Abstract
A 36-year-old man with inverse Gottron's sign was admitted for clinically amyopathic dermatomyositis (CADM) with rapidly progressive interstitial lung disease (RP-ILD). Early addition of plasma exchange (PE) to triple therapy improved severe respiratory failure and transiently decreased serum ferritin levels and anti-melanoma differentiation-associated gene 5 antibody (anti-MDA5 Ab) titers. Furthermore, switching from tacrolimus to tofacitinib resulted in disease remission. Recognition of the inverse Gottron's sign may allow for the earlier diagnosis of anti-MDA5 Ab-positive dermatomyositis, and early addition of PE to triple therapy and administration of tofacitinib in refractory cases may be effective for anti-MDA5 Ab-positive CADM with RP-ILD under life-threatening conditions.
Keywords: anti-MDA5 antibody, clinically amyopathic dermatomyositis, inverse Gottron's sign, interstitial lung disease, plasma exchange, tofacitinib
Introduction
Dermatomyositis (DM) is a common idiopathic inflammatory myopathy with heterogeneous clinical features showing various degrees of cutaneous symptoms, myositis, pulmonary involvement, and arthritis/arthralgia (1). DM has recently been categorized into several disease subsets based on various myositis-specific autoantibodies present in patients with DM (1). Anti-melanoma differentiation-associated gene 5 antibody (anti-MDA5 Ab) is a dermatomyositis-specific antibody found in 13-37% of patients (2-5). Notably, in cases of clinically amyopathic dermatomyositis (CADM) with typical DM skin eruptions but no findings of myositis or only mild evidence of myositis, anti-MDA5 Ab was found in 38-71% of patients with CADM (2-5).
Anti-MDA5 Ab-positive DM is characterized by a unique cutaneous phenotype of skin ulceration, palmar papules (inverse Gottron's sign), panniculitis, alopecia, and oral pain and/or ulceration (2). In addition, patients with anti-MDA5 Ab-positive DM were shown to be significantly more likely to have interstitial lung disease (ILD) than those with anti-MDA5 Ab-negative DM. In particular, the frequency of rapidly progressive ILD (RP-ILD) is high, leading to respiratory failure and early death (6). The frequencies of ILD and RP-ILD associated with anti-MDA5 Ab-positive DM in Asian cohorts were 86-100% and 57-79%, respectively (3-5). In contrast, in American cohorts, the frequencies of ILD and RP-ILD associated with anti-MDA5 Ab-positive DM were low, at 50-67% and 22-44%, respectively, showing clinical discrepancies (2,6).
Currently, the recommended treatment for anti-MDA5 Ab-positive DM with RP-ILD is a two-drug combination therapy comprising a high-dose corticosteroid plus a calcineurin inhibitor or three-drug combination therapy (triple therapy) with the addition of intravenous cyclophosphamide (IVCY) to the two-drug schedule (7). The 6-month survival rate for anti-MDA5 Ab-positive DM with ILD has been reported to be 70-75%, even with triple therapy (8,9). Therefore, if anti-MDA5 Ab-positive DM with RP-ILD is diagnosed based on physical, laboratory, and imaging findings, and a poor prognosis is predicted, combined immunosuppressive therapy should be initiated early (10). Additional therapies, such as plasma exchange (PE), direct hemoperfusion using a polymyxin B-immobilized fiber column, intravenous immunoglobulin, rituximab (RTX), or tofacitinib (TOF), can be added to refractory cases that do not respond to combined immunosuppressive therapy; however, no consensus has yet been reached regarding the optimal treatment (7). Furthermore, scant data are available regarding options for the intensification of treatment in anti-MDA5 Ab-positive DM patients with RP-ILD who are already in a life-threatening condition due to severe respiratory failure.
We herein report a case of CADM with RP-ILD in a patient with a life-threatening condition who had a poor prognosis due to high ferritin levels and severe hypoxemia at the initial presentation. Based on the presence of an inverse Gottron's sign, one of the characteristic cutaneous findings of anti-MDA5 Ab-positive DM, triple therapy was able to be started immediately without awaiting the results of the anti-MDA5 Ab test. Furthermore, the early addition of PE to triple therapy proved instrumental in achieving early improvement in his respiratory condition, and switching from tacrolimus (TAC) to TOF was effective in achieving disease remission.
The patient provided his written informed consent for the inclusion of his details and imaging findings in this report.
Case Report
A 36-year-old man noticed skin eruptions on his hands in mid-December of 2022. In early January 2023, he was admitted to our hospital with a 3-day history of worsening dyspnea. He had no contributory medical history and was not taking any medications.
