Abstract
Prostate cancer is one of the most commonly diagnosed cancers in men and is a major cause of cancer-related deaths worldwide. Among the molecular processes that contribute to this disease, the weight of metabolism has been placed under the limelight in recent years. Tumours exhibit metabolic adaptations to comply with their biosynthetic needs. However, metabolites also play an important role in supporting cell survival in challenging environments or remodelling the tumour microenvironment, thus being recognized as a hallmark in cancer. Prostate cancer is uniquely driven by androgen receptor signalling, and this knowledge has also influenced the paths of cancer metabolism research. This review provides a comprehensive perspective on the metabolic adaptations that support prostate cancer progression beyond androgen signalling, with a particular focus on tumour cell intrinsic and extrinsic pathways.
Subject terms: Cancer metabolism, Urological cancer
Introduction
The androgen receptor (AR) is a central player in the biology of the prostate, operating as a nuclear receptor essential for normal prostate development and function [1]. AR mediates the effects of androgens and regulates the expression of genes involved in prostate growth, maintenance, and differentiation. Beyond developmental stages, AR also influences prostate health throughout adulthood [2]. AR signalling is linked to the onset and progression of prostate cancer (PCa), where it becomes a primary driver of tumour growth. Therefore, inhibition of AR function represents the targeted therapy in this disease [3]. AR reprograms PCa cellular metabolism, creating a unique molecular scenario that has been documented for the last 100 years [4]. Nevertheless, the complexity underlying cellular metabolism extends beyond AR signalling, which is envisioned to offer innovative therapeutic opportunities. Our current understanding of cellular metabolism encompasses aspects such as the tumour microenvironment (TME) or diet. In this review, we will explore major metabolic pathways supporting PCa progression and metastasis, with special emphasis on tumour cell-intrinsic and extrinsic glucose, lipid and one-carbon metabolism (1 C metabolism), while other relevant processes including the connection between metabolism and epigenetics will be left out of the scope of this work. Furthermore, we will incorporate new evidence from other tumour types to identify shared characteristics that can apply to PCa.
Major metabolic alterations in prostate cancer cells
Glucose metabolism
Glycolysis and the Warburg effect
Glycolysis metabolises glucose to pyruvate via a series of intermediate reactions, generating ATP and NADH (Fig. 1). Cancer cells often exhibit increased glycolytic activity to generate lactate, even in the presence of oxygen, known as aerobic glycolysis or the Warburg effect [5]. Despite its lower efficiency compared to oxidative phosphorylation (OXPHOS) in the mitochondria, cancer cells heavily depend on this pathway to produce energy. It is important to state that the increase of anaerobic glucose utilisation does not imply a reduction in mitochondrial OXPHOS activity, in contrast to the initial hypothesis of Dr. Otto Warburg [6]. Although several hypotheses have been proposed, the reason why proliferating cells metabolise glucose predominantly to produce lactate remains elusive. Two complementary publications argue that when the demand for NAD+ exceeds the demand for ATP, resulting in the saturation of the mitochondrial NADH, tumour cells enforce aerobic glycolysis even in the presence of oxygen [7, 8]. These metabolic adaptations have been brought from bench to bedside through different approaches. On the one hand, aerobic glycolysis results in an elevated demand for glucose, which has inspired the development of cancer-monitoring strategies based on the uptake of 18F-fluorodeoxyglucose with positron emission tomography (PET) [9]. On the other hand, although alterations in copy number have been reported in glycolysis-promoting genes [10], deregulated mRNA expression represents a key contributing factor for aerobic glycolysis, which supported the development of transcriptomic gene signatures in different cancer types [11–13].
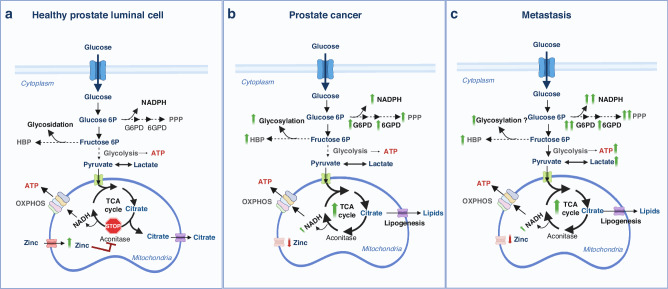
Fig. 1. Schematic overview of the metabolic rewiring occurring in prostate epithelial cells during the different stages of cancer progression.
a Healthy prostate luminal cells accumulate high levels of zinc (due to the overexpression of its transporter), leading to the inhibition of mitochondrial aconitase, the key enzyme responsible for the citrate-isocitrate conversion in the TCA cycle. This inhibition results in the truncation of the TCA cycle and citrate accumulation and secretion. As a result, normal prostate epithelial cells are characterized by an inefficient OXPHOS. b In prostate cancer cells, intracellular zinc levels are significantly reduced (due to a decreased expression of its transporter); this leads to the reactivation of aconitase, restoring the citrate-isocitrate conversion, and consequently of the TCA cycle and OXPHOS metabolic pathways. In addition, both the hexosamine biosynthesis pathway (HBP) resulting in glycosylation and the pentose phosphate pathway (PPP) that generates NADPH and nucleotides are upregulated in PCa cells. c Metastatic PCa cells exhibit the Warburg effect with persistent TCA cycle/OXPHOS and PPP activity. Created with BioRender.com.
Androgen receptor plays a predominant role in controlling the expression of growth-promoting and anti-apoptotic genes involved in various metabolic processes, such as glycolysis [14]. Glucose transporters GLUT1 and GLUT2 are regulated at the transcriptional level by the AR [15–17], whereas control by AR-independent factors such as SOX2 and MYC promotes prostate cancer progression, lineage plasticity, and therapy resistance [18, 19]. Interestingly, the Warburg effect is observed both in localised PCa [20, 21], as well as in advanced disease or metastatic lesions [22–24]. In this line, it has been reported that the highest lactate levels are found in patients with PTEN loss, a genetic feature of advanced PCa [25]. The activation of the PI3K–AKT–mTOR signalling pathway is believed to be a key factor in PTEN-deficiency-driven prostate tumorigenesis promoting aerobic glycolysis [26, 27]. Inhibition of MCT4 (a plasma membrane lactic acid transporter), has been postulated as a therapeutic strategy to reduce glycolysis and lactic acid secretion in neuroendocrine prostate cancer (NEPC), a subtype of aggressive PCa [28]. In NEPC cell lines, inhibiting MCT4 expression reduced cell proliferation in vitro and glucose metabolism by downregulating glycolytic genes. However, data about the effectiveness and toxicity of MCT4 inhibition in NEPC models in vivo are still lacking.
Despite the relevance of glycolysis for tumour cells, accumulating evidence sustains that both the tricarboxylic acid (TCA) cycle and the OXPHOS pathway are still present and active in the metastatic setting [6, 29–36], which could complicate the use of lactate-targeted therapies [27]. A promising new small molecule, BKIDC-1553 (which exhibits good safety and pharmacologic properties), has been shown to selectively inhibit the growth of PCa cell lines through its anti-glycolytic activity. This is achieved by inhibiting hexokinase 2, as reported in a preclinical xenograft model of advanced PCa. The selective growth inhibition activity of BKIDC-1553 is equivalent to that of enzalutamide [37]. All these results demonstrate the complexity and impact of metabolic interactions within tumours and in different stages, highlighting the importance of studying drug effects in diverse metabolic scenarios.
Tricarboxylic acid cycle (TCA) and oxidative phosphorylation (OXPHOS)
The TCA comprises a series of mitochondrial chemical reactions and is responsible for producing energy and metabolic intermediates. It begins with the conversion of acetyl-CoA (which is produced from the breakdown of carbohydrates, fats, and proteins) and oxaloacetate into citrate. Through a series of enzymatic reactions, citrate is transformed, resulting in the production of carbon dioxide and molecules carrying high-energy electrons, namely NADH and FADH2. These electron carriers are subsequently utilised for OXPHOS, a process that occurs in the inner mitochondrial membrane. OXPHOS involves the electron transport chain (ETC) and a proton gradient, ultimately leading to the production of ATP (Fig. 1). Despite the initial perception of a general reduction of the use of glucose derivatives in the mitochondria, recent evidence shows that there is tumour-type specificity in this reprogramming. Whereas pancreas, lung and colon tumours exhibit a slower ATP production than healthy tissues, breast cancer-derived metastases reportedly show faster TCA cycle rate than orthotopic primary tumours [38]. In line with these results, an increase in OXPHOS gene expression was detected in melanoma brain metastasis (MBM) by direct metabolite profiling and [U-13C]-glucose tracing in vivo [39], which is associated to increased sensitivity of these lesions to pharmacological OXPHOS inhibition [39]. However, later clinical trials to analyse the therapeutical potential of the same OXPHOS inhibitor in advanced solid tumours and acute myeloid leukaemia showed only modest target inhibition and limited antitumour activity at tolerated doses and led to discontinuation of the trials due to neurotoxicity [40]. Interestingly, tumour cells exhibit changes in the TCA that go beyond the regulation of its activity. Mutations in the TCA cycle or the ETC machinery induce alternative metabolic routes, such as reductive carboxylation observed in various cancer types [41–47]. Beyond the effect of mutations altering the TCA cycle, specific cell state transitions are accompanied by profound TCA reprogramming comprising the extramitochondrial use of citrate that regenerates oxaloacetate [48].
Prostate epithelial cells do not oxidise the produced citrate like most normal cells due to truncated TCA cycle [49–53]. Instead, luminal prostate cells, but not basal cells [54], accumulate high mitochondrial zinc (Zn2+). Zn2+ inhibits the mitochondrial enzyme (m)-aconitase, responsible for citrate oxidation, and the accumulated citrate is subsequently secreted into the prostatic fluid. While basal cells preferentially generate citrate through pyruvate dehydrogenase, luminal cells predominantly generate citrate through pyruvate carboxylase activity [54]. Metabolism of pyruvate, aspartate, glutamine and branched-chain amino acids (BCAA) might contribute to replenishing metabolites for the truncated TCA cycle in PCa [55, 56]. AR induces a metabolic reprogramming encompassing hZIP1 zinc transporter downregulation that leads to low mitochondrial zinc levels [57, 58] and m‐aconitase reactivation, restoring the TCA cycle [59], and increasing the susceptibility of PCa cells to OXPHOS inhibitors [60].
Oxidative phosphorylation can be targeted by restricting the supply of NADH or by directly inhibiting components of the ETC. Mutations in the mitochondrial DNA encoding for OXPHOS machinery promote Warburg-like metabolism and an anti-tumour immune response [61]. Targeting the TCA cycle by compromising mitochondrial substrate trafficking might also be an effective strategy. For example, metformin and rotenone, inhibitors of complex-I (CI) of the ETC, inhibit proliferation in several human cancer cell lines, including PCa [62–64]. Evidence has shown that metformin has multiple antineoplastic effects through AMPK-dependent and independent mechanisms, namely the alteration of IGF-1 signalling pathways, suppression of AR or mTOR pathway, and lipogenesis. In line with this notion, there is evidence for reduced mortality in PCa patients treated with metformin [64]. The rotenone derivative deguelin exhibits antitumoural activity in preclinical mouse models of PCa based on the combined loss of Pten and Trp53 [65]. This effect is associated to the alternative use of the ETC by Pten-deficient cells, which consume ATP through mitochondrial complex V instead of producing it. This observation could be translated to the use of CI inhibitors in PCa patients stratified by PTEN status. Whereas most PCa research is focused on the effect of AR signalling promoting TCA cycle [66, 67], a deeper understanding of the AR-independent metabolic alterations is lacking and could be critical when designing therapeutic strategies in castration-resistant patients.
