Abstract
We have previously shown that a plasmid (pE) encoding the Japanese encephalitis virus (JEV) envelope (E) protein conferred a high level of protection against a lethal viral challenge. In the present study, we used adoptive transfer experiments and gene knockout mice to demonstrate that the DNA-induced E-specific antibody alone can confer protection in the absence of cytotoxic T-lymphocyte (CTL) functions. Plasmid pE administered by either intramuscular or gene gun injection produced significant E-specific antibodies, helper T (Th)-cell proliferative responses, and CTL activities. Animals receiving suboptimal DNA vaccination produced low titers of anti-E antibodies and were only partially or not protected from viral challenge, indicating a strong correlation between anti-E antibodies and the protective capacity. This observation was confirmed by adoptive transfer experiments. Intravenous transfer of E-specific antisera but not crude or T-cell-enriched immune splenocytes to sublethally irradiated hosts conferred protection against a lethal JEV challenge. Furthermore, experiments with gene knockout mice showed that DNA vaccination did not induce anti-E titers and protective immunity in Igμ−/− and I-Aβ−/− mice, whereas in CD8α−/− mice the pE-induced antibody titers and protective rate were comparable to those produced in the wild-type mice. Taken together, these results demonstrate that the anti-E antibody is the most critical protective component in this JEV challenge model and that production of anti-E antibody by pE DNA vaccine is dependent on the presence of CD4+ T cells but independent of CD8+ T cells.
Japanese encephalitis virus (JEV) is a member of the Flaviviridae that causes diseases of the human central nervous system in many areas of the world, especially in Southeast Asia. Among those with clinical symptoms, the mortality rate can be as high as 10 to 30%, and a majority of patients who recover suffer severe neurological sequelae (22). Vaccination remains one of the most promising approaches to reducing JEV infections. Inactivated JEV vaccines prepared from infected mouse brains or primary hamster kidney cells and a live-attenuated SA14-14-2 vaccine have been used in many parts of Asia with measurable success (31). However, there are several disadvantages to the currently used vaccines. The mouse brain-derived inactivated JEV vaccine is costly to prepare, is unable to induce long-term immunity (26), and most importantly carries the risk of inducing allergic reactions (M. M. Andersen and T. Ronne, Letter, Lancet 337:1044, 1991). The SA14-14-2 attenuated vaccine is efficacious; however, production and regulatory standards for this vaccine are not established yet. Consequently, there has been a significant effort in recent years aimed at employing recombinant DNA technology to produce improved JEV vaccines.
Successful development of efficacious vaccines will be expedited if the immune responses that contribute to disease control are understood. In JEV infection, the immunity against membrane (M), envelope (E), and NS1 nonstructural proteins is effective in host defense. The antibody responses elicited by these viral proteins appear to play the major protective role. Passive transfer of monoclonal antibodies against E proteins protects mice against JEV encephalitis (10, 18). Recombinant vaccinia viruses expressing precursor M (pre-M) and E proteins or E protein alone are highly effective at eliciting neutralizing antibodies and protection against JEV challenge in immunized mice (9, 19) and pigs (14). The NS1 protein also evokes a strong antibody response that protects the host against challenge (16). The role of T-cell immunity in JEV protection is less well defined. In JEV-infected patients, the virus-specific CD4+ and CD8+ T lymphocytes have been isolated and found to proliferate in response to JEV stimulation (11). Vaccinees receiving the formalin-inactivated JEV vaccine (1) or the poxvirus-based JEV vaccine (13) have been shown elsewhere to produce CD4+ or CD8+ T cells, respectively, that can mediate JEV-specific cytotoxic activities. In the murine model, JEV-specific cytotoxic T lymphocytes (CTLs) are induced by JEV infection (24) and by immunization with extracellular particle-based (15) or poxvirus-based (12) JEV vaccines. Whether these specific T-cell responses are protective against JEV infection is still controversial and remains to be resolved. Adoptive transfer of immune splenocytes or T lymphocytes was reported previously to protect mice from a lethal JEV challenge (20, 25). However, under some circumstances the adoptively transferred T cells were not protective, owing to the different routes of transfer as well as the age and strain of the recipient animals (21, 25). A more comprehensive study using JEV vaccines that can efficiently induce cellular immune responses is required to address this question.
DNA vaccines have been demonstrated previously in many animal models to induce a broad range of immune responses, including antibodies, CD8+ CTLs, CD4+ helper T (Th) lymphocytes, and protective immunity against challenge with the pathogen (7, 8). Several recent clinical trials have demonstrated the ability of DNA vaccines to induce antigen-specific CTLs in humans, although their potency is limited (4, 32). The ability of DNA immunization to elicit both antibody and CTL immunity makes it an ideal vaccination approach to evaluate the relative roles of these immune responses in host defense against viral infection. We previously showed that a plasmid (pE) encoding the JEV E protein produced high titers of E-specific antibodies and provided protection against a lethal JEV challenge (6). Immunization with plasmids encoding other structural (capsid) or nonstructural (NS1-2A, NS3, and NS5) proteins was ineffective. In this study, we show that pE immunization by intramuscular or gene gun injection also produces significant Th-cell proliferation and CTL responses. Using adoptive transfer experiments and a panel of gene knockout mice, we demonstrate that DNA-induced antibody alone is able to confer protection in the absence of T-cell-mediated immunity. These results provide important information for future development of safe and efficacious JEV vaccines.
MATERIALS AND METHODS
Animals and viruses.
