Abstract
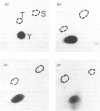
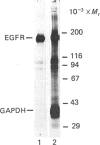
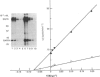
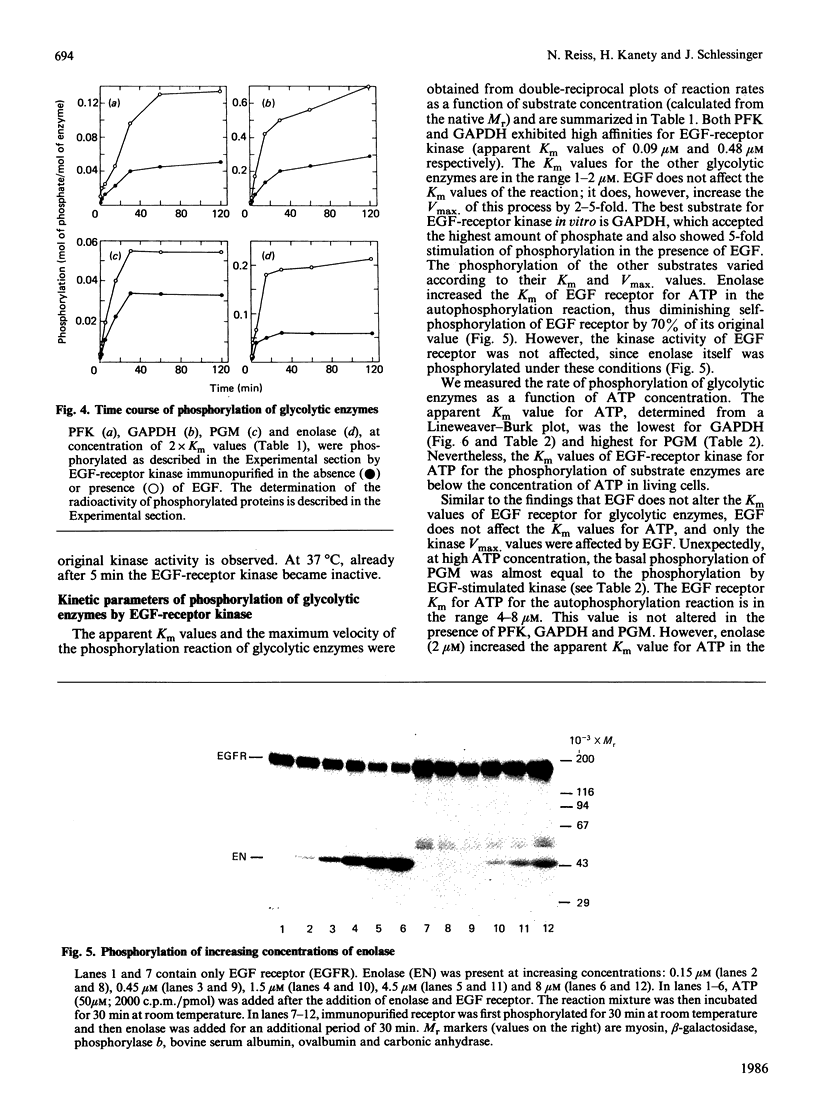
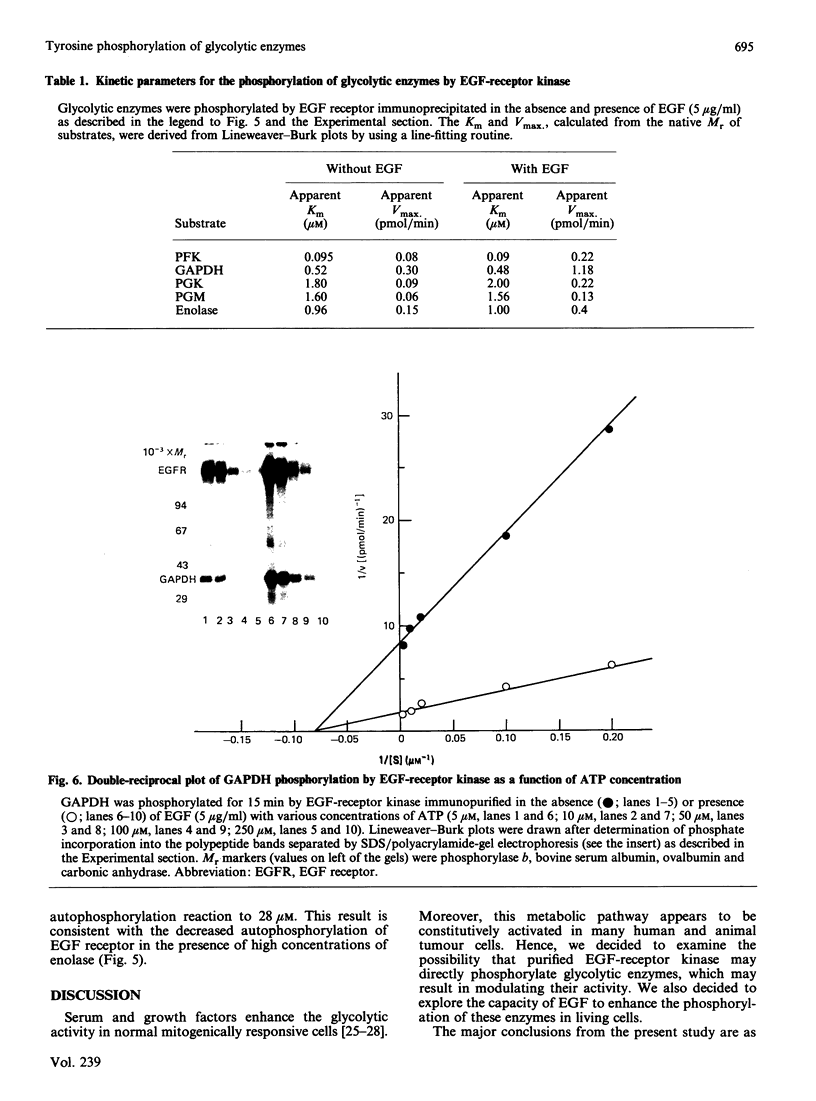
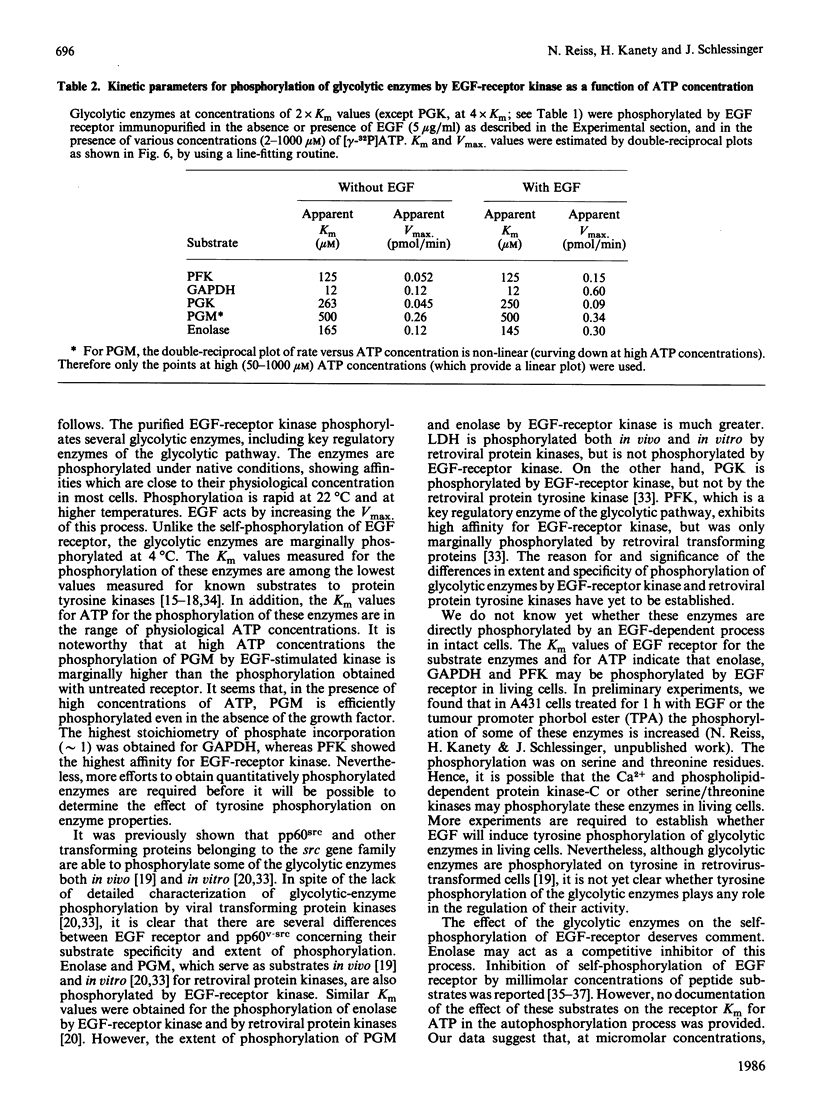
Several enzymes of the glycolytic pathway are phosphorylated in vitro and in vivo by retroviral transforming protein kinases. These substrates include the enzymes phosphoglycerate mutase (PGM), enolase and lactate dehydrogenase (LDH). Here we show that purified EGF (epidermal growth factor)-receptor kinase phosphorylates the enzymes PGM and enolase and also the key regulatory enzymes of the glycolytic pathway, phosphofructokinase and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), in an EGF-dependent manner. Stoichiometry of phosphate incorporation into GAPDH (calculated from native Mr) is the highest, reaching approximately 1. LDH and other enzymes of the glycolytic pathway are not phosphorylated by the purified EGF-receptor kinase. These enzymes are phosphorylated under native conditions, and the Km values of EGF-receptor kinase for their phosphorylation are close to the physiological concentrations of these enzymes in the cell. EGF stimulates the reaction by 2-5-fold by increasing the Vmax. without affecting the Km of this process. Phosphorylation is rapid at 22 degrees C and at higher temperatures. However, unlike the self-phosphorylation of EGF-receptor, which occurs at 4 degrees C, the glycolytic enzymes are poorly phosphorylated at this temperature. Some enzymes, in particular enolase, increase the receptor Km for ATP in the autophosphorylation process and thus may act as competitive inhibitors of EGF-receptor self-phosphorylation. On the basis of the Km values of EGF receptor for the substrate enzymes and for ATP in the phosphorylation reaction, these enzymes may also be substrates in vivo for the EGF-receptor kinase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin G. S., Burgess A. W., Kemp B. E. Phosphorylation of a synthetic gastrin peptide by the tyrosine kinase of A431 cell membranes. Biochem Biophys Res Commun. 1982 Dec 15;109(3):656–663. doi: 10.1016/0006-291x(82)91990-8. [DOI] [PubMed] [Google Scholar]