Amino acid metabolism or the pentose phosphate pathway (PPP)
The PPP is a main producer of NADPH and nucleic acid precursors [68], which helps tumour cells balance the redox status. Tumour cells exhibit deregulation of oncogenes and tumour suppressor genes that control this pathway [69]. Genetic deficiency in glucose-6-phosphate dehydrogenase (G6PD), one of the rate-limiting enzymes of the PPP, is a common inherited enzyme defect and occurs almost exclusively in males [70–72]. There is increasing evidence that this deficiency may offer protection against stomach, colon, and liver cancer. Conversely, G6PD upregulation has been associated with higher cancer risk [73]. In fast proliferating cells, a high NADP+/NADPH ratio activates G6PD to support NADPH production, leading to reductive biosynthesis of fatty acids and nucleotides. Furthermore, NADPH promotes cell survival under oxidative stress conditions such as mitochondrial dysfunction [74]. Upregulated G6PD activity is observed in various cancers, including papillary thyroid carcinoma, colorectal, renal, hepatocellular, breast, and PCa [75–81]. Mechanistic research in PCa cell lines suggests that AR-mediated regulation of the PPP occurs through upregulation of G6PD in response to mTOR complex 1 activation, leading to the production of nucleotide precursors for DNA synthesis and NADPH to promote lipogenesis [82] (Fig. 1). Indeed, PPP and G6PD have been proposed as metabolic targets for PCa bone metastasis treatment [83]. In vitro, genetic and pharmacological G6PD inhibition decreased cancer growth and migration, leading to alterations in cellular redox balance and heightened sensitivity to chemotherapy. In vivo, G6PD genetic ablation resulted in the reduction of bone metastatic burden. A recent study revealed that another PPP-related enzyme, 6PGD, plays a key role in PCa growth and survival by counteracting oxidative stress and uncovered a novel feedback mechanism linking 6PGD and the AR signalling axis that opens a new therapeutical window of co-targeting AR and the PPP [84]. Genetic or pharmacological inhibition of 6PGD using physcion and S3 showed anticancer activity in aggressive, castration-resistant disease models as well as patient-derived tumour explants, partly due to increased oxidative stress. Targeting of 6PGD was associated with two important tumour-suppressive mechanisms: firstly, it increased the activity of the AMP-activated protein kinase (AMPK); secondly, it enhanced AR ubiquitylation, leading to a reduction in AR protein levels and activity. Pharmacological co-targeting of both factors was more effective in suppressing the growth of PCa cells than single-agent therapies, indicating positive feedback between AR and 6PGD. All these findings suggest that the PPP could be a valuable source of targets for anticancer drug design and therapeutic combination.
Hexosamine biosynthetic pathway (HBP)
The HBP is a metabolic route that redirects 2–5% of glucose-derived carbons away from glycolysis in non-cancer cells. It comprises the conversion of the glycolytic intermediate fructose-6-phosphate to produce UDP-N-acetylglucosamine (UDP-GlcNAc) [85]. UDP-GlcNAc serves as a substrate for various cellular processes, including protein glycosylation—a crucial post-translational modification where sugars are attached to proteins and lipids. Cancer cells upregulate the flux towards the HBP and UDP-GlcNAc synthesis by increasing glucose and glutamine intake or in response to oncogenic-associated signals like Ras [86], mammalian target of rapamycin complex 2 (mTORC2) [87, 88], and transforming growth factor beta (TGF-β) [89]. In line with increased UDP-GlcNAc levels, breast [90, 91], lung [92], colon [92], liver [93], endometrial [94], cervical [95], pancreatic cancer [96] and PCa [97] cells exhibit increased O-GlcNAcylation (Fig. 1). There are diverse molecular alterations that converge on increased synthesis of glycans. The second-rate limiting enzyme of the HBP, UAP1 [98–100], is elevated in PCa, which protects tumour cells from ER stress-induced cell death, thus postulating it as a viable target for cancer therapy. An enzyme involved in the conjugations process, the glycosyltransferase GALNT7, is also upregulated in PCa tissues and promotes prostate tumour growth [101]. Lessons from other tumour types reveal the metabolic crosstalk that balances the use of glucose intermediary metabolites. Loss of the PHGDH, an enzyme involved in glucose-derived serine biosynthesis promotes metastasis by rewiring glucose towards HBP, thus increasing integrin glycosylation [102].
Increased glycosylation influences the structural diversity in proteins, including sialylation, fucosylation, O-β-N-acetylglucosylation, and the presence of cryptic and high-mannose N-glycans and proteoglycan alterations [103]. Based on the evidence presented, therapeutic targeting of HBP rises as an innovative strategy to selectively affect cancer cells, as non-transformed cells would be more resilient to the perturbation in O-GlcNAcylation [104, 105]. Hexosamine analogues could serve this purpose since they exhibit antitumoral properties. Other promising therapeutic strategies in preclinical models involve the pharmacological inhibition of OGT, the HBP enzyme that catalyses the addition of the GlcNAc residue to target proteins. On the one hand, inhibiting O-GlcNAcylation in PCa cells reduced the expression of matrix metalloproteinase MMP-2, MMP-9, and VEGF, resulting in inhibition of invasion and angiogenesis mediated by the regulation of the oncogenic transcription factor FoxM1 [104]. On the other hand, OGT inhibition reduced the proliferation of PCa cells due to sustained loss of c-MYC [97]. As a note of caution, the requirement of HBP for PCa cells might be tumor stage-dependent. In fact, castration-resistant PCa shows decreased HBP metabolite and enzyme levels, suggesting that targeting the pathway in this pathological setting could have unpredictable biological consequences [106]. Overall, understanding the function and composition of glycoproteins and glycans across all stages of PCa will likely be crucial to improving disease management. The relevance of the cell surface glycan profile for cell-cell interactions anticipates that HBP and glycosylation rewiring will have profound implications in the interactions of tumour cells with the TME.
Lipid metabolism
Lipid metabolic reprogramming encompasses alterations in various aspects of lipid metabolism, including synthesis, storage, and catabolism [107]. One significant adaptation is the upregulation of lipogenic pathways, where cancer cells enhance the production of fatty acids and other lipid components to sustain their rapid growth. This increase in lipogenesis often involves the activation of key enzymes such as ATP citrate lyase (ACLY) and acetyl-CoA carboxylase (ACC), driven by oncogenic signalling pathways like the PI3K/Akt/mTOR axis and MYC [108]. Additionally, cancer cells exhibit changes in lipid uptake and utilization, relying on both endogenous and exogenous lipid sources to sustain their metabolic needs.
Dysregulation of lipid metabolism is considered a hallmark in PCa [109]. These tumour cells display distinct alterations in lipid metabolism compared to normal prostate counterparts, and these changes are associated with tumour growth, survival, and metastasis [109]. Whereas alterations in oncogenes and tumour suppressor genes (p53 loss, PTEN loss, PI3K mutations) that are shared across different tumour types can alter this process, enhanced lipid metabolism in PCa is predominantly driven by AR signalling [110, 111]. Indeed, AR controls the transcription of enzymes involved in fatty acid synthesis and oxidation to fulfil the bioenergetic and anabolic demands of PCa cells, and it also regulates lipid uptake and storage, cholesterol, and phospholipid metabolisms [112] (Fig. 2).
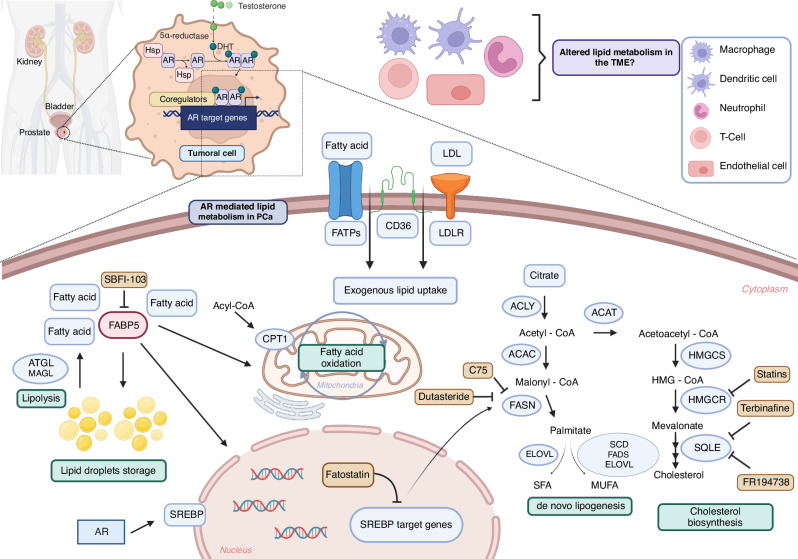
Fig. 2. The landscape of lipid metabolism in PCa.
The figure illustrates the key aspects of lipid metabolism in PCa, mainly driven by AR-mediated cellular reprogramming of tumoral cells. In response to AR signalling, PCa cells exhibit an augmented de novo lipogenesis through transcriptional regulation by SREBPs. Mitochondrial fatty acid oxidation, via upregulation of the CPT1 transporter, generates energy for proliferation. In addition, PCa cells increase exogenous fatty acid uptake through upregulated CD36 and FATPs. FABPs play a role in the intracellular compartmentalization of fatty acids. Dysregulation of cholesterol metabolism also represents a characteristic feature of prostate tumours. The main enzymes and regulators taking part in these pathways are highlighted, alongside various inhibitors studied for potential therapeutic interventions. Finally, the study of lipid metabolism in cells comprising the TME emerges as an important strategy for future research. Abbreviations: AR; androgen receptor, DHT; dihydrotestosterone. Created with BioRender.com.
De novo lipogenesis (DNL)
PCa is characterised by augmented DNL in both in early and late stages of the disease [113]. This pathway is tightly regulated and produces fatty acids from non-lipidic precursors. The primary substrate in fatty acid synthesis is acetyl-CoA, which is carboxylated by acetyl-CoA carboxylase to form malonyl-CoA [114]. Malonyl-CoA units are then sequentially added to the growing fatty acid chain by fatty acid synthase (FASN). This process continues through a series of chemical reactions until a long-chain fatty acid is synthesised. The resulting fatty acids can be further modified, incorporated into phospholipids for membrane biogenesis, or stored as triglycerides [115].
Sterol-regulatory element-binding proteins (SREBPs) are transcription factors that play a crucial role in regulating lipid synthesis. SREBP-1 is upregulated along PCa progression [111, 112], partly in an AR-dependent manner [116, 117], and it activates the expression of enzymes involved in de novo lipogenesis, including FASN [118, 119].
Different strategies have been developed to target DNL in PCa. Preclinical studies using SREBP inhibitors such as fatostatin support the idea that targeting this pathway is an interesting strategy to block PCa growth and promote apoptosis [120]. Fatostatin inhibits SREBP cleavage-activating protein (SCAP), a key regulator of lipid metabolism. SCAP is responsible for transporting the SREBPs from the endoplasmic reticulum (ER) to the Golgi apparatus, where they undergo proteolytic cleavage to activate the transcription of genes involved in cholesterol and fatty acid synthesis. By inhibiting SCAP, fatostatin prevents the translocation of SREBPs to the Golgi, thus inhibiting their activation and subsequent transcriptional regulation of lipid synthesis [120]. In addition, dutasteride and C75 are two FASN inhibitors that have been developed and tested for their effectiveness in PCa [121–123]. Dutasteride indirectly reduces FASN mRNA levels by inhibiting the enzyme 5α-reductase, which is responsible for converting testosterone into dihydrotestosterone [121]. C75 inhibits FASN through competitive binding, thus preventing the synthesis of fatty acids from acetyl-CoA and malonyl-CoA [122].