Female C3H/HeN mice were purchased from National Laboratory Animal Breeding and Research Center, Taipei, Taiwan. Female C57BL/6 mice were obtained from the Laboratory Animal Facility, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan. C57BL/6-IgH-6tm1Cgn (Igμ−/−) and C57BL/6-CD8αtm1Mak (CD8α−/−) mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). Breeding pairs of C57BL/6-I-Aβ−/− (I-Aβ−/−) and C57BL/6-β2mUnc (β2m−/−) mice, originally from the Jackson Laboratory, were kindly provided by John Kung (Academia Sinica) and maintained as a small breeding colony in our own animal facility. The phenotypes of Igμ−/− mice (absence of serum immunoglobulin [Ig]), I-Aβ−/− mice (absence of CD4+ T cells), and CD8α−/− mice (absence of CD8+ T cells) were confirmed by enzyme-linked immunosorbent assay (ELISA) and flow cytometry. β2m−/− mice were completely lacking in CD8+ T cells and had significantly less serum Ig than did other mice (0.3 ± 0.1 mg/ml versus 4.9 ± 1.6 mg/ml for wild-type mice). Animal care was provided in accordance with the guidelines approved by the Animal Committee of the Institute of Biomedical Sciences, Academia Sinica.
The JEV strain Beijing-1, prepared from suckling mouse brain, was used to make virus stock for challenge experiments (6). The 50% lethal doses (LD50) for 12- to 14-week-old C3H/HeN and C57BL/6 mice were previously determined to be 6.0 × 105 and 1 × 106 PFU, respectively.
Immunization and viral challenge.
The plasmid pE encoding the envelope protein of JEV and its parental vector pcDNA3 were previously described (6). DNA was purified from transformed Escherichia coli strain DH5α with Qiagen Plasmid Giga kits (Qiagen, Hilden, Germany) and reconstituted in sterile saline for experimental use. All mice were immunized at 6 to 8 weeks of age. The intramuscular or gene gun DNA immunization and the sublethal live virus immunization were performed as previously described (6). For intramuscular DNA immunization, animals were pretreated 1 week earlier with 100 μl of 10 μM cardiotoxin (Sigma, St. Louis, Mo.) and injected with 50 μg of DNA bilaterally in each quadriceps muscle. For gene gun DNA immunization, each animal received 1 μg of DNA in the abdominal epidermis with a helium pressure setting of 500 lb/in2. The sublethal live virus immunization was performed by intraperitoneal injection of 1.0 × 106 PFU of JEV Beijing-1 without a sham intracerebral inoculation. For booster immunization, animals were treated with the same amount of antigen at 3-week intervals. The number of injections for each group is given in the legend to each figure. The immunized animals were lethally challenged with JEV Beijing-1 at a dose of 50 times the LD50 for the respective mouse strain, followed by a sham intracerebral inoculation. The JEV-challenged animals were observed for symptoms of viral encephalitis and death every day for 30 days.
Antibody assays.
To analyze the presence of JEV E-specific antibodies, mice sera were prepared by tail bleeding and analyzed by ELISA as previously described (6). Briefly, serum samples were added to microtiter plates coated with live JEV virions produced in Vero cell cultures. The bound antibodies were detected with horseradish peroxidase-conjugated goat anti-mouse IgG Fc (1:1,000; Chemicon, Temecula, Calif.). Color was generated by adding 2,2′-azino-bis(ethylbenzthiazoline sulfonic acid) (Sigma), and the absorbance at 405 nm was measured on an ELISA reader. The readings were referenced to a standard serum, and results were expressed as arbitrary units per milliliter (1 U = 50% maximum optical density). The concentration of 1 U/ml is roughly equal to 22 ng of anti-E antibody/ml.
Lymphocyte proliferation assays.
C3H/HeN mice were immunized with DNA or live JEV vaccine as described above. To determine whether E-specific lymphoproliferation was induced in immunized animals, spleen cells were harvested 1 week after the last immunization. The splenic T lymphocytes were enriched with nylon wool columns, and 100 μl of 2 × 106 cells/ml in RPMI-5 culture medium (RPMI 1640 containing 5% fetal bovine serum, 100 nM l-glutamine, 10 nM penicillin-streptomycin, and 5 × 10−5 M 2-mercaptoethanol) was added to each well in 96-well plates. The JEV E protein preparation was added to each stimulated well at a final concentration of 0.25 μg/ml. Transferrin (120 μg/ml; Sigma) served as a negative control antigen, and concanavalin A (10 μg/ml; Sigma) served as a positive mitogenic control. Control wells received cells only. Cells in all the wells were cultured in a total volume of 200 μl of medium. After 3 days in culture, cells were pulsed with [H3]thymidine (1 μCi/well) (Amersham Pharmacia Biotech, Piscataway, N.J.) for 18 h. Cells were then harvested with FilterMate (Packard, Meriden, Conn.), and the incorporated radioactivity was determined by TopCount (Packard). The stimulation index was calculated as the mean counts per minute of the stimulated wells divided by the mean counts per minute of the control wells.
Cytotoxicity assays.
To perform the cytotoxic assay, responder splenocytes (2 × 106) were in vitro restimulated by incubation with live JEV (6 × 106 PFU) in 2 ml of RPMI-5 per well in 24-well microplates for 5 days at 37°C. Target cells were prepared by infecting L929 cells with JEV Beijing-1 at a multiplicity of infection of 100 PFU/cell or by mock infection 16 to 18 h before the assay. The viable cells (106 in 0.1 ml of RPMI-5) were labeled with 0.1 mCi of radiolabeled sodium chromate (Amersham) for 2 h at 37°C, washed three times with RPMI-5, and resuspended at a concentration of 5 × 104/ml in RPMI-5. One hundred microliters of stimulated responder splenocytes (106 cells) was added to individual wells containing 100 μl of labeled target cells (5 × 103 cells) in 96-well V-bottomed plates (Nunc, Roskilde, Denmark). The plates were incubated for 4 h at 37°C, and 51Cr release into the supernatant was measured in a gamma counter. Percentage of specific lysis was calculated by the following formula: 100% × (experimental release − minimum release)/(maximum release − minimum release), where the maximum release was obtained by lysing all the target cells with 1% Triton X-100 and the minimum release was obtained with target cells incubated alone in RPMI-5. The JEV-specific lysis of each group was calculated as percent specific lysis of infected L929 cells − percent specific lysis of uninfected L929 cells.
Adoptive transfer protocols.