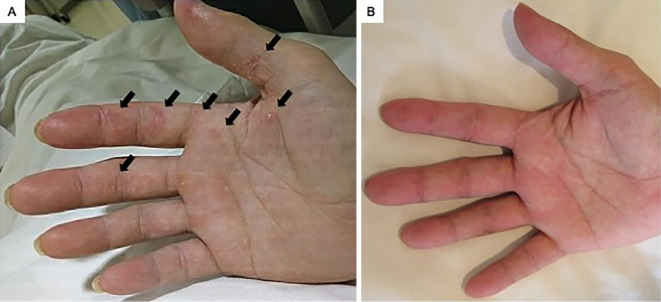
On a physical examination, he had a fever (37.7°C) and a low peripheral oxygen saturation (SpO2) of 94% on oxygen using a reservoir mask at 10 L/min. Gottron's sign was identified on the dorsal side of the metacarpophalangeal and interphalangeal joints, with an inverse Gottron's sign on the volar side of the metacarpophalangeal and interphalangeal joints and the palm (Fig. 1A), a V-neck sign on the neck and anterior chest, and a shawl sign on the posterior aspects of the shoulders and upper back. No nailfold bleeding, myalgia, or muscle weakness was detected. Fine crackles were audible in both lung fields during auscultation.
Figure 1.
Cutaneous findings of the patient. A) Inverse Gottron’s sign on the volar side of the metacarpophalangeal and interphalangeal joints and palm was observed on admission (arrows). B) Three months after starting therapy, the inverse Gottron’s sign had improved.
Blood tests on admission showed the following: white blood cells, 7,600 /μL (neutrophils, 74.0%; lymphocytes, 13.0%; monocytes, 10.0%; eosinophils, 3.0%; basophils, 0%); hemoglobin, 15.6 g/dL; platelets, 27.6×104 /μL; aspartate aminotransferase, 76 IU/L (normal, 7-38 IU/L); alanine aminotransferase, 51 IU/L (normal, 4-43 IU/L); creatine phosphokinase, 306 IU/L (normal, 57-197 IU/L); lactate dehydrogenase, 654 IU/L (normal, 124-222 IU/L); C-reactive protein (CRP), 4.24 mg/dL (normal, ≤0.3 mg/dL); ferritin, 659 ng/mL (normal, 13-301 ng/mL); procalcitonin, 0.12 ng/mL (normal, <0.50 ng/mL); and Krebs von den Lungen (KL)-6, 832 U/mL (normal, <500 U/mL). The renal function and urinalysis results were normal. The levels of β-D-glucan were below the detection limit. On an arterial blood gas (ABG) analysis, the partial pressure of arterial oxygen (PaO2) was 80.7 mmHg on oxygen by a reservoir mask at 10 L/min. Polymerase chain reaction (PCR) testing of a nasopharyngeal swab for severe acute respiratory syndrome coronavirus 2 yielded negative results.
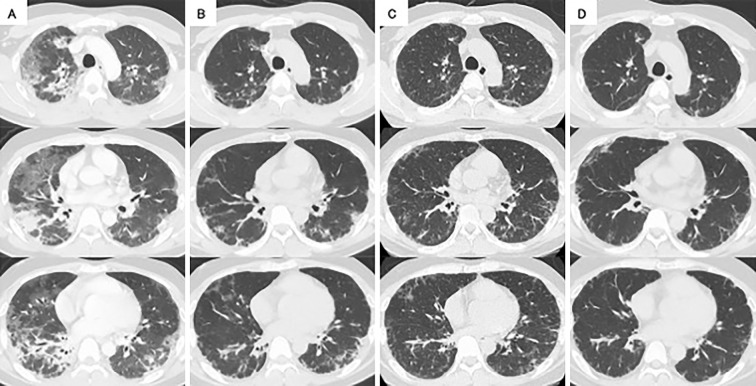
High-resolution computed tomography (HRCT) of the chest revealed non-segmental ground-glass opacities (GGOs) and consolidations, predominantly in the peripheral regions of both lungs (Fig. 2A). Therefore, CADM with RP-ILD was suspected based on the typical cutaneous findings without obvious muscle symptoms, laboratory findings, and chest HRCT findings consistent with a rapidly worsening respiratory condition. In particular, anti-MDA5 Ab-positive CADM was presumed, due to the presence of an inverse Gottron's sign, one of the characteristic cutaneous findings of anti-MDA5 Ab-positive DM.
Figure 2.
High-resolution computed tomography (HRCT) of the chest during the clinical course. A) Chest HRCT on admission showed non-segmental ground-glass opacities (GGOs) and consolidations, predominantly in the peripheral regions of both lungs. B) Immediately after four sessions of plasma exchange (PE), improvements in bilateral GGOs and consolidations could be seen. C) Eleven days after completing five sessions of PE, residual GGOs and reticular opacities were seen in both lungs. D) Six weeks after switching from tacrolimus to tofacitinib, chest HRCT showed improvements in residual GGOs and reticular opacities in both lungs.
