Abstract
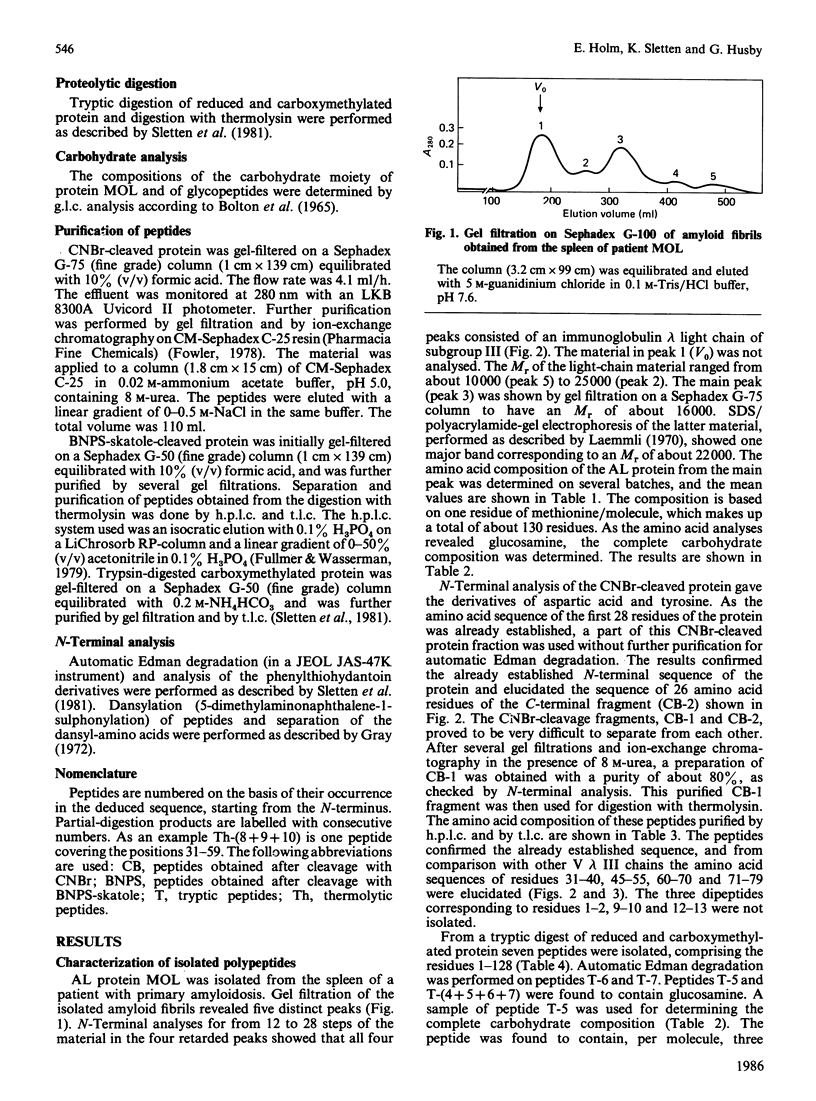
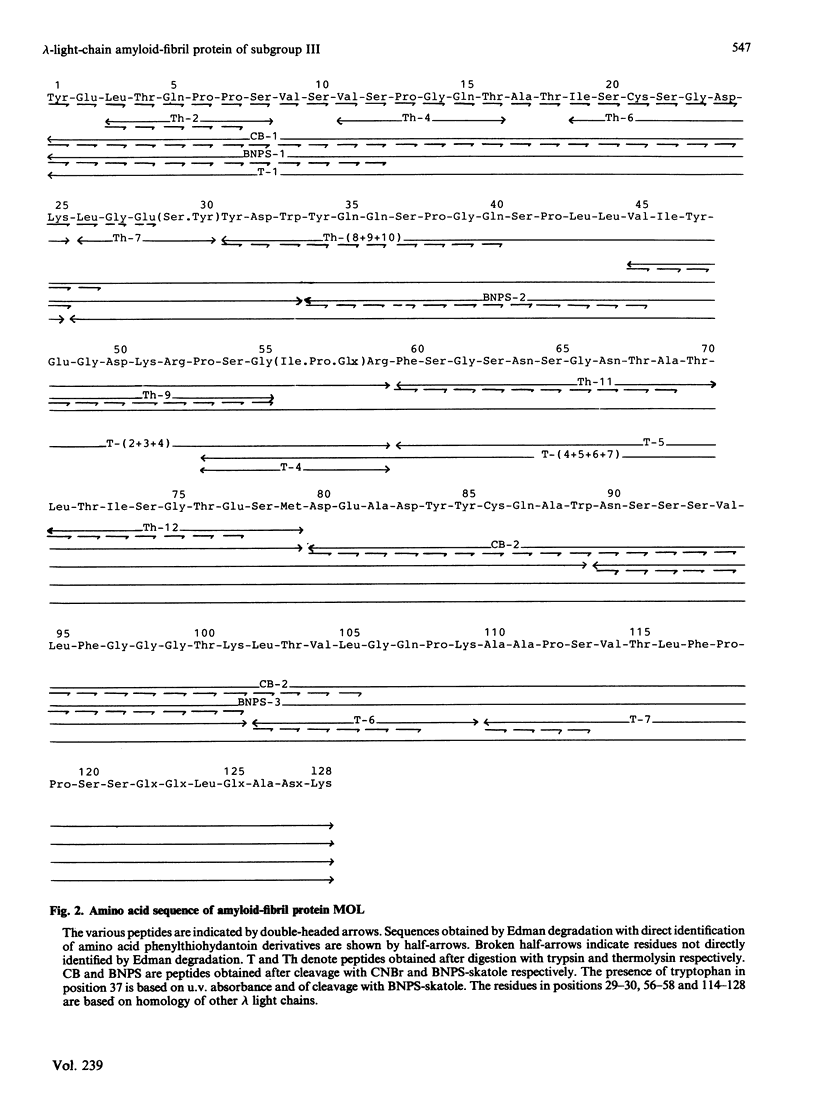
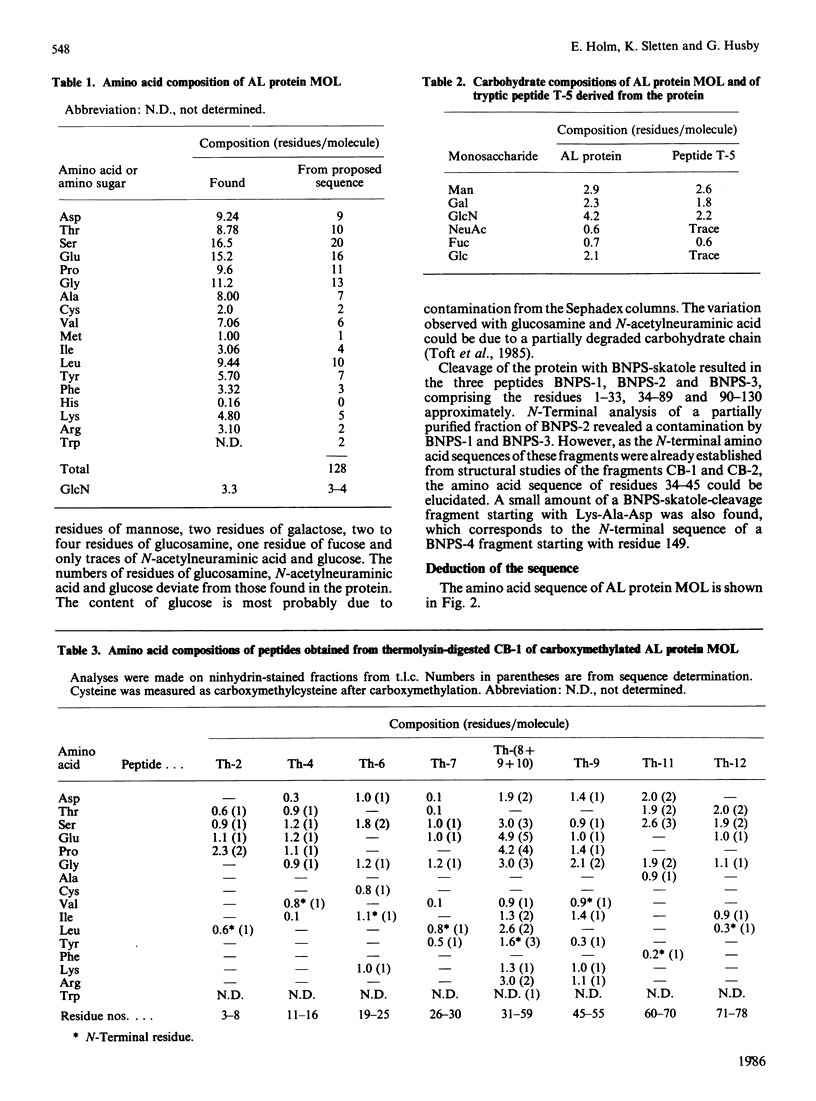
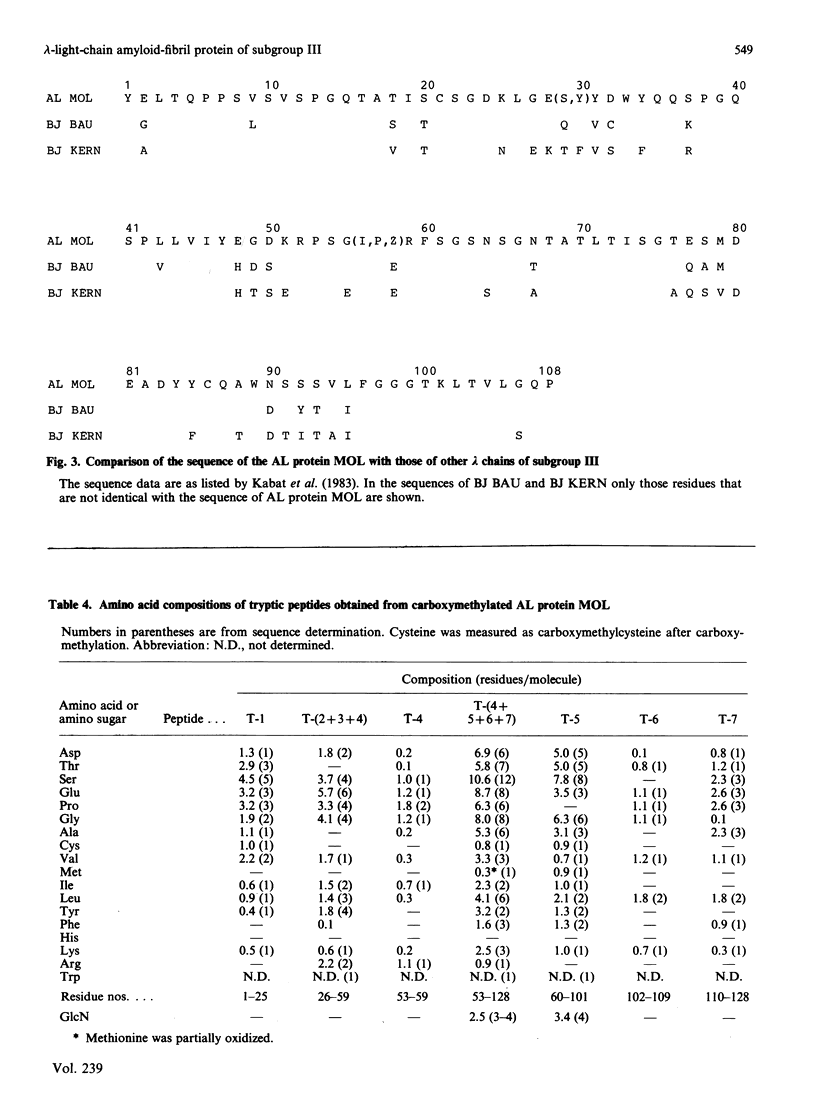
The amino acid sequence of the variable region of a carbohydrate-containing amyloid-fibril protein MOL of immunoglobulin-light-chain type (AL) was elucidated. The sequence determination involved cleaving the protein with CNBr, BNPS-skatole, thermolysin and trypsin. The sequenced protein consisted of about 130 amino acid residues; however, gel-filtration and N-terminal analysis studies revealed AL proteins ranging in Mr from about 10,000 to 25,000. The oligosaccharide chain was found to be bound in the hypervariable region. By sequence homology to other lambda chains the AL protein MOL was shown to be of the V lambda III subgroup.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baczko K., Braun D., Hilschmann N. Zur Strukturregel der Antikörper. Die Primärstruktureiner monoklonalen Immunoglobulin-L-kette vom lambda-Typ, Subgruppe IV (Bence-Jones-Protein Bau.) Hoppe Seylers Z Physiol Chem. 1974 Feb;355(2):131–154. [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hermodson M. A., Ericsson L. H. The major proteins of human and monkey amyloid substance: Common properties including unusual N-terminal amino acid sequences. FEBS Lett. 1971 Dec 1;19(2):169–173. doi: 10.1016/0014-5793(71)80506-9. [DOI] [PubMed] [Google Scholar]