For transfer experiments, female C3H/HeN mice were immunized with pE or pcDNA3 three times at 3-week intervals by intramuscular or gene gun injections or sublethally immunized twice at 3-week intervals with live JEV Beijing-1. Sera were collected from the various groups of animals 2 weeks after the last booster. Spleen cells were harvested 1 week after the last booster. The T-cell-enriched splenocytes were prepared from crude splenocytes by using nylon wool columns and were estimated to contain approximately 85% T lymphocytes by staining with fluorescein isothiocyanate-conjugated anti-Thy-1.2 (Pharmingen, San Diego, Calif.) in a fluorescence-activated cell sorting analysis. The B-lymphocyte population was isolated by incubating cells with biotinylated anti-B220 (Pharmingen) and streptavidin-coated microbeads and then separated on VarioMACS separation columns (Miltenyi Biotech, Bergisch Gladbach, Germany). Fluorescence-activated cell sorting analysis of purified B cells revealed >96% purity. For adoptive transfer experiments, 300 μl of serum or 5 × 107 crude splenocytes, 2 × 107 T-cell-enriched splenocytes, or 2 × 107 B lymphocytes were injected into the retro-orbital cavity of naïve C3H/HeN mice that were sublethally irradiated (650 rads) 24 h before transfer. All recipient animals were challenged with 50 LD50 of JEV Beijing-1 12 h after transfer.
Statistical analysis.
The statistical significance of differential findings between experimental groups of animals was determined by Student's t test. Data were considered statistically significant if P was ≤0.05.
RESULTS
Humoral and cellular immune responses induced by pE DNA vaccine.
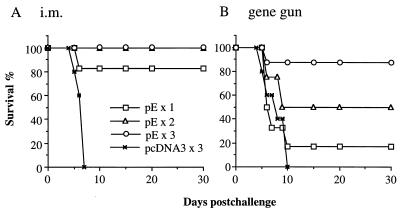
We previously showed that plasmid pE encoding the JEV envelope protein elicited protective immunity against a lethal JEV challenge (6). An equal protection rate (∼90%) was achieved by either intramuscular or gene gun delivery of three doses of the pE DNA vaccine. In this study, experiments were designed to determine more accurately the protective capacity of this DNA vaccine by different routes of immunization and to assess the relative contributions of humoral and cellular immune responses to protection. Plasmid pE was administered by intramuscular or gene gun injection to groups of C3H/HeN mice once (week 0), twice (weeks 0 and 3), or three times (weeks 0, 3, and 6). Animals receiving three doses of pcDNA3 served as negative controls. All mice were challenged 8 weeks after the first immunization with 50 LD50 (3 × 107 PFU) of JEV Beijing-1. As expected, none of the mice in the control pcDNA3 group, immunized by intramuscular or gene gun injection, survived the JEV challenge. Compared with the control group, mice that received pE by intramuscular injection once, twice, or three times were all significantly protected, with 83% (five of six, P < 0.005), 100% (six of six, P < 0.00001), and 100% (five of five, P < 0.00001) of animals, respectively, surviving the challenge (>30 days after viral challenge) (Fig. 1A). In the gene gun-injected groups, a dose response of protection was observed with increasing numbers of vaccinations (Fig. 1B). While three doses of pE by gene gun injection resulted in a high level of protection (88%, six of seven, P < 0.005 versus pcDNA3 control group), two doses of DNA conferred only partial protection (50%, three of six, P > 0.05) and one dose of DNA did not produce significant protection (17%, one of six, P > 0.05).
FIG. 1.
Effects of injection routes and numbers on JEV DNA vaccine-induced protective immunity. Groups of C3H/HeN mice (n = 5 to 7) were given intramuscular (i.m.) or gene gun injections of pE one, two, or three times at 3-week intervals. Mice receiving three doses of pcDNA3 by intramuscular or gene gun injection served as negative controls. All animals were challenged with 50 LD50 of JEV Beijing-1 8 weeks after the first immunization. Following challenge, mice were observed for 30 days, and the percentage of survivors was calculated. The data are representative of three independent experiments.
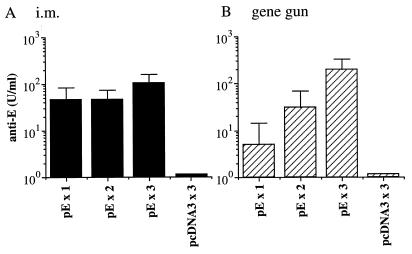
The serum samples of the different immunized groups obtained at week 8 right before viral challenge were analyzed for the presence of specific anti-E antibodies. As shown in Fig. 2, mice immunized with the control plasmid pcDNA3 by intramuscular or gene gun injection did not produce any anti-E antibodies. In contrast, intramuscular delivery of plasmid pE was effective in inducing specific antibody responses. A single dose of pE given by intramuscular injection produced a significant titer of anti-E antibody (46 ± 38 U/ml), which was comparable to that induced by two doses of vaccine (46 ± 27 U/ml) but about twofold less than that induced by three doses of DNA vaccines (105 ± 54 U/ml) (Fig. 2A). In addition, all animals in these intramuscular injection groups converted to seropositivity (E-specific titer of >1:20) before viral challenge. In the gene gun injection groups, the anti-E titers were highly dependent on the booster immunization. Mice that received a single injection of DNA produced anti-E antibodies at a barely detectable level (5 ± 4 U/ml) (Fig. 2B). One or two booster gene gun injections increased the anti-E titers to 31 ± 30 and 215 ± 147 U/ml, respectively. Furthermore, the seroconversion rate of the gene gun injection groups was also increased by booster immunization. A single gene gun injection of plasmid pE resulted in only a 17% (one of six) seroconversion rate, which increased to 50% (three of six) or 100% (seven of seven) after one or two booster immunizations, respectively. These results indicate that the presence of prechallenge anti-E antibodies was a good correlate of protection.
FIG. 2.
Anti-E antibody induced by JEV DNA vaccine. Groups of C3H/HeN mice as described in the Fig. 1 legend were analyzed for the presence of JEV E-specific antibodies before challenge. The concentration of anti-E antibodies was calculated from the standard curve generated from serially diluted reference antibodies and expressed as units per milliliter. i.m., intramuscular.