Triple therapy was immediately initiated with methylprednisolone (mPSL) pulse therapy (1,000 mg/day) for 3 days, TAC (2 mg/day), and IVCY (750 mg/body). Pulse therapy with mPSL was followed by oral prednisolone [PSL; 60 mg/day (1 mg/kg/day)]. IVCY was administered every two weeks.
Considering the likelihood of CADM with RP-ILD in this patient with a life-threatening condition and a poor prognosis due to high ferritin levels and severe hypoxemia, we decided to initiate additional PE the same day triple therapy was started after obtaining informed consent from the patient. Two hours after the end of mPSL administration [1 h after cyclophosphamide (CY) administration and 0.5 hours after TAC administration], PE was started. PE was administered 3 times a week with the following regimen: PE replacement fluid, 5% albumin, and target volume of replacement fluid, 3.0 L (corresponding to 0.94 times the plasma volume).
After admission, he was managed with nasal high-flow (NHF) therapy, with an SpO2 of 93% on NHF and 70% oxygen at 40 L/min before the initiation of triple therapy. An ABG analysis 15 min after the end of the first PE showed that the PaO2 was 144 mmHg, the alveolar-arterial oxygen difference (AaDO2) was 307 mmHg on NHF with 70% oxygen at 40 L/min, and dyspnea was somewhat alleviated. Furthermore, an ABG analysis 7 h after the end of the first PE showed a PaO2 of 64.2 mmHg and an AaDO2 of 212 mmHg on NHF with 45% oxygen at 40 L/min.
On the day of admission (day 1), because he was being managed with NHF therapy, it was challenging to perform muscle magnetic resonance imaging (MRI) to assess the presence of myositis. Therefore, a muscle biopsy specimen was obtained from the left vastus lateralis, but no significant findings were observed. On day 5, a skin biopsy of an area of erythema on the dorsal side of the metacarpophalangeal joint of the right third finger showed hyperkeratosis in the epidermis and mild perivascular infiltration of lymphocytes in the dermis, consistent with the characteristic histological findings of DM. Since an ABG analysis showed a PaO2 of 80.0 mmHg and an AaDO2 of 153 mmHg on NHF with 40% oxygen at 40 L/min, he was switched from NHF therapy to oxygen via nasal cannula. On day 6, the trough concentration of TAC was 5.4 ng/mL, and the dose of TAC was increased to 3 mg/day. On day 7, MRI of the thigh showed no high short tau time inversion recovery (STIR) signal intensity, but the results of anti-MDA5 Ab testing at the initial presentation had been positive, with an anti-MDA5 Ab titer of 5,400 index (normal, <32 index). Anti-MDA5 Ab-positive CADM with RP-ILD was therefore diagnosed according to Sontheimer's definition (11).
On day 8, chest HRCT showed improvement in bilateral GGOs and consolidations (Fig. 2B), and an ABG analysis showed a PaO2 of 67.3 mmHg and an AaDO2 of 92.3 mmHg on oxygen via nasal cannula at 2 L/min, indicating a good treatment response. Pulse therapy with mPSL was administered for the second time. On day 12 (a day after completing the fifth PE), an ABG analysis showed a PaO2 of 62.4 mmHg and an AaDO2 of 49.1 mmHg on room air, so he was successfully withdrawn from oxygen therapy. The trough TAC was 7.3 ng/mL. Serum ferritin levels and anti-MDA5 Ab titers decreased to 494 ng/mL and 1,650 index, respectively. However, PE was discontinued owing to the development of anaphylactic symptoms during transfusion of fresh-frozen plasma. No worsening of his respiratory condition was observed, even after PE was completed in five sessions. On day 15, a second dose of IVCY was administered at a dose of 1,000 mg/body. On day 22, chest HRCT revealed residual GGOs and reticular opacities in both lungs (Fig. 2C). Serum ferritin levels and anti-MDA5 Ab titers increased to 1,248 ng/mL and 2,080 index, respectively; therefore, the patient was transferred to the rheumatology department of another hospital.
After transfer, he was treated with triple therapy, including a third round of mPSL pulse therapy, two additional administrations of IVCY (1,000 mg/body), and an increasing dose of TAC. On day 25, the trough concentration of TAC decreased to 6.8 ng/mL, so the dose of TAC was increased to 4 mg/day. The trough concentration of TAC on day 32 was 8.7 ng/mL, but the trough concentration of TAC on day 39 decreased to 6.5 ng/mL, so the dose of TAC was further increased to 5 mg/day. However, the serum ferritin levels fluctuated between 600 and 800 ng/mL, the trough concentration of TAC was 7.7 ng/mL on day 46, and chest HRCT on day 47 showed no improvement in residual GGOs or reticular opacities in either lung. Furthermore, the cytomegalovirus (CMV) antigenemia assay was positive without clinical symptoms on day 34, so he was treated with valganciclovir for 2 weeks.