Despite various efforts to target lipid synthesis, a critical challenge persists in elucidating precise biomarkers and methodologies for the identification of lipogenic tumours and the stratification of patients likely to exhibit optimal responses to DNL targeting. For that reason, new targets of the DNL pathway are currently being explored for therapeutic purposes. Recently, a large-scale analysis revealed that the fatty acid elongase ELOVL5 is upregulated in PCa and its depletion leads to antitumoral responses [124]. Concomitantly, the ELOVL5 enzyme also generates polyunsaturated fatty acids (PUFAs), which have been associated with enzalutamide resistance during neuroendocrine differentiation (NED) by activating the AKT-mTOR pathway [125].
Lipolysis and fatty acid oxidation (FAO)
Lipolysis refers to the process that converts stored fats or triglycerides into glycerol and fatty acids. In the context of PCa, lipolysis is upregulated to generate fatty acids that are subsequently used as an energy source and building blocks for cellular components [126]. However, lipolysis is a more complex process than lipid synthesis. It requires a balance between fatty acid catabolism, necessary for biomass, and the need for ATP and NADPH production. Elevated levels of monoacylglycerol lipase (MAGL) in AR-independent prostate cancer contribute to malignancy through endocannabinoid and fatty acid pathways [127]. Complementarily, adipose triglyceride lipase (ATGL) expression correlates with worse prognosis in CRPC patients [128]. Inhibition of ATGL impairs PCa cell growth in vitro and in vivo, inducing a metabolic shift towards glycolysis [128].
After being released from storage units, lipids can be catabolised through fatty acid oxidation (FAO), a process where cells utilise FAO as an energy source, and that is altered in PCa [129]. CPT1, the enzyme that transports medium-long fatty acids into the mitochondria for oxidation, is upregulated in PCa [130, 131]. In addition, FAO could sustain a castration-resistant state, which has been demonstrated recently through the inhibition of 2,4-dienoyl-CoA reductase (DECR1) [132, 133].
Fatty acid uptake and transport
Fatty acid transport proteins (FATPs) and fatty acid binding proteins (FABPs) are responsible for the uptake of exogenous and intracellular transport of fatty acids, respectively. These proteins are upregulated in PCa, which theoretically increases fatty acid availability for cellular processes [134, 135]. FABP5 inhibition provides a synergistic effect in combination with chemotherapy [136], and the reported dependence of PTEN loss-driven PCa [137] on this enzyme encourages the evaluation of this therapeutic strategy in a stratified population. Indeed, SBFI-103, a competitive inhibitor of FABP5, is effective and well-tolerated both in vitro and in vivo in PCa cells resistant to ADT or taxanes [137]. Finally, CD36, a multifunctional cell surface receptor that imports fatty acids, contributes to various aspects of PCa biology, including tumour growth, angiogenesis, and metastasis. The tumour suppressive consequences of Cd36 deletion in Pten loss-induced PCa [138] suggest that knowledge and therapeutic strategies reported for other tumour types could be implemented in this disease [139, 140]. FA6.152, an anti-CD36 neutralising antibody, inhibits all known functions of CD36, including its interactions with thrombospondin, collagens, and fatty acids. Similarly, another CD36 targeting antibody named JC63.1 selectively blocks uptake of fatty acid and oxidised low-density lipoproteins. Treatment of oral squamous cell carcinoma (OSCC) models with these two antibodies impair metastasis [139].
Cholesterol metabolism
PCa cells often exhibit increased de novo cholesterol biosynthesis [141–143], and AR signalling controls the expression of cholesterol biosynthetic enzymes, such as HMG-CoA reductase (HMGCR) [144]. The relevance of this pathway in PCa spans multiple biological aspects. First, cholesterol is a critical precursor for the synthesis of steroid hormones, including androgens, which sustains the activation of AR in tumour cells after castration therapy [145–147]. Second, cholesterol is a critical component of lipid rafts, membrane microdomains that play a role in cellular signalling. Alterations in cholesterol levels affect lipid raft dynamics and the associated signalling pathways involved in PCa progression [141]. Third, cholesterol esters are abundant components of lipid droplets, whose presence is associated with PCa aggressiveness [148]. Given the relevance of cholesterol metabolism in cancer, different therapeutic strategies have been proposed for PCa. Statins are cholesterol-lowering agents that are administered chronically to millions of people around the globe. Since they inhibit HMGCR, their potential anticancer activity has been broadly studied [149]. In this regard, high doses of statins in vitro consistently reduce PCa aggressiveness [150, 151]. However, low doses of some of these drugs (equivalent to the concentrations reached in the blood of treated individuals) exhibit paradoxical effects on tumour cells in vitro and in vivo [152]. This discrepancy is evident in epidemiological studies monitoring the influence of statin treatment in PCa pathogenesis and progression [149], suggesting that we still miss critical biological information regarding how these drugs operate in cancer. Cholesterol metabolism could be particularly relevant when targeting androgen production or signalling in PCa. Indeed, inhibition of squalene epoxidase (SQLE), a crucial enzyme in cholesterol biosynthesis, has been proposed as a promising pharmacological intervention for treating CRPC [153, 154]. Targeting SQLE with terbinafine effectively inhibited orthotopic tumours growth in mice. Moreover, in a clinical setting, terbinafine demonstrated the ability to decrease prostate-specific antigen (PSA) levels in three out of four late-stage prostate cancer patients [154]. Similarly, the pharmacologic blockade of SQLE with FR194738 attenuated the growth of PC3 cells both in vitro and in mouse xenograft models [153]. Finally, a complementary strategy to support androgen synthesis in conditions of hormone deprivation is the provision of cholesterol by the TME. In this line, macrophages can serve as a source of cholesterol for PCa cells in the context of androgen deprivation, hence supporting the development of CRPC [155].
One-carbon metabolism
One-carbon (1 C) metabolism involves two central cycles: the folate cycle and the methionine cycle [156]. In the folate cycle, tetrahydrofolate (THF) acts as a carbon carrier for purine and thymidylate synthesis. Methyl groups transfer from 5-methyl THF to homocysteine, forming methionine and connecting the two cycles. Methionine is converted to S-adenosyl-methionine (SAM), a universal methyl donor for protein and DNA methylation. SAM is then metabolized to S-adenosyl-homocysteine (SAH) and later to homocysteine, completing the cycle. Homocysteine produces cystathionine in the transsulfuration pathway, a precursor of glutathione. SAM can also feed into the polyamine biosynthesis pathway through its decarboxylation by S-adenosylmethionine decarboxylase (AMD1) [157] (Fig. 3). Alterations in 1 C metabolic homeostasis are at the core of different diseases including cancer [156]. Tumour cells depend on 1 C metabolism for DNA synthesis, redox balance, methylation reactions and polyamine biosynthesis. All these processes are relevant across different cancers and contribute to tumour progression [158, 159].
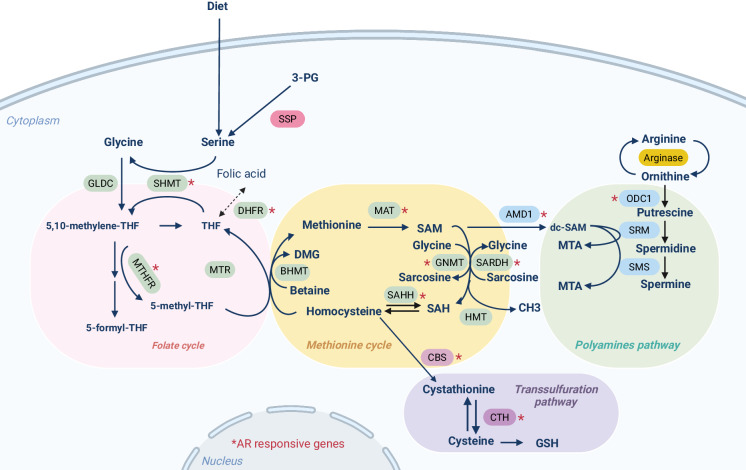
Fig. 3. 1 C metabolism in PCa.
Main metabolites and enzymes involved in 1 C metabolism. This pathway encompasses the folate and methionine cycles, essential for cellular processes like DNA synthesis and methylation. Additionally, it links to the transsulfuration pathway, maintaining redox power through glutathione synthesis, and influencing translation and proliferation via the polyamine biosynthesis pathway. Red asterisks indicate AR-responsive enzymes described in the literature. Abbreviations: 3-PG 3-phosphoglycerate, THF tetrahydrofolate, DMG dimethylglycine, SAM S-Adenosylmethionine, dc-SAM decarboxylated S-Adenosylmethionine, SAH S-adenosylhomocysteine, MTA 5’-methylthioadenosine, GSH glutathione. Created with BioRender.com.
In PCa, androgen signalling regulates the activity of 1 C enzymes involved in SAM homeostasis, the transsulfuration pathway and polyamine biosynthesis [160]. In turn, changes in AR activity occurring upon PCa progression and therapy can influence 1 C metabolism and the intricate epigenetic crosstalk [160].
SAM homeostasis
GNMT and mitochondrial SARDH are critical enzymes that control SAM availability. They are regulated by androgen signalling and are frequently altered in PCa [161, 162]. GNMT transfers a methyl group from SAM to glycine to form SAH and sarcosine, whereas SARDH demethylates sarcosine to form glycine [163]. These two reactions determine the SAM:SAH ratio for the maintenance of epigenetic responses, and the production of sarcosine in this metabolic step has been proposed as a biomarker in PCa, although this data generated intensive controversy in the field [164, 165]. GNMT, is reported to be both upregulated and downregulated depending on the study, thus suggesting a multifactorial regulation in the different stages of the disease [166, 167]. A feasible explanation relates to regulating GNMT by signalling pathways that exhibit reciprocal negative feedback regulation [168, 169]. AR has a predominant role in sustaining GNMT expression, whereas PI3K activation induces its repression [170], a process that could depend on FOXO regulation, according to studies in Drosophila melanogaster [171]. Interestingly, Gnmt levels are profoundly reduced in Pten loss-driven murine PCa, but a germline deletion of the metabolic enzyme reduced PCa incidence in this model, thus suggesting that either residual GNMT activity is essential for tumorigenesis or that this enzyme plays a critical role in the TME [170]. Finally, a recent study has shown a mTORC1/ATF4-driven downregulation of protein kinase C (PKC)λ/ι in neuroendocrine prostate cancer that increases serine biosynthesis. This metabolic shift supports cell proliferation and elevates intracellular SAM levels, promoting epigenetic changes characteristic of this aggressive form of PCa [172].
The transsulfuration pathway
The transsulfuration pathway is a branch of 1 C metabolism that converts homocysteine to cysteine. This process involves several enzymatic steps, with cystathionine beta-synthase (CBS) playing a predominant role [173]. CBS activity is controlled by SAM pools to direct homocysteine towards remethylation when SAM levels are low [173]. In PCa, studies showing both increased and decreased expression of CBS have been published [174, 175]. Lower enzyme levels are found in metastatic PCa cell lines, but these data do not correlate with clinical evidence reporting increased homocysteine and cystathionine abundance in patients with worse outcomes [176, 177]. In this line, cystine depletion sensitises PCa cells to immune checkpoint inhibitors as well as to DNA damage-inducing agents, further highlighting the importance of these intermediates in PCa [178].