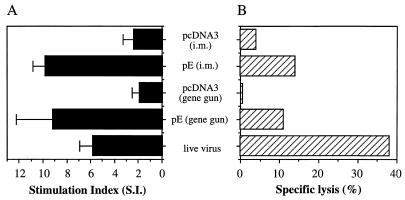
We then analyzed the T-cell immune responses elicited by pE DNA vaccine. Groups of C3H/HeN mice were immunized with pE by intramuscular or gene gun injection three times at 3-week intervals. Mice that received pcDNA3 served as negative controls. A sublethal live JEV immunization that was previously shown to induce high titers of anti-E antibodies and a high level of protection was included as a positive control (6). One week after the last booster, splenocytes were examined for proliferation in response to specific antigen stimulation. As shown in Fig. 3A, immunization with pE DNA vaccine by intramuscular or gene gun injection induced a significant proliferative response to the E protein, with a mean stimulation index of 10 ± 1 or 9 ± 3, respectively. Live virus immunization also stimulated cellular proliferation, with a mean stimulation index of 6 ± 1. Mice vaccinated with the control pcDNA3 vector by intramuscular or gene gun injection did not respond to the E protein (Fig. 3A), and all mice in the different immunized groups failed to respond to transferrin included as a control antigen (data not shown), indicating that the observed T-cell proliferation was JEV E protein specific. Splenocytes from the different immunized groups were also restimulated in vitro and examined for cytotoxicity against JEV-infected L929 cells. Mice immunized with live JEV induced a substantial level of JEV-specific lysis (Fig. 3B). Immunization with the pE vaccine by intramuscular or gene gun injection produced a low JEV-specific CTL activity at a high effector/target ratio (200:1). We performed the CTL experiments several times, and in each case pE immunization induced a low but significant CTL activity.
FIG. 3.
T-cell immunity generated by DNA or live viral vaccines. Groups of C3H/HeN mice (n = 3) were immunized three times at 3-week intervals with pE or pcDNA3 or sublethally immunized twice at 3-week intervals with 6.0 × 105 PFU of JEV Beijing-1 as described in Materials and Methods. One week after the last immunization, splenocytes were examined for JEV E-specific proliferative responses (A) and CTL activities (B) as described in Materials and Methods. The data are representative of three independent experiments. i.m., intramuscular.
Adoptively transferred immune sera but not lymphocytes conferred protection against JEV challenge.
As shown in above, immunization with pE DNA vaccine elicited a substantial humoral as well as cellular immune response against JEV. To identify which DNA-induced immune responses were protective in the JEV challenge model, pooled immune sera or splenocytes obtained from C3H/HeN mice receiving three doses of DNA vaccine or two doses of live JEV vaccine were adoptively transferred into irradiated naïve recipients. Each animal received 300 μl of immune sera or 5 × 107 splenocytes by intravenous injection and was subsequently challenged with 50 LD50 of JEV Beijing-1. The results are summarized in Table 1. Transfer of sera from animals immunized with live JEV or pE DNA vaccine via intramuscular or gene gun injection elicited significant levels of protection, with 100% (seven of seven), 75% (six of eight), and 67% (six of nine) of recipients, respectively, surviving the challenge. In contrast, adoptively transferred splenocytes from pE-vaccinated donors did not provide protection to recipient animals against JEV challenge; and splenocytes from live-JEV-immunized donors conferred only a low level of protection (27%, 3 of 11). Adoptive transfer of sera or splenocytes from pcDNA3-immunized donors by either intramuscular or gene gun injection had no effect on the survival rate or mean survival time of the recipient animals. We also adoptively transferred T-cell-enriched splenocytes (2 × 107 cells) or B lymphocytes (2 × 107 cells) from the pE DNA- or live-JEV-vaccinated animals and found that neither of these cell populations provided protection against JEV challenge (Table 1). Since the recipients were sublethally irradiated and thus cleared of host immune cells, our adoptive transfer experiments strongly suggest that the JEV-specific antibody alone can mediate viral clearance, whereas the cellular immunity did not play a role in protection.
TABLE 1.
Passive protection by adoptive transfer of antibodies but not splenocytes
| Transferred materiala | % Survivors (no. of survivors/no. total)
|
|||
|---|---|---|---|---|
| Serac | Splenocytesc | T-cell-enriched splenocytes | B cells | |
| Live virus | 100 (7/7) | 27 (3/11) | 0 (0/4) | 0 (0/4) |
| pE (i.m.e) | 75 (6/8) | 0 (0/6) | 0 (0/5) | 0 (0/5) |
| pE (gene gun) | 67 (6/9) | 0 (0/6) | 0 (0/5) | 0 (0/4) |
| pcDNA3b | 0 (0/7) | 0 (0/6) | NDd | ND |
| Naïve | 0 (0/5) | 0 (0/9) | ND | ND |
Transferred materials were prepared from C3H/HeN mice that were immunized with DNA or live JEV vaccines as described in the legend to Fig. 4.
Data are included for both intramuscular and gene gun immunization.
Data are from two independent experiments.
ND, not done.
i.m., intramuscular.
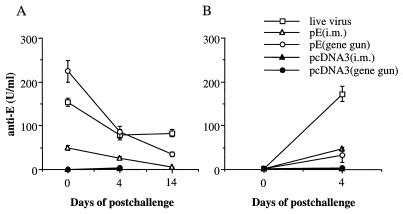
Since the antibody is the primary mediator of protection, we monitored JEV-specific antibody titers in recipient animals at day 0 (prechallenge) and days 4 and 14 postchallenge. At the time of viral challenge, groups of mice receiving immune sera from live-JEV-, pE (intramuscular)-, and pE (gene gun)-vaccinated animals had specific anti-E antibody titers of 153 ± 9, 49 ± 5, and 224 ± 24 U/ml, respectively (Fig. 4A). These antibody titers were gradually decreased at days 4 and 14 post-viral challenge. Mice to which antisera or splenocytes from pcDNA3-immunized donors were adoptively transferred did not show detectable anti-E titers at all times of analysis. In contrast, mice to which splenocytes from pE (intramuscular)-, pE (gene gun)-, or live-JEV-vaccinated animals were adoptively transferred showed a significant increase of anti-E titers from day 0 to day 4, indicating a secondary immune response due to the challenge virus (Fig. 4B). However, these secondary induced antibodies present at 4 days after infection were not protective; all animals except three in the group receiving splenocytes from live-JEV-immunized mice succumbed to the challenge by day 10 (Table 1). Interestingly, animals in this group had the highest anti-E antibodies at day 4 among animals in the different groups to which immune cells were transferred.