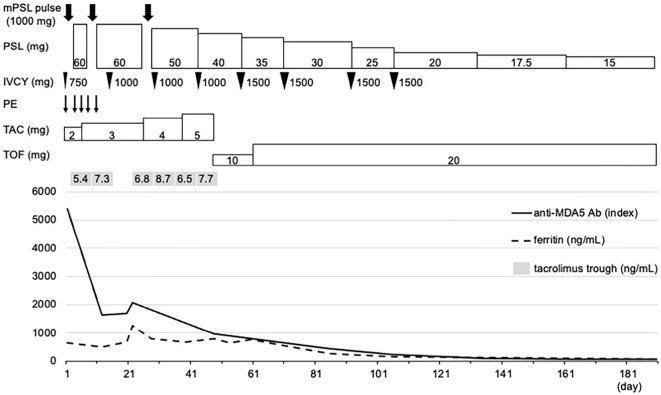
On day 48, the anti-MDA5 Ab titers decreased to 965 index but remained high. Considering that the addition of TOF to triple therapy would increase the risk of infection due to severe immunosuppression, it was decided to switch from TAC to TOF (10 mg/day) with the patient's informed consent for the administration of TOF, although this was not reviewed by the local ethics committee. On day 57, the fifth dose of IVCY was administered at a dose of 1,500 mg/body. However, since serum ferritin levels remained high at 773 ng/mL on day 60, the TOF dose was increased to 20 mg/day on day 61. Thereafter, serum ferritin levels tended to decrease, and the patient was discharged on day 71. We administered IVCY three more times at 1,500 mg/body while tapering oral PSL and continuing TOF at 20 mg/day. On day 85, the serum ferritin levels and anti-MDA5 Ab titers decreased to 254 ng/mL and 450 index, respectively. On day 91, chest HRCT showed improvement of residual GGOs and reticular opacities in both lungs (Fig. 2D), and an ABG analysis showed that PaO2 was 87.1 mmHg, and AaDO2 was 15.9 mmHg on room air. The cutaneous findings also improved (Fig. 1B). Four months after discharge, using PSL (15 mg/day) and TOF (20 mg/day), no increase was observed in serum ferritin levels or anti-MDA5 Ab titers, and his respiratory condition and imaging findings remained stable, with no adverse events (Fig. 3).
Figure 3.
Clinical course of the patient, a 36-year-old man. Serum ferritin levels, anti-MDA5 Ab titers, trough concentrations of TAC, and treatment interventions are shown. Serum ferritin levels and anti-MDA5 Ab titers decreased during the PE period but re-increased after PE was completed in five sessions. Despite continuing triple therapy, serum ferritin levels fluctuated around baseline levels, and anti-MDA5 Ab titers decreased but remained high. These levels gradually decreased after switching from TAC to TOF. IVCY: intravenous cyclophosphamide, mPSL: methylprednisolone, PE: plasma exchange, PSL: prednisolone, TAC: tacrolimus, TOF: tofacitinib
Discussion
The inverse Gottron's sign refers to papules or erythema that develop around the finger joints on the palmar side (12). Fiorentino et al. reported that, of 77 patients with DM, including 13 CADM patients, 10 patients were positive for anti-MDA5 Ab, and 5 of those anti-MDA5 Ab-positive patients showed an inverse Gottron's sign compared to only 2 of the MDA5 Ab-negative DM patients (50% sensitivity and 97% specificity) (2). Another study found that six of seven DM patients with an inverse Gottron's sign were positive for anti-MDA5 Ab and showed complications of ILD (13). Thus, the inverse Gottron's sign is considered a cutaneous finding that is highly specific for anti-MDA5 Ab-positive DM.
Chest HRCT can also help predict the presence of anti-MDA5 Abs in patients with DM and ILD. Tanizawa et al. reported that, of 25 patients with DM with ILD, chest HRCT findings of anti-MDA5 Ab-positive DM with ILD were characterized by a low consolidation/GGO pattern, random GGO pattern, and the absence of a low reticulation pattern (14). Notably, low consolidation/GGO patterns, which were characterized by lower lobe-predominant non-segmental consolidations or GGOs with subpleural or peribronchovascular distribution, were more frequently observed in the anti-MDA5 Ab-positive group (6 of 12 patients; 50%) than in the anti-MDA5 Ab-negative group (2 of 13 patients; 15%) (14). In the present case, chest HRCT at the initial presentation showed non-segmental GGOs and consolidations predominantly in the peripheral regions of both lungs, features that corresponded to a low consolidation/GGO pattern, suggesting the presence of anti-MDA5 Ab. Given the presence of an inverse Gottron's sign in addition to the chest HRCT findings in the present case, it was decided to immediately initiate triple therapy without awaiting the results of anti-MDA5 Ab testing. Recognizing cutaneous findings specific to anti-MDA5 Ab-positive DM, such as the inverse Gottron's sign, may aid physicians in the early diagnosis and treatment of anti-MDA5 Ab-positive DM with RP-ILD.