- Bertics P. J., Gill G. N. Self-phosphorylation enhances the protein-tyrosine kinase activity of the epidermal growth factor receptor. J Biol Chem. 1985 Nov 25;260(27):14642–14647. [PubMed] [Google Scholar]
- Bissell M. J., White R. C., Hatie C., Bassham J. A. Dynamics of metabolism of normal and virus-transformed chick cells in culture. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2951–2955. doi: 10.1073/pnas.70.10.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner P., Resnick R. J., Racker E. Stimulation of glycolysis and amino acid uptake in NRK-49F cells by transforming growth factor beta and epidermal growth factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1350–1353. doi: 10.1073/pnas.82.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S., Raymond W. E., Racker E. Synthetic tyrosine polymers as substrates and inhibitors of tyrosine-specific protein kinases. J Biol Chem. 1984 Feb 25;259(4):2051–2054. [PubMed] [Google Scholar]
- Buhrow S. A., Cohen S., Staros J. V. Affinity labeling of the protein kinase associated with the epidermal growth factor receptor in membrane vesicles from A431 cells. J Biol Chem. 1982 Apr 25;257(8):4019–4022. [PubMed] [Google Scholar]
- Cassel D., Pike L. J., Grant G. A., Krebs E. G., Glaser L. Interaction of epidermal growth factor-dependent protein kinase with endogenous membrane proteins and soluble peptide substrate. J Biol Chem. 1983 Mar 10;258(5):2945–2950. [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Reiss N. A., Schwartz R. J., Hunter T. Three glycolytic enzymes are phosphorylated at tyrosine in cells transformed by Rous sarcoma virus. Nature. 1983 Mar 17;302(5905):218–223. doi: 10.1038/302218a0. [DOI] [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I., Legg A., Schneider J. A., Rozengurt E. Glycolysis in quiescent cultures of 3T3 cells. Stimulation by serum, epidermal growth factor, and insulin in intact cells and persistence of the stimulation after cell homogenization. J Biol Chem. 1978 Feb 10;253(3):866–871. [PubMed] [Google Scholar]