FIG. 4.
Anti-E antibodies in recipient animals to which was transferred immune serum (A) or splenocytes (B). C3H/HeN mice that were immunized with pE or pcDNA3 three times at 3-week intervals or sublethally immunized twice at 3-week intervals with live JEV Beijing-1 were used as donor animals. Immune splenocytes and sera were prepared as described in Materials and Methods. Naïve animals that were sublethally irradiated 24 h before were inoculated intravenously with 300 μl of sera or 5 × 107 splenocytes and challenged with 50 LD50 of JEV Beijing-1 12 h after transfer. Serum samples were collected 4 h, 4 days, or 14 days after transfer and analyzed for the presence of E-specific antibodies. i.m., intramuscular.
Immunization with pE of gene knockout mice devoid of various populations of immune cells.
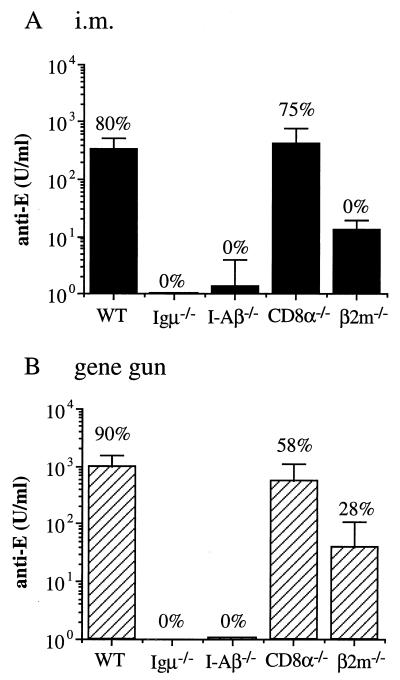
In addition to the adoptive transfer experiments, we used a panel of gene knockout mice to more clearly define the role of humoral and cellular immune responses in the pE DNA vaccine-induced protective immunity. C57BL/6 mice that were targeted for disruption of the H2-I-A beta chain (I-Aβ−/−), CD8 alpha chain (CD8α−/−), or Igμ heavy chain (Igμ−/−) were used to assess the relative contribution of CD4+, CD8+, and B lymphocytes and/or antibodies in JEV clearance and recovery. Groups of the different gene knockout mice and their wild-type littermates were immunized with pE by intramuscular or gene gun injections three times at 3-week intervals. Serum from each mouse was then analyzed for JEV E-specific antibody titers. As shown in Fig. 5, pE vaccination of Igμ−/− and I-Aβ−/− mice did not produce detectable anti-E antibodies in any of the serum samples tested and all animals in these groups succumbed to the JEV challenge. In contrast, immunization of CD8α−/− mice with pE DNA vaccine by either intramuscular or gene gun injection induced significant titers of anti-E antibodies, which were comparable to those produced in wild-type C57BL/6 mice. The pE-immunized CD8α−/− mice were well protected from JEV infection, with 75% (6 of 8) in the intramuscularly vaccinated group and 58% (7 of 12) in the gene gun-vaccinated group surviving the lethal viral challenge. We also used the β2m gene knockout mice (β2m−/−) to analyze the pE-induced protective immunity. The β2m−/− mice were defective in the development of both CD8+ CTL (27) and antibody (29) responses. Injection of pE DNA vaccine in the β2m−/− mice elicited only low titers of anti-E antibodies and conferred either no protection (zero of seven) or a low level of protection (two of seven) in the intramuscularly or gene gun-immunized group, respectively. Taken together, these results show that the pE-induced anti-E antibodies are sufficient to provide protection against JEV challenge, whereas the JEV-specific CD8+ T cells are not effective. These data also demonstrate that induction of antibody responses by pE DNA vaccine requires CD4+ T cells, whereas CD8+ T cells are not important.
FIG. 5.
DNA-induced anti-E antibody and protective immunity in gene knockout mice. Gene knockout mice and wild-type (WT) controls (C57BL/6) were given intramuscular (i.m.) or gene gun injections of pE three times at 3-week intervals. Two weeks after the last immunization, mice were challenged with 50 LD50 of JEV Beijing-1. The concentration of anti-E antibodies was determined as described in the Fig. 2 legend. The percentage of animals in each group that survived JEV challenge is indicated above each column.
DISCUSSION
Successful development of safe and efficacious JEV vaccines will be aided if their protective immune mechanisms are understood. It is generally accepted that neutralizing antibodies play a critical role in the prevention of and recovery from JEV infection. The role of CTL activity in protection is less well defined. In this study, we used two vaccination approaches, DNA vaccines and live viral vaccines, both stimulating potent humoral and cellular immune responses, to address this issue. In the adoptive transfer experiments, we showed that E-specific antiserum but not crude or T-cell-enriched immune splenocytes conferred protection against a lethal JEV challenge. Immunization of a panel of gene knockout mice also demonstrated that DNA-induced protection was lost in B-cell- or CD4+ Th-cell-deficient animals but was not affected in CD8+ CTL-deficient mice. These results together established that E-specific antibodies are sufficient by themselves to mediate the clearance of JEV infection, whereas the JEV-specific CD8+ T cells are not required for protection.