High ferritin levels and respiratory impairment are key markers of a poor prognosis in patients with anti-MDA5 Ab-positive DM and RP-ILD. Isoda et al. reported pretreatment ferritin levels of ≥600 ng/mL and AaDO2 levels of ≥45 mmHg as predictors of poor prognosis for DM with RP-ILD, and the 6-month survival rate for patients with both factors was 0% (15). Fujiki et al. showed that initial ferritin levels of ≥450 ng/mL, AaDO2 ≥30 mmHg, and GGO >5% in the middle lobe were factors associated with a poor prognosis for patients with anti-MDA5 Ab-positive DM with ILD (16). In an analysis of 497 patients with myositis-associated ILD, an age at onset of ≥60 years old, CRP level of ≥1 mg/dL, SpO2 <95%, and anti-MDA5 Ab positivity were identified as initial predictors of a poor prognosis (17). Therefore, when considering the early initiation of combined immunosuppressive therapy or intensification of treatment for patients with anti-MDA5 Ab-positive DM with RP-ILD, in addition to serum ferritin levels and oxygen demand, evaluations of the age at the onset, CRP levels, and chest HRCT findings are also critical. In the present case, a CRP level of ≥1 mg/dL, serum ferritin level of ≥600 ng/mL, and high oxygen demand were observed at the initial presentation. In addition, based on the presence of an inverse Gottron's sign and chest HRCT findings of GGO >5% in the middle lobe, a diagnosis of anti-MDA5 Ab-positive CADM with RP-ILD and a very poor prognosis was presumed.
The early initiation of combined immunosuppressive therapy appears to provide the highest chance of a survival for patients with anti-MDA5 Ab-positive DM with RP-ILD, and additional therapies, such as PE, TOF, or RTX, may play critical roles in the therapeutic armamentarium for refractory patients who do not respond to combined immunosuppressive therapy (10). However, data on the intensification of treatment for patients with anti-MDA5 Ab-positive DM and RP-ILD who are already in a life-threatening condition due to severe respiratory failure are lacking.
Compared with anti-MDA5 Ab-negative DM patients, pre-treatment serum levels of interleukin (IL)-6, IL-10, IL-18, macrophage colony-stimulating factor, interferon (IFN)-α, and ferritin were significantly elevated in anti-MDA5 Ab-positive DM patients, suggesting that the activation of monocytes and macrophages underlies the pathophysiology of anti-MDA5 Ab-positive DM (8). Gono et al. reported that serum ferritin and IL-18 levels were significantly reduced in patients with anti-MDA5 Ab-positive DM and ILD who responded to treatment (18). In addition, the anti-MDA5 Ab titer is reported to be directly related to the disease activity and prognosis in anti-MDA5 Ab-positive DM with RP-ILD (4). PE, which can reduce anti-MDA5 Ab titers and levels of various cytokines, may thus be effective against anti-MDA5 Ab-positive DM with RP-ILD. Several recent reports have described the efficacy of PE in patients with anti-MDA5 Ab-positive DM and RP-ILD who have proven refractory to combined immunosuppressive therapy. Abe et al. showed that, in 10 patients with anti-MDA5 Ab-positive DM with PR-ILD who were refractory to triple therapy, the 1-year survival rate of the 6 patients treated with PE was significantly higher (100%) than that of the 4 patients without PE (25%) (19). Similarly, Shirakashi et al. showed that, of 13 patients with anti-MDA5 Ab-positive DM with ILD who were refractory to triple therapy, the 3-year survival rate of the 8 patients treated with PE (62.5%) was significantly higher than that of the 5 patients without PE (0%) (20). Saito et al. reported that, in 6 patients with anti-MDA5 Ab-positive CADM with RP-ILD who were refractory to triple therapy and underwent PE (median time from diagnosis to start of PE, 11.5 days), the 2 who died had higher AaDO2 levels, more severe lung lesions at initiation of PE, and a longer period before initiation of PE than the 4 who survived, suggesting that the early initiation of PE may improve the prognosis by preventing ILD progression (21).
However, in PE for patients with MDA5 Ab-positive DM with ILD, the indication criteria for PE, timing of PE initiation, and the ideal number and frequency of PE sessions have not been established. Takahashi et al. reported that, in patients with anti-MDA5 Ab-positive DM with RP-ILD, high ferritin levels and oxygen demand may be factors influencing the initiation of PE, and the early initiation of PE may be useful until effects are achieved from CY, which might be the key drug in triple therapy (22).