Polyamine biosynthesis
Polyamines (PA) are small polycations essential for normal cell growth in all eukaryotic organisms [179]. Putrescine is generated from the urea cycle through decarboxylation of ornithine by ornithine decarboxylase (ODC1), whereas AMD1 decarboxylates SAM to dcSAM. This reaction provides the propyl amines necessary to form spermidine and spermine from putrescine through the action of spermidine synthase (SRM) and spermine synthase (SMS) [180]. The prostate epithelium synthesises high levels of polyamines that are secreted into the seminal fluid. Androgens control this process through transcriptional regulation of ODC1 and AMD1 [181, 182]. Accordingly, androgen deprivation therapies reduce the abundance of spermidine and spermine [183]. However, regulation of PA biosynthesis in PCa extends beyond AR signalling. On the one hand, ODC1 is a main target of MYC, which associates MYC amplification and overexpression with elevated polyamine biosynthesis [184]. The regulation of polyamine biosynthesis downstream MYC contributes to the tumour suppressive activity of PGC1α, which was recently reported to repress this oncogene [185–187]. On the other hand, PI3K-mTORC1-dependent regulation of AMD1 stability influences polyamine synthesis [188], an observation that is extensible to other pathophysiological contexts beyond cancer [189].
Tumor cell-extrinsic metabolic influences
Prostate cancer is associated with ageing, and in turn, the organism and cellular environment represent an important modifiable factor in the pathogenesis and progression of the disease. There is an emerging interest in studying the metabolic properties of the tumour microenvironment, as well as how exogenous factors like the diet may impact tumour progression. The TME closely interacts with tumour cells and comprises immune cells, fibroblasts, blood vessels, and the extracellular matrix [190]. Immune cells within the TME can either trigger pro-tumoral or anti-tumoral responses [191], while the extracellular matrix and stromal cells within the TME provide structural and biochemical support to tumours, influencing their ability to invade surrounding tissues and metastasize [190]. Advances in high-throughput, single-cell resolution technologies have significantly enhanced our comprehension of cellular diversity in PCa [192–194]. However, there is still very little knowledge about the metabolic adaptations in PCa stromal cells, and a glimpse at other tumour types can provide critical information on what is to come (Fig. 4).
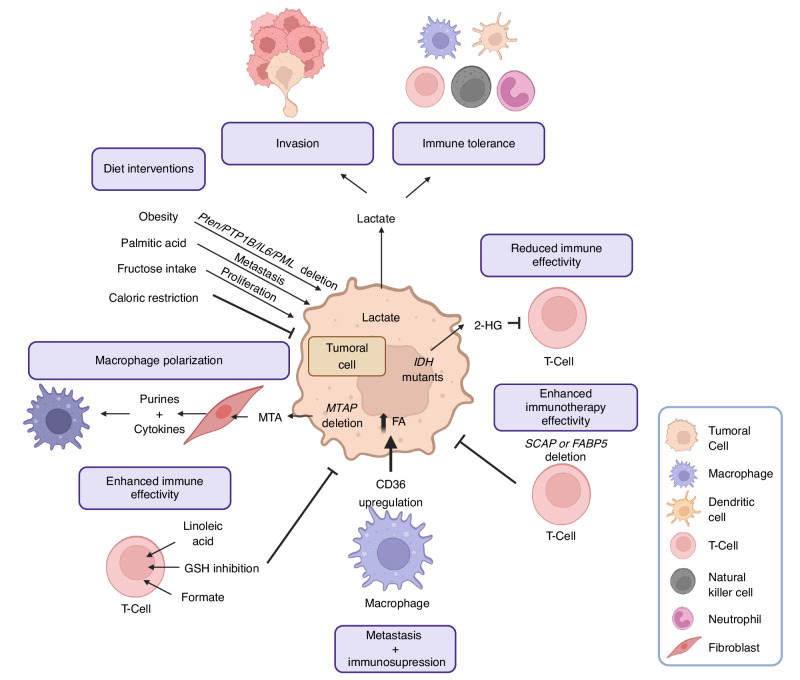
Fig. 4. The role of metabolism in the cancer tumour microenvironment.
Summary of the recent concepts regarding the interaction of tumour metabolism with the tumour microenvironment to support cancer progression. Abbreviations: 2-HG 2-Hydroxyglutarate, GSH glutathione, IDH isocitrate dehydrogenase, MTAP S-methyl-5’-thioadenosine phosphorylase, MTA 5′-deoxy-5′-methylthioadenosine. Created with BioRender.com.
Glucose metabolism in the TME
The elevated glycolytic rate of tumour cells is directly responsible for creating the acidic and nutrient-depleted conditions of the TME, which have profound consequences for immune activity [195, 196]. One of the most significant effects of aerobic glycolysis is the acidification of the TME due to lactate secretion [197, 198], which supports increased migration and invasion [199] and promotes immune reprogramming towards a tolerant phenotype [196, 200]. Glycolysis in the tumour stroma is also required for adequate antitumoral response, which has led to the development of metabolite-based formulations in the presence of a glycolytic inhibitor that specifically targets cancer cells [201]. Cancer and T cells compete for glucose among several other metabolites, and the avidity of cancer cells for this nutrient diminishes the cytolytic activity [195, 202, 203]. Glycolytic capacity in T cells is also influenced by oncometabolites such as 2-hydroxyglutarate, which is produced at high concentrations in isocitrate dehydrogenase mutant cancers and inhibits their proliferation, cytokine production, and ability to kill tumour cells [204].
Lipid metabolism in the TME
Lipid metabolism is similarly required in stromal cells. SREBP activity orchestrates the immune responses in cancer. Inhibition of SREBP function in regulatory T cells (Treg) enhances antitumour immune responses [205]. Particularly, SREBP-cleavage-activating protein deletion in intra-tumoral Tregs inhibits tumour growth and improves PD-1-triggered immunotherapy by regulating interferon-γ production [205]. Similarly, deletion of FABP5 in Treg affects mitochondrial integrity and triggers cGAS-STING-dependent type I IFN signalling [206]. Macrophages are regulated by tumour cells at multiple levels and their polarisation contributes to the acquisition of aggressive features. Upregulation of CD36 in metastasis-associated macrophages (MAMs) promotes tumour cell-derived fatty acid uptake, protumoural polarization and their supportive role in the establishment of liver metastasis [207]. Thus, targeting CD36 emerges as a two-hit strategy targeting both tumour and immune cells in the treatment of metastasis. Lipids can also support the activation of lymphocytes. As an illustrative example, linoleic acid activates CD8 + T cells, enhancing metabolic fitness and preventing exhaustion [208], highlighting its role as a potential adjuvant to potentiate adoptive T cell therapy.
One-carbon metabolism in the TME
Very little is known about the contribution of 1 C metabolism to the TME in PCa. In turn, scattered evidence in other tumour types can provide an idea of the processes influenced by this metabolic route in cancer. Deficiencies in one-carbon metabolism impair the effectiveness of PD-1 blockade in melanoma. Coherently, augmenting 1 C metabolism through formate supplementation during anti-PD-1 therapy improves CD8 + T-cell fitness and facilitates CD8 + T-cell-mediated tumour clearance [209]. These results indicate that formate supplementation has the potential to enhance the function of exhausted CD8 + T cells. Importantly, the acidification of the extracellular milieu also influences T cell function, eliciting a reduction in methionine metabolism via SLC7A5 downregulation that results in a ‘stem-like memory’ state. This reprogramming enhances T cell persistence and anti-tumour efficacy in mice, revealing a novel influence of acidic conditions on T cell characteristics [210]. The relevance of 1 C metabolism for glutathione production and redox balance is an additional factor controlling the activity of the TME. Disrupting glutathione synthesis in Tregs impairs their ability to regulate serine metabolism, leading to severe autoimmunity and improved anti-tumour responses [211]. Tumour-intrinsic 1 C metabolism produces secreted metabolic intermediates that can remodel the TME. Tumour cells exhibit frequent loss of methylthioadenosine phosphorylase (MTAP), which leads to the accumulation of its substrate MTA [212]. Secreted MTA is uptaken and metabolized by fibroblasts, which will produce and secrete both purine products and cytokines that induce macrophage polarization.
Diet and obesity
Nutrition represents the tightest interaction of our organism with the environment. As such, it is closely linked to the development of diseases, including cancer. Studies in other tumour types have unveiled additional molecular processes responsible for the high-fat diet-induced phenotype. In oral carcinoma and melanoma models dietary palmitic acid, but not oleic or linoleic acid, promotes metastasis in mice [139, 140]. Molecularly, palmitic acid induces a pro-metastatic memory involving CD36, histone modifications, and a neural signature linked to Schwann cells, leading to both metastasis initiation and long-term metastatic memory. These same modifications may also play a role in PCa [140]. In line with the role of CD36, a high-fat diet has been shown to promote metastasis by enhancing saturated fatty acid uptake via this receptor in breast cancer [213]. Modifications in dietary habits could also be beneficial for cancer patients. Caloric restriction induces anti-proliferative effects in mouse xenografts, an effect that is limited to tumours without mutations causing constitutive activation of the PI3K pathway [214]. More recently, caloric restriction has been shown to inhibit the growth of certain tumours in mice by lowering lipid levels in both plasma and tumours [215]. This dietary modification reduces stearoyl-CoA desaturase activity in cancer cells, causing an imbalance between unsaturated and saturated fatty acids and impairing tumour growth.
In PCa obesity has been linked to an increased risk and progression of the disease in epidemiological studies [216–218], owing to the contribution of factors such as insulin resistance, chronic inflammation, or hormonal dysregulation, among others. However, the causal contribution of obesity to PCa and the mechanistic foundations of this effect remains elusive. Murine models have shed some light on these questions. Obesity and high calorie-induced hyperinsulinemia promote PCa in prostate-specific Pten-/- mice by increasing cell proliferation and activating insulin/IGF1/PI3K/AKT signalling pathways [219, 220]. In line with this notion, mutations in PCa that activate PI3K (such as prostate-specific Pten loss) prime or promote obesity-driven PCa aggressiveness in conjunction with other signalling pathways, such as loss of Ptpn1 [221], IL6/pSTAT3 signalling activation [222] or Pml co-deletion [223]. This knowledge offers new therapeutic opportunities for targeting PTP1B, IL6 or PML-loss induced SREBP signalling in the context of obesity.
Although much of the emphasis on the influence of obesity has been put on lipid availability and chronic inflammation, sugars could also play a relevant role. Indeed, increased expression of fructose transporters in PCa has been suggested to promote fructose uptake and metabolism to support cancer cell fitness [224].
Collectively, dietary interventions may also play a role in both the progression and treatment of PCa, and further studies are required to extend the knowledge of molecular and biological effectors that can be translated into preventive and therapeutic actions.
Concluding remarks and open questions
Over the past decade, there have been extensive efforts to understand the mechanisms and biological consequences of metabolic reprogramming in cancer. Although currently there are no drugs approved for PCa treatment that target specific metabolic pathways, there are multiple agents in development. Metabolic reprogramming is essential for the biology of cancer cells. Tumour metabolism is influenced by cancer cell-specific metabolic adaptations as well as by metabolic alterations in the TME. Modern technologies to study metabolism, including new imaging techniques, spatial metabolomics and single-cell RNA sequencing have redefined our knowledge of cancer metabolism. However, despite extensive research in PCa metabolism, there is still a gap in knowledge on the therapeutically-actionable metabolic pathways that are relevant to each stage of the disease. Further research into the metabolic dependencies of the primary tumour and those of the metastatic lesions, including the role of ferroptosis, hypoxia and microbiota, might lead to new metabolic interventions to prevent metastatic dissemination of prostate cancer, and to significant improvements in the curation rate of this disease.