DNA vaccines represent a novel vaccination technique that shows great promise in eliciting potent humoral and cytotoxic cellular immune responses (7, 8). We previously showed that plasmid pE encoding the JEV E protein elicited a high level of protection against a lethal JEV challenge, whereas other structural and nonstructural JEV protein genes, including those for capsid, NS1-2A, NS3, and NS5, were not protective (6). An equal protection rate (∼90%) was achieved by either intramuscular or gene gun delivery of three doses of the pE DNA vaccine (Fig. 1) (6). In this study, we show that these two routes of DNA immunization vary in vaccine efficacy in terms of the number of vaccine doses administered. While a single intramuscular injection of DNA led to near-complete protection (83%, five of six mice [Fig. 1A]) against lethal JEV challenge, three doses given by gene gun injection were required to achieve a similar protection rate (88%, six of seven mice [Fig. 1B]). One and two doses of gene gun DNA vaccinations produced only a 50% (three of six) and a 17% (one of six) protection rate, respectively. We found that the presence of prechallenge anti-E antibodies was a good correlate of protection. All animals that received one, two, or three doses of DNA vaccines by intramuscular injection and those that received three doses of DNA by gene gun injection produced significant amounts of anti-E antibodies (Fig. 2), and these animals were well protected. In contrast, mice that received one and two doses of DNA by gene gun vaccination produced much smaller amounts of anti-E titers (Fig. 2B), with 17% (five of six) and 50% (three of six) of animals, respectively, in these two groups remaining seronegative (E-specific titer of <1:20) before JEV challenge. Other factors that might affect the protective efficacy were the isotype profile and avidity of the anti-E antibodies induced by these two routes of DNA immunization. In a previous study, we demonstrated that intramuscular immunization with pE generated high-avidity anti-E antibodies predominantly of the IgG2a isotype, while gene gun DNA immunization produced predominantly IgG1 anti-E antibodies of significantly lower avidity (6). Since the antibody avidity has been directly correlated with effector functions such as the abilities to neutralize virus (2) and to fix complement (30), the high-avidity anti-E antibodies generated by intramuscular DNA immunization were expected to provide better protection than the low-avidity antibodies produced by gene gun immunization.
The role of T-cell immunity in JEV infection is not yet clearly defined. JEV-specific CD4+ and CD8+ T lymphocytes have been detected in animal models and humans who were infected by JEV (11, 24). The formalin-inactivated JEV vaccines (1) and extracellular particle-based (15) or poxvirus-based (12, 13) vaccines were also reported previously to stimulate specific T-cell responses. In this study, we showed that immunization with plasmid pE by intramuscular or gene gun injections produced a significant E-specific T-cell-proliferative response (Fig. 3A). The magnitude of the proliferative response induced by these two routes of DNA immunization was comparable to that elicited by a sublethal live JEV immunization (Fig. 3A). In contrast, immunization with pE DNA vaccine by either route of injection produced only low JEV-specific CTL activity even at a high effector/target ratio (200:1), whereas live JEV immunization induced much stronger JEV-specific lysis (Fig. 3B). The CTL targets in our study were prepared from JEV-infected cells and thus should contain epitopes from both structural and nonstructural JEV proteins. The increased CTL activity induced by live JEV immunization is likely due to its ability to induce CTLs against both structural and nonstructural JEV proteins, whereas the pE DNA vaccine can induce only E-specific CTLs. Indeed, dominant CTL epitopes for several nonstructural proteins of JEV (23) and other flaviviruses (17) have been previously identified.
Previous studies have shown that passive transfer of monoclonal antibodies against E proteins protects mice against JEV infection (10, 18). Our results in this study also showed that the immune sera from DNA- or live-virus-immunized animals mediated significant protection against JEV challenge (Table 1). The recipient animals used in this study were sublethally irradiated before transfer to exclude the participation of host immune cells in protection. Thus, our transfer results suggest that antibody can act as an independent protective component in JEV infection. We observed that immune sera from live-virus-immunized mice consistently achieved better protection than those from animals receiving pE DNA vaccine (Table 1). This was likely due to the ability of live JEV vaccine to induce antibodies against all three protective antigens (pre-M, E, and NS1), whereas immunization with pE DNA vaccine produced only anti-E antibodies. Another factor that might contribute to the better protection by live-JEV-immunized mouse sera was the presence of neutralizing activity that was not detected in sera from pE-immunized animals. We showed in a previous study that the pE-encoded E protein did not adopt a proper structural conformation and thus failed to generate neutralizing antibodies (6). Instead, the antiviral activity of pE-induced anti-E antibodies is likely produced through activation of complement and Fcγ receptor-bearing phagocytic cells in vivo (28).
In contrast to the highly effective antibodies, transfer of bulk or T-cell-enriched immune splenocytes from pE- or live-JEV-immunized animals did not provide significant protection against a lethal JEV challenge (Table 1). This result suggests that both the CD4+ and CD8+ T cells specific for the E protein are not directly involved in clearance of JEV. Mathur et al. (20) and Murali Krishna et al. (25) previously reported that adoptive transfer of immune splenocytes or T lymphocytes from JEV-infected mice protected naïve mice from JEV infection. The reason for the discrepancy between our study and previous studies is not clear but may be related to the contribution of recipients' immune cells in protection. In our studies, the contribution of recipients' immune cells to protection was minimal, since all animals were pretreated with sublethal irradiation before adoptive transfer. In contrast, the recipients' immune system was intact in the previous studies. Thus, in their studies the challenge JEV might stimulate increased antibody titers or faster kinetics of the antibody response with the help of the transferred JEV-specific T cells. In fact, we observed an increase of anti-E antibodies in mice to which were adoptively transferred bulk immune splenocytes (containing both B and T cells) from pE- or live-JEV-vaccinated animals (Fig. 4B), indicating that a secondary immune response occurred following JEV infection. Interestingly, animals in the group receiving splenocytes from live-JEV-immunized mice had the highest level of anti-E antibodies at day 4 following JEV infection (Fig. 4B), and in this group only a low percentage of animals (27%, 3 of 11) survived the JEV challenge (Table 1). Another possibility is that the nonstructural protein-specific cytotoxic T cells, which could be induced by live JEV immunization (17) but not by pE DNA vaccine, contributed to the low level of protection. Nevertheless, these cytotoxic T cells are less effective and not sufficient to provide protection, since transfer of T-cell-enriched splenocytes from live-JEV-immunized animals conferred no protection against subsequent JEV challenge (Table 1).