To our knowledge, no case report has described cases where PE and triple therapy were started on the same day. However, in the present case, since the patient was considered to have CADM with RP-ILD, specifically anti-MDA5 Ab-positive CADM, and had a life-threatening condition with a poor prognosis due to high ferritin levels and severe hypoxemia at the initial presentation, PE was initiated immediately after starting triple therapy. In a report by Shirakashi et al., in 8 patients treated with PE 1-3 times a week for 3-15 consecutive weeks, there was no significant difference in the number of PE sessions between the survival and fatal outcome groups [median of 8.0 sessions (interquartile range 8.0-14.5) and 12.0 sessions (11.0-15.0), respectively] (20). Furthermore, in the aforementioned reports, the number and frequency of PE sessions were a median of 9.5 sessions (interquartile range 4-10) every other day to 16 sessions (14-22) approximately 3 times a week after the initial performance on 2 consecutive days (19,21). In the present case, PE was performed for 5 sessions over 11 days, 3 times a week, with no consecutive days, and the number of PE sessions was less than that in previous reports. However, the improvement in chest HRCT findings and decrease in serum ferritin levels, anti-MDA5 Ab titers, and AaDO2 levels during the PE period are indicative of the beneficial effects of PE. The present case thus suggests that the early addition of PE to triple therapy, such as on the day of starting triple therapy, may be effective for patients with anti-MDA5 Ab-positive DM with RP-ILD who have a life-threatening condition due to severe respiratory failure, with treatment continued until the effects of immunosuppressive drugs appear.
Concerns have been raised regarding the efficacy of extracorporeal elimination of administered drugs when performing PE. The extracorporeal elimination efficacy of a given drug depends on the volume of distribution (Vd) and protein binding (PB) of the drug, as well as the exchanged plasma volume, intercompartmental equilibration of the drug, and endogenous clearance (23). In general, drugs with low Vd (<0.2 L/kg) and/or high PB (>80%) are theoretically more likely to be removed by PE than others (23). For a 50% reduction in pretreatment levels, the replacement volume of a given PE treatment would have to be equal to 0.7 times the Vd of the drug (24). Prednisone, a corticosteroid that is converted to PSL, is highly bound to plasma proteins (90-95%) and has a moderate Vd (0.6-0.7 L/kg). Stigelman et al. reported that the amount of combined prednisone and PSL removed by PE in 2 patients starting at 0.42 and 1.16 h after oral prednisone administration was 0.83% and 0.74%, respectively, of the administered prednisone dose (25). Szefler et al. reported that mPSL was also highly bound to plasma proteins (77%) and had a moderate Vd (1.4 L/kg), and the disposition rate of mPSL was similar to that of PSL (26). Therefore, similar to PSL, the amount of mPSL removed by PE was expected to be minimal. TAC is highly bound to plasma proteins (75-99%) and has a large and variable Vd (0.85-65 L/kg), and its levels appear relatively unaffected by PE (27,28). CY was shown to be weakly bound to plasma proteins (13.4±5.3%) with a moderate Vd (0.7 L/kg), suggesting that the amount of CY removed by PE was minimal (24,29). Drug dosing should preferably be performed after PE; however, given the minimal removal of PSL, mPSL, TAC, and CY by PE, administration of PE for anti-MDA5 Ab-positive DM with RP-ILD may be feasible even on the day of starting triple therapy.
TOF is a Janus kinase (JAK) inhibitor that mainly inhibits JAK1 and JAK3 and suppresses the signaling of various cytokines, such as type I IFNs (IFN-α/β), IL-6, IL-7, IL-10, IL-15, and IL-21, by inhibiting the JAK-signal transducer and activator of the transcription signaling pathway (30). Several reports have demonstrated the efficacy of TOF in patients with anti-MDA5 Ab-positive DM and ILD. Chen et al. reported that, in 18 patients with early stage anti-MDA5 Ab-positive amyopathic DM with ILD who received glucocorticoid combined with TOF (10 mg/day) as initial treatment, the 6-month survival rate was 100%, and significant improvements in serum ferritin levels and chest HRCT findings were observed (31). Kurasawa et al. reported that, among 5 patients with anti-MDA5 Ab-positive DM with ILD who were refractory to triple therapy and received TOF (10 mg/day) 5-15 days after starting triple therapy, 3 survived, while 2 died (32). They also reported that the survival rate of patients who received TOF was significantly better than that of historical controls before TOF (32). However, patients treated with combined immunosuppressive therapy and TOF reportedly experience complicated adverse events, particularly viral infections. Chen et al. reported that infectious adverse events were observed in two cases (one of urinary tract infection and one of possible invasive fungal infection) (31). Kurasawa et al. reported that infectious adverse events were frequently observed, including viral infections (all five cases of CMV reactivation, three cases of herpes zoster, and one case of adenovirus cystitis), four cases of bacterial infection, two cases of fungal infections, and one case of lymphoproliferative disease with Epstein Barr virus activation (32). They also reported that, in all three survivors, the conditions of the patients were well controlled by reducing the number of administered immunosuppressive drugs, including TOF, from four within three to six months of starting treatment (32). Another report confirmed the efficacy of switching from IVCY and TAC to TOF (10 mg/day) in anti-MDA5 Ab-positive DM with ILD refractory to triple therapy with additional PE (33).