Acknowledgements
We are grateful to the Carracedo lab for valuable input, and to Kathrin Keim for the help with English editing. A. Carracedo is funded by the Basque Department of Industry, Tourism and Trade (Elkartek), the BBVA foundation (Becas Leonardo), the MICINN (PID2022-141553OB-I0 (FEDER/EU); La Caixa Foundation (ID 100010434), under the agreement LCF/PR/HR17, Fundación Cris Contra el Cáncer (PR_EX_2021-22), Severo Ochoa Excellence Accreditation CEX2021-001136-S), 2023 AstraZeneca Award for Young scientists in oncology, European Training Networks Project (H2020-MSCA-ITN-308 2016 721532), Vencer el Cáncer Foundation, iDIFFER network of Excellence (RED2022-134792-T), Asociación Española Contra el Cáncer (GCTRA18006CARR) and the European Research Council (Consolidator Grant 819242). L. Bozal was supported by the AECC Foundation (POSTD19048BOZA). CIBERONC was co-funded with FEDER funds and funded by ISCIII.
Author contributions
MP performed bibliography search, contributed to structuring the review, preparing the figures and writing specific sections of the manuscript. LB coordinated the design of the review with AC, performed bibliography search, contributed to structuring the review, preparing the figures and writing sections of the manuscript. AC supervised the structure, design and writing of the review, and perform critical reading and editing of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Laura Bozal-Basterra, Email: lbozal@cicbiogune.es.
Arkaitz Carracedo, Email: acarracedo@cicbiogune.es.
References
- 1.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. [DOI] [PubMed] [Google Scholar]
- 2.Gibson DA, Saunders PTK, McEwan IJ. Androgens and androgen receptor: above and beyond. Mol Cell Endocrinol. 2018;465:1–3. [DOI] [PubMed] [Google Scholar]
- 3.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333–44. [DOI] [PubMed] [Google Scholar]
- 4.Barfeld SJ, Itkonen HM, Urbanucci A, Mills IG. Androgen-regulated metabolism and biosynthesis in prostate cancer. Endocr Relat Cancer. 2014;21:T57–66. [DOI] [PubMed] [Google Scholar]
- 5.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBerardinis RJ, Chandel NS. We need to talk about the Warburg effect. Nat Metab. 2020;2:127–9. [DOI] [PubMed] [Google Scholar]
- 7.Luengo A, Li Z, Gui DY, Sullivan LB, Zagorulya M, Do BT, et al. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol Cell. 2021;81:691–707.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Stancliffe E, Fowle-Grider R, Wang R, Wang C, Schwaiger-Haber M, et al. Saturation of the mitochondrial NADH shuttles drives aerobic glycolysis in proliferating cells. Mol Cell. 2022;82:3270–83.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaarwerk B, Breunis WB, Haveman LM, de Keizer B, Jehanno N, Borgwardt L, et al. Fluorine-18-fluorodeoxyglucose (FDG) positron emission tomography (PET) computed tomography (CT) for the detection of bone, lung, and lymph node metastases in rhabdomyosarcoma. Cochrane Database Syst Rev. 2021;11:CD012325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham NA, Minasyan A, Lomova A, Cass A, Balanis NG, Friedman M, et al. Recurrent patterns of DNA copy number alterations in tumors reflect metabolic selection pressures. Mol Syst Biol. 2017;13:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei J, Huang K, Chen Z, Hu M, Bai Y, Lin S, et al. Characterization of glycolysis-associated molecules in the tumor microenvironment revealed by pan-cancer tissues and lung cancer single cell data. Cancers (Basel). 2020;12:1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell KG, Amini B, Wang Y, Carter BW, Godoy MCB, Parra ER, et al. 18)F-fluorodeoxyglucose positron emission tomography correlates with tumor immunometabolic phenotypes in resected lung cancer. Cancer Immunol Immunother. 2020;69:1519–34. [DOI] [PMC free article] [PubMed]
- 13.Mathews EH, Liebenberg L, Pelzer R. High-glycolytic cancers and their interplay with the body’s glucose demand and supply cycle. Med Hypotheses. 2011;76:157–65. [DOI] [PubMed] [Google Scholar]
- 14.Uo T, Sprenger CC, Plymate SR. Androgen receptor signaling and metabolic and cellular plasticity during progression to castration resistant prostate cancer. Front Oncol. 2020;10:580617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White MA, Tsouko E, Lin C, Rajapakshe K, Spencer JM, Wilkenfeld SR, et al. GLUT12 promotes prostate cancer cell growth and is regulated by androgens and CaMKK2 signaling. Endocr Relat Cancer. 2018;25:453–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu M, Sakamoto S, Matsushima J, Kimura T, Ueda T, Mizokami A, et al. Up-regulation of LAT1 during antiandrogen therapy contributes to progression in prostate cancer cells. J Urol. 2016;195:1588–97. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Xu W, Wang B, Lin G, Wei Y, Abudurexiti M, et al. GLUT1 is an AR target contributing to tumor growth and glycolysis in castration-resistant and enzalutamide-resistant prostate cancers. Cancer Lett. 2020;485:45–55. [DOI] [PubMed] [Google Scholar]
- 18.de Wet L, Williams A, Gillard M, Kregel S, Lamperis S, Gutgesell LC, et al. SOX2 mediates metabolic reprogramming of prostate cancer cells. Oncogene. 2022;41:1190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowell PD, Giafaglione JM, Jones AE, Nunley NM, Hashimoto T, Delcourt AML, et al. MYC is a regulator of androgen receptor inhibition-induced metabolic requirements in prostate cancer. Cell Rep. 2023;42:113221. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zuckier LS, Ghesani NV. Dominant uptake of fatty acid over glucose by prostate cells: a potential new diagnostic and therapeutic approach. Anticancer Res. 2010;30:369–74. [PubMed] [Google Scholar]
- 21.Sadeghi RN, Karami-Tehrani F, Salami S. Targeting prostate cancer cell metabolism: impact of hexokinase and CPT-1 enzymes. Tumour Biol. 2015;36:2893–905. [DOI] [PubMed] [Google Scholar]
- 22.Twum-Ampofo J, Fu D-X, Passaniti A, Hussain A, Siddiqui MM. Metabolic targets for potential prostate cancer therapeutics. Curr Opin Oncol. 2016;28:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jadvar H. PET of glucose metabolism and cellular proliferation in prostate cancer. J Nucl Med. 2016;57:25S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaz CV, Alves MG, Marques R, Moreira PI, Oliveira PF, Maia CJ, et al. Androgen-responsive and nonresponsive prostate cancer cells present a distinct glycolytic metabolism profile. Int J Biochem Cell Biol. 2012;44:2077–84. [DOI] [PubMed] [Google Scholar]
- 25.Granlund KL, Tee S-S, Vargas HA, Lyashchenko SK, Reznik E, Fine S, et al. Hyperpolarized MRI of human prostate cancer reveals increased lactate with tumor grade driven by monocarboxylate transporter 1. Cell Metab. 2020;31:105–14.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen M-L, Xu P-Z, Peng X, Chen WS, Guzman G, Yang X, et al. The deficiency of Akt1 is sufficient to suppress tumor development in Pten + /− mice. Genes Dev. 2006;20:1569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SYC, Ettinger SL, Lin D, Xue H, Ci X, Nabavi N, et al. Targeting MCT4 to reduce lactic acid secretion and glycolysis for treatment of neuroendocrine prostate cancer. Cancer Med. 2018;7:3385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porporato PE, Filigheddu N, Pedro JMB-S, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia D, Lu M, Jung KH, Park JH, Yu L, Onuchic JN, et al. Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci USA. 2019;116:3909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassim S, Vučetić M, Ždralević M, Pouyssegur J. Warburg and beyond: the power of mitochondrial metabolism to collaborate or replace fermentative glycolysis in cancer. Cancers (Basel). 2020;12:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grasso D, Zampieri LX, Capelôa T, Van de Velde JA, Sonveaux P. Mitochondria in cancer. Cell Stress. 2020;4:114–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth KG, Mambetsariev I, Kulkarni P, Salgia R. The mitochondrion as an emerging therapeutic target in cancer. Trends Mol Med. 2020;26:119–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosier JA, Schwager SC, Boyajian DA, Reinhart-King CA. Cancer cell metabolic plasticity in migration and metastasis. Clin Exp Metastasis. 2021;38:343–59. [DOI] [PubMed] [Google Scholar]
- 35.Tan YQ, Zhang X, Zhang S, Zhu T, Garg M, Lobie PE, et al. Mitochondria: the metabolic switch of cellular oncogenic transformation. Biochim Biophys Acta Rev Cancer. 2021;1876:188534. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Su Q, Zhou J, Yang Z, Liu Z, Ji L, et al. To betray or to fight? The dual identity of the mitochondria in cancer. Future Oncol. 2021;17:723–43. [DOI] [PubMed] [Google Scholar]
- 37.Uo T, Ojo KK, Sprenger CCT, Soriano Epilepsia K, Perera BGK, Damodarasamy, M et al. A Compound that Inhibits Glycolysis in Prostate Cancer Controls Growth of Advanced Prostate Cancer. Mol Cancer Ther. 2024; 10.1158/1535-7163.MCT-23-0540. [DOI] [PMC free article] [PubMed]
- 38.Bartman CR, Weilandt DR, Shen Y, Lee WD, Han Y, TeSlaa T, et al. Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature. 2023;614:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer GM, Jalali A, Kircher DA, Lee W-C, McQuade JL, Haydu LE, et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 2019;9:628–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap TA, Daver N, Mahendra M, Zhang J, Kamiya-Matsuoka C, Meric-Bernstam F, et al. Complex I inhibitor of oxidative phosphorylation in advanced solid tumors and acute myeloid leukemia: phase I trials. Nat Med. 2023;29:115–26. [DOI] [PubMed] [Google Scholar]
- 41.Mullen AR, Hu Z, Shi X, Jiang L, Boroughs LK, Kovacs Z, et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014;7:1679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomlinson IPM, Alam NA, Rowan AJ, Barclay E, Jaeger EEM, Kelsell D, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–10. [DOI] [PubMed] [Google Scholar]
- 43.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–51. [DOI] [PubMed] [Google Scholar]
- 44.Mullen AR, Wheaton WW, Jin ES, Chen P-H, Sullivan LB, Cheng T, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481:385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3:648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niemann S, Müller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268–70. [DOI] [PubMed] [Google Scholar]
- 48.Arnold PK, Jackson BT, Paras KI, Brunner JS, Hart ML, Newsom OJ, et al. A non-canonical tricarboxylic acid cycle underlies cellular identity. Nature. 2022;603:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cutruzzolà F, Giardina G, Marani M, Macone A, Paiardini A, Rinaldo S, et al. Glucose metabolism in the progression of prostate cancer. Front Physiol. 2017;8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costello LC, Franklin RB. Citrate metabolism of normal and malignant prostate epithelial cells. Urology. 1997;50:3–12. [DOI] [PubMed] [Google Scholar]
- 51.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper JF, Farid I. The role of citric acid in the physiology of the prostate. 3. Lactate/citrate ratios in benign and malignant prostatic homogenates as an index of prostatic malignancy. J Urol. 1964;92:533–6. [DOI] [PubMed] [Google Scholar]