The importance of humoral immune responses in the defense against JEV infection was confirmed by our experiments with gene knockout mice. CD8α−/− mice immunized with pE DNA vaccine by either intramuscular or gene gun injection were well protected, and these animals produced significant titers of anti-E antibodies comparable to those produced in wild-type mice (Fig. 5). In contrast, pE vaccination of Igμ−/− and I-Aβ−/− mice did not produce detectable anti-E antibodies and all animals in these groups succumbed to the challenge, indicating a critical role of B cells and CD4+ T cells in protection against JEV infection. Given the fact that adoptive transfer of T-cell-enriched splenocytes is not protective, we believe that an antibody response driven by CD4+ T lymphocytes is the likely protective mechanism in this JEV challenge model. Using a truncated DNA construct capable of inducing CTL but not antibody responses, Konishi et al. also demonstrated the importance of antibody response in JEV protection (15a). In accordance with this hypothesis, pE immunization of β2m−/− mice elicited only low titers of pE antibodies, and these animals either were not protected or were protected at a much lower rate (Fig. 5). Likewise, live JEV immunization of Igμ−/− mice did not produce detectable anti-E titers and all animals succumbed to the JEV challenge (data not shown). Our study of gene knockout mice also demonstrated that the antibody responses raised by pE DNA vaccine by either intramuscular or gene gun injection were dependent on CD4+ T cells but independent of CD8+ T cells. Using anti-CD4 and anti-CD8 antibodies to deplete the respective T-cell population, Boyle and Robinson (3) reported a similar result in a study of influenza virus DNA vaccine. In another study, Chan et al. (5) demonstrated that DNA immunization failed to generate a CTL response in major histocompatibility complex class II−/− mice. Together, these studies demonstrate that major histocompatibility complex class II-restricted CD4+ T-cell help is required for induction of both antibody and CTL immune responses to DNA vaccines.
In summary, we demonstrate in this study that humoral immunity, particularly E-specific antibodies, plays a critical role in clearance of JEV infection, whereas the CD8+ CTL activity is not required for protective immunity. Furthermore, induction of optimal antibody responses by DNA or live JEV vaccines is entirely dependent on the presence of CD4+ Th cells. This information should be valuable for future development of safe and efficacious JEV vaccines.
ACKNOWLEDGMENTS
We thank John Kung (Institute of Molecular Biology, Academia Sinica) for providing I-Aβ−/− and β2m−/− mice and Sho-Tone Lee, Mei-Shang Ho, and Yi-Ling Lin (Institute of Biomedical Sciences, Academia Sinica) for many helpful discussions.
This work was supported by grant 89-2318-B001-006-M51 from National Science Council, Taiwan, Republic of China.
REFERENCES
- 1.Aihara H, Takasaki T, Matsutani T, Suzuki R, Kurane I. Establishment and characterization of Japanese encephalitis virus-specific, human CD4+ T-cell clones: flavivirus cross-reactivity, protein recognition, and cytotoxic activity. J Virol. 1998;72:8032–8036. doi: 10.1128/jvi.72.10.8032-8036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blank S E, Leslie G A, Clem L W. Antibody affinity and valence in viral neutralization. J Immunol. 1972;108:665–673. [PubMed] [Google Scholar]
- 3.Boyle C M, Robinson H L. Basic mechanisms of DNA-raised antibody responses to intramuscular and gene gun immunizations. DNA Cell Biol. 2000;19:157–165. doi: 10.1089/104454900314546. [DOI] [PubMed] [Google Scholar]
- 4.Calarota S, Bratt G, Nordlund S, Hinkula J, Leandersson A C, Sandstrom E, Wahren B. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet. 1998;351:1320–1325. doi: 10.1016/S0140-6736(97)09440-3. [DOI] [PubMed] [Google Scholar]
- 5.Chan K, Lee D J, Schubert A, Tang C M, Crain B, Schoenberger S P, Corr M. The roles of MHC class II, CD40, and B7 costimulation in CTL induction by plasmid DNA. J Immunol. 2001;166:3061–3066. doi: 10.4049/jimmunol.166.5.3061. [DOI] [PubMed] [Google Scholar]
- 6.Chen H W, Pan C H, Liau M Y, Jou R, Tsai C J, Wu H J, Lin Y L, Tao M H. Screening of protective antigens of Japanese encephalitis virus by DNA immunization: a comparative study with conventional viral vaccines. J Virol. 1999;73:10137–10145. doi: 10.1128/jvi.73.12.10137-10145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 8.Gurunathan S, Klinman D M, Seder R A. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 9.Jan L R, Yang C S, Henchal L S, Sumiyoshi H, Summers P L, Dubois D R, Lai C J. Increased immunogenicity and protective efficacy in outbred and inbred mice by strategic carboxyl-terminal truncation of Japanese encephalitis virus envelope glycoprotein. Am J Trop Med Hyg. 1993;48:412–423. doi: 10.4269/ajtmh.1993.48.412. [DOI] [PubMed] [Google Scholar]
- 10.Kimura-Kuroda J, Yasui K. Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J Immunol. 1988;141:3606–3610. [PubMed] [Google Scholar]
- 11.Konishi E, Kurane I, Mason P W, Innis B L, Ennis F A. Japanese encephalitis virus-specific proliferative responses of human peripheral blood T lymphocytes. Am J Trop Med Hyg. 1995;53:278–283. doi: 10.4269/ajtmh.1995.53.278. [DOI] [PubMed] [Google Scholar]
- 12.Konishi E, Kurane I, Mason P W, Shope R E, Ennis F A. Poxvirus-based Japanese encephalitis vaccine candidates induce JE virus-specific CD8+ cytotoxic T lymphocytes in mice. Virology. 1997;227:353–360. doi: 10.1006/viro.1996.8331. [DOI] [PubMed] [Google Scholar]