- Eulitz M., Linke R. P. Primary structure of the variable part of an amyloidogenic Bence-Jones Protein (Mev.). An unusual insertion in the third hypervariable region of a human kappa-immunoglobulin light chain. Hoppe Seylers Z Physiol Chem. 1982 Nov;363(11):1347–1358. doi: 10.1515/bchm2.1982.363.2.1347. [DOI] [PubMed] [Google Scholar]
- Eulitz M., Linke R. Amyloid fibrils derived from V-region together with C-region fragments from a lambda II-immunoglobulin light chain (HAR). Biol Chem Hoppe Seyler. 1985 Sep;366(9):907–915. doi: 10.1515/bchm3.1985.366.2.907. [DOI] [PubMed] [Google Scholar]
- Fowler A. V. Amino acid sequence of beta-galactosidase. VII. Isolation of the 24 cyanogen bromide peptides. J Biol Chem. 1978 Aug 10;253(15):5499–5504. [PubMed] [Google Scholar]
- Fullmer C. S., Wasserman R. H. Analytical peptide mapping by high performance liquid chromatography. Application to intestinal calcium-binding proteins. J Biol Chem. 1979 Aug 10;254(15):7208–7212. [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med. 1980 Jun 12;302(24):1333–1343. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- Husby G., Sletten K., Blumenkrantz N., Danielsen L. Characterization of an amyloid fibril protein from localized amyloidosis of the skin as lambda immunoglobulin light chains of variable subgroup I (A lambda I). Clin Exp Immunol. 1981 Jul;45(1):90–96. [PMC free article] [PubMed] [Google Scholar]
- Husby G., Sletten K. Chemical and clinical classification of amyloidosis 1985. Scand J Immunol. 1986 Mar;23(3):253–265. doi: 10.1111/j.1365-3083.1986.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten K., Husby G., Natvig J. B. N-terminal amino acid sequence of amyloid fibril protein AR, prototype of a new lambda-variable subgroup, V lambda V. Scand J Immunol. 1974;3(6):833–836. doi: 10.1111/j.1365-3083.1974.tb01319.x. [DOI] [PubMed] [Google Scholar]