- 54.Giafaglione JM, Crowell PD, Delcourt AML, Hashimoto T, Ha SM, Atmakuri A, et al. Prostate lineage-specific metabolism governs luminal differentiation and response to antiandrogen treatment. Nat Cell Biol. 2023;25:1821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costello LC, Franklin RB, Feng P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion. 2005;5:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frégeau-Proulx L, Lacouture A, Berthiaume L, Weidmann C, Harvey M, Gonthier K, et al. Multiple metabolic pathways fuel the truncated tricarboxylic acid cycle of the prostate to sustain constant citrate production and secretion. Mol Metab. 2022;62:101516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou J, Milon BC, Desouki MM, Costello LC, Franklin RB. hZIP1 zinc transporter down-regulation in prostate cancer involves the overexpression of ras responsive element binding protein-1 (RREB-1). Prostate. 2011;71:1518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milon BC, Agyapong A, Bautista R, Costello LC, Franklin RB. Ras responsive element binding protein-1 (RREB-1) down-regulates hZIP1 expression in prostate cancer cells. Prostate. 2010;70:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shao Y, Ye G, Ren S, Piao H-L, Zhao X, Lu X, et al. Metabolomics and transcriptomics profiles reveal the dysregulation of the tricarboxylic acid cycle and related mechanisms in prostate cancer. Int J Cancer. 2018;143:396–407. [DOI] [PubMed] [Google Scholar]
- 60.Ahmad F, Cherukuri MK, Choyke PL. Metabolic reprogramming in prostate cancer. Br J Cancer. 2021;125:1185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahmood M, Liu EM, Shergold AL, Tolla E, Tait-Mulder J, Huerta-Uribe A, et al. Mitochondrial DNA mutations drive aerobic glycolysis to enhance checkpoint blockade response in melanoma. Nat Cancer. 2024. 10.1038/s43018-023-00721-w. [DOI] [PMC free article] [PubMed]
- 62.Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heinz S, Freyberger A, Lawrenz B, Schladt L, Schmuck G, Ellinger-Ziegelbauer H. Mechanistic investigations of the mitochondrial complex I inhibitor rotenone in the context of pharmacological and safety evaluation. Sci Rep. 2017;7:45465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaidi S, Gandhi J, Joshi G, Smith NL, Khan SA. The anticancer potential of metformin on prostate cancer. Prostate Cancer Prostatic Dis. 2019;22:351–61. [DOI] [PubMed] [Google Scholar]
- 65.Naguib A, Mathew G, Reczek CR, Watrud K, Ambrico A, Herzka T, et al. Mitochondrial complex I inhibitors expose a vulnerability for selective killing of Pten-null cells. Cell Rep. 2018;23:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bader DA, McGuire SE. Tumour metabolism and its unique properties in prostate adenocarcinoma. Nat Rev Urol. 2020;17:214–31. [DOI] [PubMed] [Google Scholar]
- 67.Bader DA, Hartig SM, Putluri V, Foley C, Hamilton MP, Smith EA, et al. Mitochondrial pyruvate import is a metabolic vulnerability in androgen receptor-driven prostate cancer. Nat Metab. 2019;1:70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin L, Zhou Y. Crucial role of the pentose phosphate pathway in malignant tumors. Oncol Lett. 2019;17:4213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ronquist G, Theodorsson E. Inherited, non-spherocytic haemolysis due to deficiency of glucose-6-phosphate dehydrogenase. Scand J Clin Lab Invest. 2007;67:105–11. [DOI] [PubMed] [Google Scholar]
- 71.Mehta A, Mason PJ, Vulliamy TJ. Glucose-6-phosphate dehydrogenase deficiency. Baillieres Best Pr Res Clin Haematol. 2000;13:21–38. [DOI] [PubMed] [Google Scholar]
- 72.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. [DOI] [PubMed] [Google Scholar]
- 73.Dore MP, Davoli A, Longo N, Marras G, Pes GM. Glucose-6-phosphate dehydrogenase deficiency and risk of colorectal cancer in Northern Sardinia: a retrospective observational study. Medicine. 2016;95:e5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kowalik MA, Columbano A, Perra A. Emerging role of the pentose phosphate pathway in hepatocellular carcinoma. Front Oncol. 2017;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen M, Shen M, Li Y, Liu C, Zhou K, Hu W, et al. GC-MS-based metabolomic analysis of human papillary thyroid carcinoma tissue. Int J Mol Med. 2015;36:1607–14. [DOI] [PubMed] [Google Scholar]
- 76.Cohen HJ, Elizalde A, Miller SP. Cytologic studies of glucose-6-phosphate dehydrogenase in malignancy. Cancer. 1968;21:1055–60. [DOI] [PubMed] [Google Scholar]
- 77.Langbein S, Frederiks WM, zur Hausen A, Popa J, Lehmann J, Weiss C, et al. Metastasis is promoted by a bioenergetic switch: new targets for progressive renal cell cancer. Int J Cancer. 2008;122:2422–8. [DOI] [PubMed] [Google Scholar]
- 78.Lu M, Lu L, Dong Q, Yu G, Chen J, Qin L, et al. Elevated G6PD expression contributes to migration and invasion of hepatocellular carcinoma cells by inducing epithelial-mesenchymal transition. Acta Biochim Biophys Sin (Shanghai). 2018;50:370–80. [DOI] [PubMed] [Google Scholar]
- 79.Dong T, Kang X, Liu Z, Zhao S, Ma W, Xuan Q, et al. Altered glycometabolism affects both clinical features and prognosis of triple-negative and neoadjuvant chemotherapy-treated breast cancer. Tumour Biol. 2016;37:8159–68. [DOI] [PubMed] [Google Scholar]
- 80.Pu H, Zhang Q, Zhao C, Shi L, Wang Y, Wang J, et al. Overexpression of G6PD is associated with high risks of recurrent metastasis and poor progression-free survival in primary breast carcinoma. World J Surg Oncol. 2015;13:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zampella EJ, Bradley ELJ, Pretlow TG 2nd. Glucose-6-phosphate dehydrogenase: a possible clinical indicator for prostatic carcinoma. Cancer. 1982;49:384–7. [DOI] [PubMed] [Google Scholar]
- 82.Tsouko E, Khan AS, White MA, Han JJ, Shi Y, Merchant FA, et al. Regulation of the pentose phosphate pathway by an androgen receptor-mTOR-mediated mechanism and its role in prostate cancer cell growth. Oncogenesis. 2014;3:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whitburn J, Rao SR, Morris EV, Tabata S, Hirayama A, Soga T, et al. Metabolic profiling of prostate cancer in skeletal microenvironments identifies G6PD as a key mediator of growth and survival. Sci Adv. 2022;8:eabf9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gillis JL, Hinneh JA, Ryan NK, Irani S, Moldovan M, Quek L-E, et al. A feedback loop between the androgen receptor and 6-phosphogluoconate dehydrogenase (6PGD) drives prostate cancer growth. Elife. 2021;10:e62592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role hexosamine biosynth induction insulin resistance. J Biol Chem. 1991;266:4706–12. [PubMed] [Google Scholar]
- 86.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moloughney JG, Kim PK, Vega-Cotto NM, Wu C-C, Zhang S, Adlam M, et al. mTORC2 responds to glutamine catabolite levels to modulate the hexosamine biosynthesis enzyme GFAT1. Mol Cell. 2016;63:811–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moloughney JG, Vega-Cotto NM, Liu S, Patel C, Kim PK, Wu C-C, et al. mTORC2 modulates the amplitude and duration of GFAT1 Ser-243 phosphorylation to maintain flux through the hexosamine pathway during starvation. J Biol Chem. 2018;293:16464–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lucena MC, Carvalho-Cruz P, Donadio JL, Oliveira IA, de Queiroz RM, Marinho-Carvalho MM, et al. Epithelial mesenchymal transition induces aberrant glycosylation through hexosamine biosynthetic pathway activation. J Biol Chem. 2016;291:12917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29:2831–42. [DOI] [PubMed] [Google Scholar]
- 91.Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, et al. Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer. Proteomics. 2013;13:2088–99. [DOI] [PubMed] [Google Scholar]
- 92.Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, et al. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta. 2011;1812:514–9. [DOI] [PubMed] [Google Scholar]
- 93.Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L, et al. O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med Oncol. 2012;29:985–93. [DOI] [PubMed] [Google Scholar]
- 94.Krześlak A, Wójcik-Krowiranda K, Forma E, Bieńkiewicz A, Bryś M. Expression of genes encoding for enzymes associated with O-GlcNAcylation in endometrial carcinomas: clinicopathologic correlations. Ginekol Pol. 2012;83:22–26. [PubMed] [Google Scholar]
- 95.Kim MJ, Choi MY, Lee DH, Roh GS, Kim HJ, Kang SS, et al. O-linked N-acetylglucosamine transferase enhances secretory clusterin expression via liver X receptors and sterol response element binding protein regulation in cervical cancer. Oncotarget. 2018;9:4625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-κB activity in pancreatic cancer cells. J Biol Chem. 2013;288:15121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, et al. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res. 2013;73:5277–87. [DOI] [PubMed] [Google Scholar]
- 98.Munkley J, Vodak D, Livermore KE, James K, Wilson BT, Knight B, et al. Glycosylation is an androgen-regulated process essential for prostate cancer cell viability. EBioMedicine. 2016;8:103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Albitar M, Ma W, Lund L, Albitar F, Diep K, Fritsche HA, et al. Predicting prostate biopsy results using a panel of plasma and urine biomarkers combined in a scoring system. J Cancer. 2016;7:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Itkonen HM, Engedal N, Babaie E, Luhr M, Guldvik IJ, Minner S, et al. UAP1 is overexpressed in prostate cancer and is protective against inhibitors of N-linked glycosylation. Oncogene. 2015;34:3744–50. [DOI] [PubMed] [Google Scholar]
- 101.Scott E, Hodgson K, Calle B, Turner H, Cheung K, Bermudez A, et al. Upregulation of GALNT7 in prostate cancer modifies O-glycosylation and promotes tumour growth. Oncogene. 2023;42:926–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rossi M, Altea-Manzano P, Demicco M, Doglioni G, Bornes L, Fukano M, et al. PHGDH heterogeneity potentiates cancer cell dissemination and metastasis. Nature. 2022;605:747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Samaržija I. Post-translational modifications that drive prostate cancer progression. Biomolecules. 2021;11:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Itkonen HM, Gorad SS, Duveau DY, Martin SES, Barkovskaya A, Bathen TF, et al. Inhibition of O-GlcNAc transferase activity reprograms prostate cancer cell metabolism. Oncotarget. 2016;7:12464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Queiroz RM, Madan R, Chien J, Dias WB, Slawson C. Changes in O-linked N-acetylglucosamine (O-GlcNAc) homeostasis activate the p53 pathway in ovarian cancer cells. J Biol Chem. 2016;291:18897–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaushik AK, Shojaie A, Panzitt K, Sonavane R, Venghatakrishnan H, Manikkam M, et al. Inhibition of the hexosamine biosynthetic pathway promotes castration-resistant prostate cancer. Nat Commun. 2016;7:11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353–63. [DOI] [PMC free article] [PubMed]
- 108.Broadfield LA, Pane AA, Talebi A, Swinnen JV, Fendt SM. Lipid metabolism in cancer: new perspectives and emerging mechanisms. Dev Cell. 2021;56:1363–93. [DOI] [PubMed] [Google Scholar]
