradable polyurethane matrix. Eplasty. 2016;16:e17. [PMC free article] [PubMed] [Google Scholar]
- 10. Concannon E, Damkat‐Thomas L, Rose E, Coghlan P, Solanki N, Wagstaff M. Use of a synthetic dermal matrix for reconstruction of 55 patients with Nongraftable wounds and Management of Complications. J Burn Care Res. 2023;44:894‐904. [DOI] [PubMed] [Google Scholar]
- 11. Solanki NS, York B, Gao Y, Baker P, Wong She RB. A consecutive case series of defects reconstructed using NovoSorb® biodegradable Temporising matrix: initial experience and early results. J Plastic Reconstr Aesthetic Surg. 2020;73(10):1845‐1853. [DOI] [PubMed] [Google Scholar]
- 12. Cereceda‐Monteoliva N, Rela M, Borges A, Dheansa B. Early results and initial experience of reconstructing defects with NovoSorb® biodegradable Temporising matrix (BTM): a UK case series. Eur J Plast Surg. 2023;46:1331‐1338. [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferreira MC, Tuma P, Carvalho VF, Kamamoto F. Complex wounds. Clinics. 2006;61(6):571‐578. [DOI] [PubMed] [Google Scholar]
- 15. McCartan B, Dinh T. The use of Split‐thickness skin grafts on diabetic foot ulcerations: a literature review. Plast Surg Int. 2012;14:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagstaff MJD, Schmitt BJ, Coghlan P, Finkemeyer JP, Caplash Y, Greenwood JE. A biodegradable polyurethane dermal matrix in reconstruction of free flap donor sites: a pilot study. Eplasty. 2015;15:e13. [PMC free article] [PubMed] [Google Scholar]
- 17. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. [Internet]. Ottawa Hospital Research Institute; 2012. Accessed July 29, 2024. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 18. Austin CL, Draper B, Larson KW, Thompson SJ. Biodegradable temporising matrix: use of negative pressure wound therapy shows a significantly higher success rate. J Wound Care. 2023;32(3):159‐166. [DOI] [PubMed] [Google Scholar]
- 19. Kuang B, Pena G, Cowled P, et al. Use of biodegradable Temporising matrix (BTM) in the reconstruction of diabetic foot wounds: a pilot study. Scars Burn Heal. 2022;8:20595131221122272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu SS, Wells M, Ascha M, Gatherwright J, Chepla K. Performance of biodegradable temporizing matrix vs collagen‐chondroitin silicone bilayer dermal regeneration substitutes in soft tissue wound healing: a retrospective analysis. Wounds. 2022;34(4):106‐115. [PubMed] [Google Scholar]
- 21. Chen A, Lin TW, Chang KC, Chang DH. Strategic use of biodegradable temporizing matrix (BTM) in wound healing: a case series in Asian patients. J Funct Biomater. 2024;15(5):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devine M, Edmondson M, Gearing P, et al. NovoSorb® biodegradable temporising matrix (BTM) in the reconstruction of cutaneous malignancies in a major cancer centre: a case series. ANZ J Surg. 2024. [DOI] [PubMed] [Google Scholar]
- 23. Guerriero FP, Clark RA, Miller M, Delaney CL. Overcoming barriers to wound healing in a neuropathic and neuro‐Ischaemic diabetic foot cohort using a novel bilayer biodegradable synthetic matrix. Biomedicine. 2023;11(3):721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kidd T, Kolaityte V, Bajaj K, Wallace D, Izadi D, Bechar J. The use of NovoSorb biodegradable temporising matrix in wound management: a literature review and case series. J Wound Care. 2023;32(8):470‐478. [DOI] [PubMed] [Google Scholar]
- 25. Struble SL, Patel NK, Graham EM, et al. Outcomes of biodegradable temporizing matrix for soft tissue reconstruction of the hand and extremities. Plast Reconstr Surg Glob Open. 2024;12(7):e5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heard J, Sen S, Greenhalgh D, Palmieri T, Romanowski K. Use of cultured epithelial autograft in conjunction with biodegradable temporizing matrix in massive burns: a case series. J Burn Care Res. 2023;44(6):1434‐1439. [DOI] [PubMed] [Google Scholar]
- 27. Wagstaff MJD, Schmitt BJ, Caplash Y, Greenwood JE. Free flap donor site reconstruction: a prospective case series using an optimized polyurethane biodegradable temporizing matrix. Eplasty. 2015;15:e27. [PMC free article] [PubMed] [Google Scholar]