In the present case, despite triple therapy with additional PE, serum ferritin levels fluctuated around baseline levels, the anti-MDA5 Ab titers decreased but remained high, and chest HRCT revealed residual GGOs and reticular opacities in both lungs, suggesting that the patient was not in remission. Several studies have reported that the efficacy and safety are correlated with trough concentrations. Suzuka et al. showed that early therapeutic intervention with a combination of glucocorticoids, and an initial high-trough level TAC (15-20 ng/mL) was effective for DM with acute/subacute ILD (34). Tsuji et al. showed that triple therapy, with the trough concentration of TAC adjusted to within the range of 10-12 ng/mL, was effective for anti-MDA5 Ab-positive DM with ILD (9). In the present case, the TAC dose was increased from 2 to 5 mg/day, but the trough concentration of TAC remained below 10 ng/mL, suggesting that the insufficient dose of TAC may have been related to resistance to triple therapy with additional PE. An attempt to increase the dose of TAC may have been a treatment option, but the effect of TAC was thought to be limited, since the blood concentration of TAC was unstable. After carefully evaluating the risk-benefit balance of adding TOF to triple therapy, we decided to switch from TAC to TOF (10 mg/day).
Ida et al. reported a case series of anti-MDA5 Ab-positive DM patients with RP-ILD treated with TOF (20 mg/day) due to an inadequate response to triple therapy and TOF (10 mg/day), in which 4 of 6 patients improved (35). They also reported that all six patients developed at least one infection, including five cases of CMV reactivation, one case of pulmonary aspergillosis, one case of herpes zoster, and one case of herpes simplex keratitis; however, all infections responded well to treatment (35). In the present case, no decrease in serum ferritin levels was observed even after switching from TAC to TOF (10 mg/day); therefore, the TOF dose was increased to 20 mg/day. Serum ferritin levels and anti-MDA5 Ab titers then decreased, chest HRCT findings resolved, and the dose of glucocorticoids was tapered smoothly with no adverse events, such as infection, achieving disease remission. Switching from TAC to TOF may thus be effective in anti-MDA5 Ab-positive CADM with RP-ILD, which is refractory to triple therapy with additional PE.
In conclusion, when treating CADM with RP-ILD in a patient who already has a life-threatening condition with a poor prognosis due to high ferritin levels and severe hypoxemia, recognizing the characteristic cutaneous findings of anti-MDA5 Ab-positive DM may help physicians initiate triple therapy immediately without waiting for the results of anti-MDA5 Ab testing. Additional PE on the day of starting triple therapy may be effective not only for reducing serum ferritin levels and anti-MDA5 Ab titers but also for achieving early improvement of the respiratory condition. Furthermore, switching from TAC to TOF may be effective in achieving disease remission in anti-MDA5 Ab-positive CADM with RP-ILD refractory to intensive immunosuppressive therapy. Evolutions in treatment strategies using additional therapies, such as PE and TOF, will hopefully lead to better clinical outcomes for anti-MDA5 Ab-positive CADM with RP-ILD in patients who already have a life-threatening condition due to severe respiratory failure.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Selva-O'Callaghan A, Pinal-Fernandez I, Trallero-Araguás E, Milisenda JC, Grau-Junyent JM, Mammen AL. Classification and management of adult inflammatory myopathies. Lancet Neurol 17: 816-828, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol 65: 25-34, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 32: 3909-3915, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Sato S, Kuwana M, Fujita T, Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol 23: 496-502, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Motegi S, Sekiguchi A, Toki S, et al. Clinical features and poor prognostic factors of anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis with rapid progressive interstitial lung disease. Eur J Dermatol 29: 511-517, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical spectrum in North American patients with dermatomyositis. J Rheumatol 44: 319-325, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Romero-Bueno F, Diaz del Campo P, Trallero-Araguás E, et al. Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin Arthritis Rheum 50: 776-790, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakashima R, Hosono Y, Mimori T. Clinical significance and new detection system of autoantibodies in myositis with interstitial lung disease. Lupus 25: 925-933, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol 72: 488-498, 2020. [DOI] [PubMed] [Google Scholar]
- 10.McPherson M, Economidou S, Liampas A, Zis P, Parperis K. Management of MDA-5 antibody positive clinically amyopathic dermatomyositis associated interstitial lung disease: a systematic review. Semin Arthritis Rheum 53: 151959, 2022. [DOI] [PubMed] [Google Scholar]