- Sletten K., Husby G. The complete amino-acid sequence of non-immunoglobulin amyloid fibril protein AS in rheumatoid arthritis. Eur J Biochem. 1974 Jan 3;41(1):117–125. doi: 10.1111/j.1432-1033.1974.tb03251.x. [DOI] [PubMed] [Google Scholar]
- Sletten K., Natvig J. B., Husby G., Juul J. The complete amino acid sequence of a prototype immunoglobulin-lambda light-chain-type amyloid-fibril protein AR. Biochem J. 1981 Jun 1;195(3):561–572. doi: 10.1042/bj1950561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten K., Westermark P., Pitkänen P., Thyresson N., Olstad O. K. Amino acid sequences in amyloid proteins of kappa III immunoglobulin light-chain origin. Scand J Immunol. 1983 Dec;18(6):557–560. doi: 10.1111/j.1365-3083.1983.tb00891.x. [DOI] [PubMed] [Google Scholar]
- Solomon A., Frangione B., Franklin E. C. Bence Jones proteins and light chains of immunoglobulins. Preferential association of the V lambda VI subgroup of human light chains with amyloidosis AL (lambda). J Clin Invest. 1982 Aug;70(2):453–460. doi: 10.1172/JCI110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sox H. C., Jr, Hood L. Attachment of carbohydrate to the variable region of myeloma immunoglobulin light chains. Proc Natl Acad Sci U S A. 1970 Jul;66(3):975–982. doi: 10.1073/pnas.66.