- 109.Giunchi F, Fiorentino M, Loda M. The metabolic landscape of prostate cancer. Eur Urol Oncol. 2019;2:28–36. [DOI] [PubMed] [Google Scholar]
- 110.Butler LM, Centenera MM, Swinnen JV. Androgen control of lipid metabolism in prostate cancer: novel insights and future applications. Endocr Relat Cancer. 2016;23:R219–27. [DOI] [PubMed] [Google Scholar]
- 111.Swinnen JV, Verhoeven G. Androgens and the control of lipid metabolism in human prostate cancer cells. J Steroid Biochem Mol Biol. 1998;65:191–8. [DOI] [PubMed] [Google Scholar]
- 112.Pardo JC, de Porras VR, Gil J, Font A, Puig-Domingo M, Jordà M. Lipid metabolism and epigenetics crosstalk in prostate cancer. Nutrients. 2022;14:851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lounis MA, Péant B, Leclerc-Desaulniers K, Ganguli D, Daneault C, Ruiz M, et al. Modulation of de novo lipogenesis improves response to enzalutamide treatment in prostate cancer. Cancers (Basel). 2020;12:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Butler LM, Mah CY, Machiels J, Vincent AD, Irani S, Mutuku SM, et al. Lipidomic profiling of clinical prostate cancer reveals targetable alterations in membrane lipid composition. Cancer Res. 2021;81:4981–93. [DOI] [PubMed] [Google Scholar]
- 115.Mounier C, Bouraoui L, Rassart E. Lipogenesis in cancer progression (review). Int J Oncol. 2014;45:485–92. [DOI] [PubMed] [Google Scholar]
- 116.Huang WC, Li X, Liu J, Lin J, Chung LWK. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res. 2012;10:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee MY, Moon JS, Park SW, Koh YK, Ahn YH, Kim KS. KLF5 enhances SREBP-1 action in androgen-dependent induction of fatty acid synthase in prostate cancer cells. Biochem J. 2009;417:313–22. [DOI] [PubMed] [Google Scholar]
- 118.Hamada S, Horiguchi A, Kuroda K, Ito K, Asano T, Miyai K, et al. Increased fatty acid synthase expression in prostate biopsy cores predicts higher Gleason score in radical prostatectomy specimen. BMC Clin Pathol. 2014;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bastos DC, Ribeiro CF, Ahearn T, Nascimento J, Pakula H, Clohessy J, et al. Genetic ablation of FASN attenuates the invasive potential of prostate cancer driven by Pten loss. J Pathol. 2021;253:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li X, Chen YT, Hu P, Huang WC. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol Cancer Ther. 2014;13:855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmidt LJ, Ballman KV, Tindall DJ. Inhibition of fatty acid synthase activity in prostate cancer cells by dutasteride. Prostate. 2007;67:1111–20. [DOI] [PubMed] [Google Scholar]
- 122.Rae C, Fragkoulis GI, Chalmers AJ. Cytotoxicity and radiosensitizing activity of the fatty acid synthase inhibitor C75 is enhanced by blocking fatty acid uptake in prostate cancer cells. Adv Radiat Oncol. 2020;5:994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chuang HY, Lee YP, Lin WC, Lin YH, Hwang JJ. Fatty acid inhibition sensitizes androgen-dependent and -independent prostate cancer to radiotherapy via FASN/NF-κB pathway. Sci Rep. 2019;9:13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Centenera MM, Scott JS, Machiels J, Nassar ZD, Miller DC, Zinonos I, et al. ELOVL5 is a critical and targetable fatty acid elongase in prostate cancer. Cancer Res. 2021;81:1704–18. [DOI] [PubMed] [Google Scholar]
- 125.Xu H, Li S, Sun Y, Xu L, Hong X, Wang Z, et al. ELOVL5-mediated long chain fatty acid elongation contributes to enzalutamide resistance of prostate cancer. Cancers (Basel). 2021;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaini RR, Sillerud LO, Zhaorigetu S, Hu CAA. Autophagy regulates lipolysis and cell survival through lipid droplet degradation in androgen-sensitive prostate cancer cells. Prostate. 2012;72:1412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nomura DK, Lombardi DP, Chang JW, Niessen S, Ward AM, Long JZ, et al. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011;18:846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Awad D, Cao PHA, Pulliam TL, Spradlin M, Subramani E, Tellman TV, et al. Adipose triglyceride lipase is a therapeutic target in advanced prostate cancer that promotes metabolic plasticity. Cancer Res. 2024;84:703–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–4. [DOI] [PubMed] [Google Scholar]
- 130.Flaig TW, Salzmann-Sullivan M, Su LJ, Zhang Z, Joshi M, Gijón MA, et al. Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen blockade. Oncotarget. 2017;8:56051–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schlaepfer IR, Rider L, Rodrigues LU, Gijón MA, Pac CT, Romero L, et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol Cancer Ther. 2014;13:2361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Blomme A, Ford CA, Mui E, Patel R, Ntala C, Jamieson LE, et al. 2,4-dienoyl-CoA reductase regulates lipid homeostasis in treatment-resistant prostate cancer. Nat Commun. 2020;11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nassar ZD, Mah CY, Dehairs J, Burvenich IJG, Irani S, Centenera MM, et al. Human DECR1 is an androgen-repressed survival factor that regulates PUFA oxidation to protect prostate tumor cells from ferroptosis. Elife. 2020;9:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Sullivan SE, Kaczocha M. FABP5 as a novel molecular target in prostate cancer. Drug Discov Today. 2020;25:2056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hillowe A, Gordon C, Wang L, Rizzo RC, Trotman LC, Ojima I, et al. Fatty acid binding protein 5 regulates docetaxel sensitivity in taxane-resistant prostate cancer cells. PLoS One. 2023;18:e0292483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carbonetti G, Converso C, Clement T, Wang C, Trotman LC, Ojima I, et al. Docetaxel/cabazitaxel and fatty acid binding protein 5 inhibitors produce synergistic inhibition of prostate cancer growth. Prostate. 2020;80:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.M Swamynathan M, Mathew G, Aziz A, Gordon C, Hillowe A, Wang H, et al. FABP5 inhibition against PTEN-mutant therapy resistant prostate cancer. Cancers (Basel). 2023;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Watt MJ, Clark AK, Selth LA, Haynes VR, Lister N, Rebello R, et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci Transl Med. 2019;11:1–12. [DOI] [PubMed]
- 139.Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CSO, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. [DOI] [PubMed] [Google Scholar]
- 140.Pascual G, Domínguez D, Elosúa-Bayes M, Beckedorff F, Laudanna C, Bigas C, et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature. 2021;599:485–90. [DOI] [PubMed] [Google Scholar]
- 141.Stopsack KH, Gerke TA, Sinnott JA, Penney KL, Tyekucheva S, Sesso HD, et al. Cholesterol metabolism and prostate cancer lethality. Cancer Res. 2016;76:4785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tibbo AJ, Hartley A, Vasan R, Shaw R, Galbraith L, Mui E, et al. MBTPS2 acts as a regulator of lipogenesis and cholesterol synthesis through SREBP signalling in prostate cancer. Br J Cancer. 2023;128:1991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jiang S, Wang X, Song D, Liu XJ, Gu Y, Xu Z, et al. Cholesterol induces epithelial-to-mesenchymal transition of prostate cancer cells by suppressing degradation of EGFR through APMAP. Cancer Res. 2019;79:3063–75. [DOI] [PubMed] [Google Scholar]
- 144.Solanki AA, Liauw SL. Role of HMG-CoA reductase inhibitors with curative radiotherapy in men with prostate cancer. Open Access J Urol. 2011;3:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Locke JA, Nelson CC, Adomat HH, Hendy SC, Gleave ME, Guns EST. Steroidogenesis inhibitors alter but do not eliminate androgen synthesis mechanisms during progression to castration-resistance in LNCaP prostate xenografts. J Steroid Biochem Mol Biol. 2009;115:126–36. [DOI] [PubMed] [Google Scholar]
- 147.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. [DOI] [PubMed] [Google Scholar]
- 148.Raftopulos NL, Washaya TC, Niederprüm A, Egert A, Hakeem-Sanni MF, Varney B, et al. Prostate cancer cell proliferation is influenced by LDL-cholesterol availability and cholesteryl ester turnover. Cancer Metab. 2022;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Murtola TJ, Siltari A. Statins for prostate cancer: when and how much? Clin Cancer Res. 2021;27:4947–9. [DOI] [PubMed] [Google Scholar]
- 150.Pan T, Lin SC, Lee YC, Yu G, Song JH, Pan J, et al. Statins reduce castration-induced bone marrow adiposity and prostate cancer progression in bone. Oncogene. 2021;40:4592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoque A, Chen H, Xu XC. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol Biomark Prev. 2008;17:88–94. [DOI] [PubMed] [Google Scholar]
- 152.Caro-Maldonado A, Camacho L, Zabala-Letona A, Torrano V, Fernández-Ruiz S, Zamacola-Bascaran K, et al. Low-dose statin treatment increases prostate cancer aggressiveness. Oncotarget. 2017;9:1494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shangguan X, Ma Z, Yu M, Ding J, Xue W, Qi J. Squalene epoxidase metabolic dependency is a targetable vulnerability in castration-resistant prostate cancer. Cancer Res. 2022;82:3032–44. [DOI] [PubMed] [Google Scholar]
- 154.Kalogirou C, Linxweiler J, Schmucker P, Snaebjornsson MT, Schmitz W, Wach S, et al. MiR-205-driven downregulation of cholesterol biosynthesis through SQLE-inhibition identifies therapeutic vulnerability in aggressive prostate cancer. Nat Commun. 2021;12:5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.El-Kenawi A, Dominguez-Viqueira W, Liu M, Awasthi S, Abraham-Miranda J, Keske A, et al. Macrophage-derived cholesterol contributes to therapeutic resistance in prostate cancer. Cancer Res. 2021;81:5477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab. 2017;25:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Islam A, Shaukat Z, Hussain R, Gregory SL. One-carbon and polyamine metabolism as cancer therapy targets. Biomolecules. 2022;12:1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Newman AC, Maddocks ODK. One-carbon metabolism in cancer. Br J Cancer. 2017;116:1499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16:650–62. [DOI] [PubMed] [Google Scholar]
- 160.Corbin JM, Ruiz-Echevarría MJ. One-carbon metabolism in prostate cancer: the role of androgen signaling. Int J Mol Sci. 2016;17:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Green T, Chen X, Ryan S, Asch AS, Ruiz-Echevarría MJ. TMEFF2 and SARDH cooperate to modulate one-carbon metabolism and invasion of prostate cancer cells. Prostate. 2013;73:1561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Song YH, Shiota M, Kuroiwa K, Naito S, Oda Y. The important role of glycine N-methyltransferase in the carcinogenesis and progression of prostate cancer. Mod Pathol. 2011;24:1272–80. [DOI] [PubMed] [Google Scholar]
- 163.Khan AP, Rajendiran TM, Ateeq B, Asangani IA, Athanikar JN, Yocum AK, et al. The role of sarcosine metabolism in prostate cancer progression. Neoplasia. 2013;15:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jentzmik F, Stephan C, Lein M, Miller K, Kamlage B, Bethan B, et al. Sarcosine in prostate cancer tissue is not a differential metabolite for prostate cancer aggressiveness and biochemical progression. J Urol. 2011;185:706–11. [DOI] [PubMed] [Google Scholar]