- 11.Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis siné myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol 46: 626-636, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Muro Y, Sugiura K, Akiyama M. Cutaneous manifestations in dermatomyositis: key clinical and serological features - a comprehensive review. Clin Rev Allergy Immunol 51: 293-302, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Irie K, Matsumura N, Hoshi M, Yamamoto T. Inverse Gottron's papules in patients with dermatomyositis: an underrecognized but important sign for interstitial lung disease. Int J Dermatol 60: e62-e65, 2021. [DOI] [PubMed] [Google Scholar]
- 14.Tanizawa K, Handa T, Nakashima R, et al. HRCT features of interstitial lung disease in dermatomyositis with anti-CADM-140 antibody. Respir Med 105: 1380-1387, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Isoda K, Takeuchi T, Kotani T, et al. Pre-treatment ferritin level and alveolar-arterial oxygen gradient can predict mortality rate due to acute/subacute interstitial pneumonia in dermatomyositis treated by cyclosporine A/glucocorticosteroid combination therapy: a case control study. PLoS One 9: e89610, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujiki Y, Kotani T, Isoda K, et al. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod Rheumatol 28: 133-140, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Masui K, Nishina N, et al. Initial predictors of poor survival in myositis-associated interstitial lung disease: a multicentre cohort of 497 patients. Rheumatology (Oxford) 57: 1212-1221, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Gono T, Sato S, Kawaguchi Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology (Oxford) 51: 1563-1570, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Abe Y, Kusaoi M, Tada K, Yamaji K, Tamura N. Successful treatment of anti-MDA5 antibody-positive refractory interstitial lung disease with plasma exchange therapy. Rheumatology (Oxford) 59: 767-771, 2020. [DOI] [PubMed] [Google Scholar]
- 20.Shirakashi M, Nakashima R, Tsuji H, et al. Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatology (Oxford) 59: 3284-3292, 2020. [DOI] [PubMed] [Google Scholar]
- 21.Saito T, Mizobuchi M, Miwa Y, et al. Anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis with rapidly progressive interstitial lung disease treated with therapeutic plasma exchange: a case series. J Clin Apher 36: 196-205, 2021. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi R, Yoshida T, Morimoto K, et al. Successful treatment of anti-MDA5 antibody-positive dermatomyositis-associated rapidly progressive-interstitial lung disease by plasma exchange: two case reports. Clin Med Insights Case Rep 14: 11795476211036322, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim RB, Balogun RA. Medications in patients treated with therapeutic plasma exchange: prescription dosage, timing, and drug overdose. Semin Dial 25: 176-189, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan AA. Therapeutic plasma exchange: core curriculum 2008. Am J Kidney Dis 52: 1180-1196, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Stigelman WH, Henry DH, Talbert RL, Townsend RJ. Removal of prednisone and prednisolone by plasma exchange. Clin Pharm 3: 402-407, 1984. [PubMed] [Google Scholar]
- 26.Szefler SJ, Ebling WF, Georgitis JW, Jusko WJ. Methylprednisolone versus prednisolone pharmacokinetics in relation to dose in adults. Eur J Clin Pharmacol 30: 323-329, 1986. [DOI] [PubMed] [Google Scholar]
- 27.Przepiorka D, Suzuki J, Ippoliti C, Hester JP, Fritsche HA. Blood tacrolimus concentration unchanged by plasmapheresis. Am J Hosp Pharm 1: 1708, 1994. [PubMed] [Google Scholar]
- 28.Jacobson P, Uberti J, Davis W, Ratanatharathorn V. Tacrolimus: a new agent for the prevention of graft-versus-host disease in hematopoietic stem cell transplantation. Bone Marrow Transplant 22: 217-225, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Juma FD, Rogers HJ, Trounce JR. The kinetics of salivary elimination of cyclophosphamide in man. Br J Clin Pharmacol 8: 455-458, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodge JA, Kawabata TT, Krishnaswami S, et al. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 34: 318-328, 2016. [PubMed] [Google Scholar]
- 31.Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N Engl J Med 381: 291-293, 2019. [DOI] [PubMed] [Google Scholar]
- 32.Kurasawa K, Arai S, Namiki Y, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology (Oxford) 57: 2114-2119, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Hosokawa Y, Oiwa H. A case of refractory interstitial lung disease in anti-MDA5-positive dermatomyositis that improved after switching to tofacitinib. J Clin Rheumatol 27: S661-S662, 2021. [DOI] [PubMed] [Google Scholar]
- 34.Suzuka T, Kotani T, Takeuchi T, et al. Efficacy and safety of oral high-trough level tacrolimus in acute/subacute interstitial pneumonia with dermatomyositis. Int J Rheum Dis 22: 303-313, 2019. [DOI] [PubMed] [Google Scholar]
- 35.Ida T, Furuta S, Takayama A, et al. Efficacy and safety of dose escalation of tofacitinib in refractory anti-MDA5 antibody-positive dermatomyositis. RMD Open 9: e002795, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]