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft K. G., Sletten K., Husby G. The amino-acid sequence of the variable region of a carbohydrate-containing amyloid fibril protein EPS (immunoglobulin light chain, type lambda). Biol Chem Hoppe Seyler. 1985 Jul;366(7):617–625. doi: 10.1515/bchm3.1985.366.2.617. [DOI] [PubMed] [Google Scholar]
- Tonoike H., Kametani F., Hoshi A., Shinoda T., Isobe T. Amino acid sequence of an amyloidogenic Bence Jones protein in myeloma-associated systemic amyloidosis. FEBS Lett. 1985 Jun 3;185(1):139–141. doi: 10.1016/0014-5793(85)80757-2. [DOI] [PubMed] [Google Scholar]
- Tonoike H., Kametani F., Hoshi A., Shinoda T., Isobe T. Primary structure of the variable region of an amyloidogenic Bence Jones protein NIG-77. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1228–1234. doi: 10.1016/0006-291x(85)90317-1. [DOI] [PubMed] [Google Scholar]
- Tveteraas T., Sletten K., Westermark P. The amino acid sequence of a carbohydrate-containing immunoglobulin-light-chain-type amyloid-fibril protein. Biochem J. 1985 Nov 15;232(1):183–190. doi: 10.1042/bj2320183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. C., 2nd, Jacobson R. J., Binder R. A., Linke R. P., Glenner G. G. Immunoglobulin D myeloma and amyloidosis: immunochemical and structural studies of Bence Jones and amyloid fibrillar proteins. Blood. 1975 Nov;46(5):713–722. [PubMed] [Google Scholar]
- Winkelhake J. L. Immunoglobulin structure and effector functions. Immunochemistry. 1978 Sep;15(9):695–714. doi: 10.1016/0161-5890(78)90044-5. [DOI] [PubMed] [Google Scholar]