- 28. Wu SS, Wells M, Ascha M, Duggal R, Gatherwright J, Chepla K. Head and neck wound reconstruction using biodegradable temporizing matrix versus collagen‐chondroitin silicone bilayer. Eplasty. 2022;22:e31. [PMC free article] [PubMed] [Google Scholar]
- 29. Lo CH, Wagstaff MJD, Barker TM, et al. Long‐term scarring outcomes and safety of patients treated with NovoSorb® biodegradable temporizing matrix (BTM): an observational cohort study. JPRAS Open. 2023;37:42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grande PK, Hill D, McElfresh J, Velamuri R, Liu X. Systematic review and meta‐analysis of biodegradable temporizing matrix application for complex wound reconstruction. J Burn Care Res. 2024:irae081. [DOI] [PubMed] [Google Scholar]
- 31. Burke JF, Yannas OV, Quinby WC, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194(4):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryssel H, Gazyakan E, Germann G, Öhlbauer M. The use of MatriDerm® in early excision and simultaneous autologous skin grafting in burns‐a pilot study. Burns. 2008;34(1):93‐97. [DOI] [PubMed] [Google Scholar]
- 33. Lagus H, Sarlomo‐Rikala M, Bohling T, Vuola J. Prospective study on burns treated with Integra®, a cellulose sponge and split thickness skin graft: comparative clinical and histological study‐randomized controlled trial. Burns. 2013;39(8):1577‐1587. [DOI] [PubMed] [Google Scholar]
- 34. van Zuijlen PPM, van Trier AJM, Vloemans JFPM, Groenevelt F, Kreis RW, Middelkoop E. Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a one‐stage grafting model. Plast Reconstr Surg. 2000;106(3):615‐623. [DOI] [PubMed] [Google Scholar]
- 35. Cheshire PA, Herson MR, Cleland H, Akbarzadeh S. Artificial dermal templates: a comparative study of NovoSorb™ biodegradable Temporising matrix (BTM) and Integra® dermal regeneration template (DRT). Burns. 2016;42(5):1088‐1096. [DOI] [PubMed] [Google Scholar]
- 36. Banakh I, Cheshire P, Rahman M, et al. A comparative study of engineered dermal templates for skin wound repair in a mouse model. Int J Mol Sci. 2020;21(12):4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res. 1980;14(1):65‐81. [DOI] [PubMed] [Google Scholar]
- 38. Hicks KE, Huynh MN, Jeschke M, Malic C. Dermal regenerative matrix use in burn patients: a systematic review. J Plast Reconstr Aesthet Surg. 2019;72(11):1741‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heimbach DM, Warden GD, Luterman A, et al. Multicenter Postapproval clinical trial of Integra® dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24(1):42‐48. [DOI] [PubMed] [Google Scholar]
- 40. Eriksson A, Burcharth J, Rosenberg J. Animal derived products may conflict with religious patients' beliefs. BMC Med Ethics. 2013;14(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonzalez SR, Wolter KG, Yuen JC. Infectious complications associated with the use of Integra: a systematic review of the literature. Plast Reconstr Surg Glob Open. 2020;8(7):e2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phillips GSA, Nizamoglu M, Wakure A, Barnes D, El‐Muttardi N, Dziewulski P. The use of dermal regeneration templates for primary burns surgery in a UK regional burns Centre. Ann Burns Fire Disasters. 2020;33(3):245‐252. [PMC free article] [PubMed] [Google Scholar]
- 43. Parker A, de Berker H, Kiely A, et al. The use of NovoSorb™ biodegradable Temporising matrix (BTM™) in the reconstruction of complex soft tissue defects—an oncological, aesthetic, and practical solution. Eur J Plast Surg. 2023;46(6):1339‐1348. [Google Scholar]
- 44. Tapking C, Thomas BF, Hundeshagen G, et al. NovoSorb® biodegradable Temporising matrix (BTM): what we learned from the first 300 consecutive cases. J Plast Reconstr Aesthet Surg. 2024;92:190‐197. [DOI] [PubMed] [Google Scholar]
- 45. Jou C, Chepla KJ. Novosorb biodegradable temporizing matrix for reconstruction of complex upper‐extremity wounds. J Hand Surg Glob Online. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fuest L, Vögelin E. Biodegradable Temporising matrix: the rising star in synthetic skin substitutes for the hand? J Surg. 2024;9(5):11055. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