- 165.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 166.Luka Z, Mudd SH, Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J Biol Chem. 2009;284:22507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ottaviani S, Brooke GN, O’Hanlon-Brown C, Waxman J, Ali S, Buluwela L. Characterisation of the androgen regulation of glycine N-methyltransferase in prostate cancer cells. J Mol Endocrinol. 2013;51:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zabala-Letona A, Arruabarrena-Aristorena A, Fernandez-Ruiz S, Viera C, Carlevaris O, Ercilla A, et al. PI3K-regulated Glycine N-methyltransferase is required for the development of prostate cancer. Oncogenesis. 2022;11:10. [DOI] [PMC free article] [PubMed]
- 171.Obata F, Miura M. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat Commun. 2015;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Reina-Campos M, Linares JF, Duran A, Cordes T, L’Hermitte A, Badur MG, et al. Increased serine and one-carbon pathway metabolism by PKCλ/ι deficiency promotes neuroendocrine prostate cancer. Cancer Cell. 2019;35:385–400.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang HF, Klein Geltink RI, Parker SJ, Sorensen PH. Transsulfuration, minor player or crucial for cysteine homeostasis in cancer. Trends Cell Biol. 2022;32:800–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Prudova A, Albin M, Bauman Z, Lin A, Vitvitsky V, Banerjee R. Testosterone regulation of homocysteine metabolism modulates redox status in human prostate cancer cells. Antioxid Redox Signal. 2007;9:1875–81. [DOI] [PubMed] [Google Scholar]
- 175.Guo H, Gai JW, Wang Y, Jin HF, Du JB, Jin J. Characterization of hydrogen sulfide and its synthases, cystathionine β-synthase and cystathionine γ-lyase, in human prostatic tissue and cells. Urology. 2012;79:483.e1–483.e5. [DOI] [PubMed] [Google Scholar]
- 176.Zhang W, Braun A, Bauman Z, Olteanu H, Madzelan P, Banerjee R. Expression profiling of homocysteine junction enzymes in the NCI60 panel of human cancer cell lines. Cancer Res. 2005;65:1554–60. [DOI] [PubMed] [Google Scholar]
- 177.Stabler S, Koyama T, Zhao Z, Martinez-Ferrer M, Allen RH, Luka Z et al. Serum methionine metabolites are risk factors for metastatic prostate cancer progression. PLoS One 2011;6:8. [DOI] [PMC free article] [PubMed]
- 178.Saha A, Zhao S, Kindall A, Wilder C, Friedman CA, Clark R et al. Cysteine depletion sensitizes prostate cancer cells to agents that enhance DNA damage and to immune checkpoint inhibition. J Exp Clin Cancer Res. 2023;42:119. [DOI] [PMC free article] [PubMed]
- 179.Pegg AE. Functions of polyamines in mammals. J Biol Chem. 2016;291:14904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nowotarski SL, Woster PM, Casero RA Jr. Polyamines and cancer: implications for chemoprevention and chemotherapy. Expert Rev Mol Med. 2014;15:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jänne OA, Crozat A, Palvimo J, Eisenberg LM. Androgen-regulation of ornithine decarboxylase and S-adenosylmethionine decarboxylase genes. J Steroid Biochem Mol Biol. 1991;40:307–15. [DOI] [PubMed] [Google Scholar]
- 182.Fjösne HE, Strand H, Sunde A. Dose-dependent induction of ornithine decarboxylase and S-adenosyl-methionine decarboxylase activity by testosterone in the accessory sex organs of male rats. Prostate. 1992;21:239–45. [DOI] [PubMed] [Google Scholar]
- 183.Cohen RJ, Fujiwara K, Holland JW, McNeal JE. Polyamines in prostatic epithelial cells and adenocarcinoma; the effects of androgen blockade. Prostate. 2001;49:278–84. [DOI] [PubMed] [Google Scholar]
- 184.Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci USA. 1993;90:7804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kaminski L, Torrino S, Dufies M, Djabari Z, Haider R, Roustan FR, et al. PGC1α inhibits polyamine synthesis to suppress prostate cancer aggressiveness. Cancer Res. 2019;79:3268–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Torrano V, Valcarcel-Jimenez L, Cortazar AR, Liu X, Urosevic J, Castillo-Martin M, et al. The metabolic co-regulator PGC1α suppresses prostate cancer metastasis. Nat Cell Biol. 2016;18:645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Valcarcel-Jimenez L, Macchia A, Crosas-Molist E, Schaub-Clerigue A, Camacho L, Martín-Martín N, et al. PGC1α suppresses prostate cancer cell invasion through ERRα transcriptional control. Cancer Res. 2019;79:6153–65. [DOI] [PubMed] [Google Scholar]
- 188.Zabala-Letona A, Arruabarrena-Aristorena A, Martín-Martín N, Fernandez-Ruiz S, Sutherland JD, Clasquin M, et al. MTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature. 2017;547:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Monelli E, Villacampa P, Zabala-Letona A, Martinez-Romero A, Llena J, Beiroa D, et al. Angiocrine polyamine production regulates adiposity. Nat Metab. 2022;4:327–43. [DOI] [PubMed] [Google Scholar]
- 190.de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374–403. [DOI] [PubMed] [Google Scholar]
- 191.Qin Y, Lu F, Lyu K, Chang AE, Li Q. Emerging concepts regarding pro- and anti tumor properties of B cells in tumor immunity. Front Immunol. 2022;13:881427. [DOI] [PMC free article] [PubMed]
- 192.Chen S, Zhu G, Yang Y, Wang F, Xiao YT, Zhang N, et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat Cell Biol. 2021;23:87–98. [DOI] [PubMed] [Google Scholar]
- 193.Wong HY, Sheng Q, Hesterberg AB, Croessmann S, Rios BL, Giri K, et al. Single cell analysis of cribriform prostate cancer reveals cell intrinsic and tumor microenvironmental pathways of aggressive disease. Nat Commun. 2022;13:6036. [DOI] [PMC free article] [PubMed]
- 194.Lopez-Bujanda ZA, Haffner MC, Chaimowitz MG, Chowdhury N, Venturini NJ, Patel RA, et al. Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression. Nat Cancer. 2021;2:803–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chang C-H, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Beckermann KE, Dudzinski SO, Rathmell JC. Dysfunctional T cell metabolism in the tumor microenvironment. Cytokine Growth Factor Rev. 2017;35:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73:1524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Niu D, Wu Y, Lei Z, Zhang M, Xie Z, Tang S. Lactic acid, a driver of tumor-stroma interactions. Int Immunopharmacol. 2022;106:108597. [DOI] [PubMed] [Google Scholar]
- 200.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–9. [DOI] [PubMed] [Google Scholar]
- 201.Inamdar S, Suresh AP, Mangal JL, Ng ND, Sundem A, Wu C, et al. Rescue of dendritic cells from glycolysis inhibition improves cancer immunotherapy in mice. Nat Commun. 2023;14:5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chang C-H, Curtis JD, Maggi LBJ, Faubert B, Villarino AV, O’Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Notarangelo G, Spinelli JB, Perez EM, Baker GJ, Kurmi K, Elia I, et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8( + ) T cell function. Science. 2022;377:1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lim SA, Wei J, Nguyen TLM, Shi H, Su W, Palacios G, et al. Lipid signalling enforces functional specialization of Treg cells in tumours. Nature. 2021;591:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Field CS, Baixauli F, Kyle RL, Puleston DJ, Cameron AM, Sanin DE, et al. Mitochondrial integrity regulated by lipid metabolism is a cell-intrinsic checkpoint for treg suppressive function. Cell Metab. 2020;31:422–37.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yang P, Qin H, Li Y, Xiao A, Zheng E, Zeng H, et al. CD36-mediated metabolic crosstalk between tumor cells and macrophages affects liver metastasis. Nat Commun. 2022;13:5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nava Lauson CB, Tiberti S, Corsetto PA, Conte F, Tyagi P, Machwirth M, et al. Linoleic acid potentiates CD8 + T cell metabolic fitness and antitumor immunity. Cell Metab. 2023;35:633–50.e9. [DOI] [PubMed] [Google Scholar]
- 209.Rowe JH, Elia I, Shahid O, Gaudiano EF, Sifnugel NE, Johnson S, et al. Formate supplementation enhances antitumor CD8 + T-cell fitness and efficacy of PD-1 blockade. Cancer Discov. 2023;13:2566–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cheng H, Qiu Y, Xu Y, Chen L, Ma K, Tao M, et al. Extracellular acidosis restricts one-carbon metabolism and preserves T cell stemness. Nat Metab. 2023;5:314–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kurniawan H, Franchina DG, Guerra L, Bonetti L, Baguet LS, Grusdat M, et al. Glutathione restricts serine metabolism to preserve regulatory T cell function. Cell Metab. 2020;31:920–36.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Valera PS, Plou J, García I, Astobiza I, Viera C, Aransay AM, et al. SERS analysis of cancer cell-secreted purines reveals a unique paracrine crosstalk in MTAP-deficient tumors. Proc Natl Acad Sci USA. 2023;120:52. [DOI] [PMC free article] [PubMed]
- 213.Terry AR, Nogueira V, Rho H, Ramakrishnan G, Li J, Kang S, et al. CD36 maintains lipid homeostasis via selective uptake of monounsaturated fatty acids during matrix detachment and tumor progression. Cell Metab. 2023;35:2060–2076.e9. [DOI] [PubMed] [Google Scholar]
- 214.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lien EC, Westermark AM, Zhang Y, Yuan C, Li Z, Lau AN, et al. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature. 2021;599:302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Vidal AC, Freedland SJ. Obesity and prostate cancer: a focused update on active surveillance, race, and molecular subtyping. Eur Urol. 2017;72:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cantarutti A, Bonn SE, Adami HO, Grönberg H, Bellocco R, Bälter K. Body mass index and mortality in men with prostate cancer. Prostate. 2015;75:1129–36. [DOI] [PubMed] [Google Scholar]
- 218.Zhong S, Yan X, Wu Y, Zhang X, Chen L, Tang J, et al. Body mass index and mortality in prostate cancer patients: a dose-response meta-analysis. Prostate Cancer Prostatic Dis. 2016;19:122–31. [DOI] [PubMed] [Google Scholar]
- 219.Liu J, Ramakrishnan SK, Khuder SS, Kaw MK, Muturi HT, Lester SG, et al. High-calorie diet exacerbates prostate neoplasia in mice with haploinsufficiency of Pten tumor suppressor gene. Mol Metab. 2015;4:186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang H, Yan W, Sun Y, Yang CS. High-fat diet-induced hyperinsulinemia promotes the development of prostate adenocarcinoma in prostate-specific Pten−/− mice. Carcinogenesis. 2022;43:504–16. [DOI] [PubMed] [Google Scholar]
- 221.Labbé DP, Uetani N, Vinette V, Lessard L, Aubry I, Migon E, et al. PTP1B deficiency enables the ability of a high-fat diet to drive the invasive character of PTEN-deficient prostate cancers. Cancer Res. 2016;76:3130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hayashi T, Fujita K, Nojima S, Hayashi Y, Nakano K, Ishizuya Y, et al. High-fat diet-induced inflammation accelerates prostate cancer growth via IL6 signaling. Clin Cancer Res. 2018;24:4309–18. [DOI] [PubMed] [Google Scholar]
- 223.Chen M, Zhang J, Sampieri K, Clohessy JG, Mendez L, Gonzalez-Billalabeitia E, et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat Genet. 2018;50:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Carreño DV, Corro NBNB, Cerda-Infante JF, Echeverría CE, Asencio-Barría CA, Torres-Estay VAVA, et al. Dietary fructose promotes prostate cancer growth. Cancer Res. 2021;81:2824–32. [DOI] [PubMed] [Google Scholar]