- Downward J., Waterfield M. D., Parker P. J. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J Biol Chem. 1985 Nov 25;260(27):14538–14546. [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Activation of phosphofructokinase by stimulants of cell multiplication. Nat New Biol. 1973 Dec 12;246(154):181–183. doi: 10.1038/newbio246181a0. [DOI] [PubMed] [Google Scholar]
- Ghosh-Dastidar P., Coty W. A., Griest R. E., Woo D. D., Fox C. F. Progesterone receptor subunits are high-affinity substrates for phosphorylation by epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1654–1658. doi: 10.1073/pnas.81.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Synthetic peptide substrates for a tyrosine protein kinase. J Biol Chem. 1982 May 10;257(9):4843–4848. [PubMed] [Google Scholar]
- Inman W. H., Colowick S. P. Stimulation of glucose uptake by transforming growth factor beta: evidence for the requirement of epidermal growth factor-receptor activation. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1346–1349. doi: 10.1073/pnas.82.5.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Fujita-Yamaguchi Y., Nishida E., Takaku F., Akiyama T., Kathuria S., Akanuma Y., Kasuga M. Phosphorylation of tubulin and microtubule-associated proteins by the purified insulin receptor kinase. J Biol Chem. 1985 Apr 10;260(7):4016–4020. [PubMed] [Google Scholar]
- Karess R. E., Hayward W. S., Hanafusa H. Cellular information in the genome of recovered avian sarcoma virus directs the synthesis of transforming protein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3154–3158. doi: 10.1073/pnas.76.7.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Fujita-Yamaguchi Y., Blithe D. L., White M. F., Kahn C. R. Characterization of the insulin receptor kinase purified from human placental membranes. J Biol Chem. 1983 Sep 25;258(18):10973–10980. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Gallis B., Casnellie J. E., Bornstein P., Krebs E. G. Epidermal growth factor stimulates the phosphorylation of synthetic tyrosine-containing peptides by A431 cell membranes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1443–1447. doi: 10.1073/pnas.79.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J., Kuenzel E. A., Casnellie J. E., Krebs E. G. A comparison of the insulin- and epidermal growth factor-stimulated protein kinases from human placenta. J Biol Chem. 1984 Aug 10;259(15):9913–9921. [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Reiss N. A., Kaye A. M. Identification of the major component of the estrogen-induced protein of rat uterus as the BB isozyme of creatine kinase. J Biol Chem. 1981 Jun 10;256(11):5741–5749. [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Diamond I., Rozengurt E. Glycolysis of quiescent cultures of 3T3 cells. Addition of serum, epidermal growth factor, and insulin increases the activity of phosphofructokinase in a protein synthesis-independent manner. J Biol Chem. 1978 Feb 10;253(3):872–877. [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Singh V. N., Singh M., August J. T., Horecker B. L. Alterations in glucose metabolism in chick-embryo cells transformed by Rous sarcoma virus: intracellular levels of glycolytic intermediates. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4129–4132. doi: 10.1073/pnas.71.10.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- White M. F., Haring H. U., Kasuga M., Kahn C. R. Kinetic properties and sites of autophosphorylation of the partially purified insulin receptor from hepatoma cells. J Biol Chem. 1984 Jan 10;259(1):255–264. [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Harari I., Schlessinger J. Purification of an active EGF receptor kinase with monoclonal antireceptor antibodies. J Biol Chem. 1985 Jan 10;260(1):315–319. [PubMed] [Google Scholar]