- 13.Konishi E, Kurane I, Mason P W, Shope R E, Kanesa Thasan N, Smucny J J, Hoke C H, Jr, Ennis F A. Induction of Japanese encephalitis virus-specific cytotoxic T lymphocytes in humans by poxvirus-based JE vaccine candidates. Vaccine. 1998;16:842–849. doi: 10.1016/s0264-410x(97)00265-x. [DOI] [PubMed] [Google Scholar]
- 14.Konishi E, Pincus S, Paoletti E, Laegreid W W, Shope R E, Mason P W. A highly attenuated host range-restricted vaccinia virus strain, NYVAC, encoding the prM, E, and NS1 genes of Japanese encephalitis virus prevents JEV viremia in swine. Virology. 1992;190:454–458. doi: 10.1016/0042-6822(92)91233-k. [DOI] [PubMed] [Google Scholar]
- 15.Konishi E, Win K S, Kurane I, Mason P W, Shope R E, Ennis F A. Particulate vaccine candidate for Japanese encephalitis induces long-lasting virus-specific memory T lymphocytes in mice. Vaccine. 1997;15:281–286. doi: 10.1016/s0264-410x(96)00180-6. [DOI] [PubMed] [Google Scholar]
- 15a.Konishi E, Yamaoka M, Win K S, Kurane I, Takada K, Mason P W. The anamnestic neutralizing antibody response is critical for protection of mice from challenge following vaccination with a plasmid encoding the Japanese encephalitis virus premembrane and envelope genes. J Virol. 1999;73:5527–5534. doi: 10.1128/jvi.73.7.5527-5534.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y L, Chen L K, Liao C L, Yeh C T, Ma S H, Chen J L, Huang Y L, Chen S S, Chiang H Y. DNA immunization with Japanese encephalitis virus nonstructural protein NS1 elicits protective immunity in mice. J Virol. 1998;72:191–200. doi: 10.1128/jvi.72.1.191-200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobigs M, Arthur C E, Mullbacher A, Blanden R V. The flavivirus nonstructural protein NS3 is a dominant source of cytotoxic T cell peptide determinants. Virology. 1994;202:195–201. doi: 10.1006/viro.1994.1335. [DOI] [PubMed] [Google Scholar]
- 18.Mason P W, Dalrymple J M, Gentry M K, McCown J M, Hoke C H, Burke D S, Fournier M J, Mason T L. Molecular characterization of a neutralizing domain of the Japanese encephalitis virus structural glycoprotein. J Gen Virol. 1989;70:2037–2049. doi: 10.1099/0022-1317-70-8-2037. [DOI] [PubMed] [Google Scholar]
- 19.Mason P W, Pincus S, Fournier M J, Mason T L, Shope R E, Paoletti E. Japanese encephalitis virus-vaccinia recombinants produce particulate forms of the structural membrane proteins and induce high levels of protection against lethal JEV infection. Virology. 1991;180:294–305. doi: 10.1016/0042-6822(91)90034-9. [DOI] [PubMed] [Google Scholar]
- 20.Mathur A, Arora K L, Chaturvedi U C. Host defence mechanisms against Japanese encephalitis virus infection in mice. J Gen Virol. 1983;64:805–811. doi: 10.1099/0022-1317-64-4-805. [DOI] [PubMed] [Google Scholar]
- 21.Miura K, Onodera T, Nishida A, Goto N, Fujisaki Y. A single gene controls resistance to Japanese encephalitis virus in mice. Arch Virol. 1990;112:261–270. doi: 10.1007/BF01323170. [DOI] [PubMed] [Google Scholar]
- 22.Monath T P, Heinz F X. Flaviviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 961–1034. [Google Scholar]
- 23.Murali Krishna K, Ramireddy B, Ravi V, Manjunath R. Recognition of nonstructural protein peptides by cytotoxic T lymphocytes raised against Japanese encephalitis virus. Microbiol Immunol. 1995;39:1021–1024. doi: 10.1111/j.1348-0421.1995.tb03294.x. [DOI] [PubMed] [Google Scholar]
- 24.Murali Krishna K, Ravi V, Manjunath R. Cytotoxic T lymphocytes raised against Japanese encephalitis virus: effector cell phenotype, target specificity and in vitro virus clearance. J Gen Virol. 1994;75:799–807. doi: 10.1099/0022-1317-75-4-799. [DOI] [PubMed] [Google Scholar]
- 25.Murali Krishna K, Ravi V, Manjunath R. Protection of adult but not newborn mice against lethal intracerebral challenge with Japanese encephalitis virus by adoptively transferred virus-specific cytotoxic T lymphocytes: requirement for L3T4+ T cells. J Gen Virol. 1996;77:705–714. doi: 10.1099/0022-1317-77-4-705. [DOI] [PubMed] [Google Scholar]
- 26.Poland J D, Cropp C B, Craven R B, Monath T P. Evaluation of the potency and safety of inactivated Japanese encephalitis vaccine in US inhabitants. J Infect Dis. 1990;161:878–882. doi: 10.1093/infdis/161.5.878. [DOI] [PubMed] [Google Scholar]
- 27.Raulet D H. MHC class I-deficient mice. Adv Immunol. 1994;55:381–421. doi: 10.1016/s0065-2776(08)60514-3. [DOI] [PubMed] [Google Scholar]
- 28.Schlesinger J J, Foltzer M, Chapman S. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology. 1993;192:132–141. doi: 10.1006/viro.1993.1015. [DOI] [PubMed] [Google Scholar]
- 29.Spriggs M K, Koller B H, Sato T, Morrissey P J, Fanslow W C, Smithies O, Voice R F, Widmer M B, Maliszewski C R. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci USA. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steward M W, Lew A M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985;78:173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- 31.Tsai T F, Chang G J, Yu Y X. Japanese encephalitis vaccines. In: Stanley A P, Orenstein W A, editors. Vaccines. 3rd ed. Philadelphia, Pa: W. B. Saunders; 1999. pp. 672–710. [Google Scholar]
- 32.Wang R, Doolan D L, Le T P, Hedstrom R C, Coonan K M, Charoenvit Y, Jones T R, Hobart P, Margalith M, Ng J, Weiss W R, Sedegah M, de Taisne C, Norman J A, Hoffman S L. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]