Abstract
COVID-19 survivors concerning about the rehabilitation and sustained sequelae of Coronavirus Disease 2019 (COVID-19) infection. We aimed to investigate the sequelae of patients’ psychological and physical condition and its related factors in the early and late stages. This longitudinal study tracked 281 COVID-19 patients discharged from hospitals in Guangdong, China, for one year. Assessments occurred at 2,4,12,24 and 48 weeks post-discharge. We define 2 weeks, 4 weeks, and 12 weeks as early stage, and 24 weeks and 48 weeks as late stage. Psychological health was measured using the Patient Health Questionnaire-9 (PHQ-9), Generalized Anxiety Disorder-7 (GAD-7), Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5), and Pittsburgh Sleep Quality Index (PSQI) scales. Physical health was assessed through laboratory tests, chest computed tomography (CT) scans, and pulmonary function tests. Data were analyzed using multivariate regression models to evaluate the influence of demographic and clinical variables on health outcomes. COVID-19 survivors exhibited psychological and physical sequelae in both the early and late stages. Compared to the early stage, the proportions of patients with depression (early stage 14.6%, late stage 4.6%), anxiety (early stage 8.9%, late stage 5.3%), PTSD(early stage 3.6%, late stage 0.7%), abnormal liver function (early stage 24.6%, late stage 11.0%), abnormal cardiac function (early stage 10.0%, late stage 7.8%), abnormal renal function (early stage 20.6%, late stage 11.0%) and abnormal pulmonary function (early stage 40.9%, late stage 13.5%) were significantly reduced in the late stage. Factors such as gender, age, severity of COVID-19, hospitalization duration, and various comorbidities were significantly associated with these sequelae. We noticed that psychological and physical sequelae occurred to COVID-19 survivors in short and long stages, and these would gradually decrease as time went on. Male gender, age > 50 years old, severe clinical condition, longer hospitalization time and comorbidity history were related factors that significantly affected the rehabilitation of COVID-19 patients.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-76013-9.
Keywords: COVID-19 sequelae, Prognosis, Psychological condition, Physical condition, Risk factors
Subject terms: Diseases, Health care, Medical research
Introduction
Coronavirus disease, commonly referred to as COVID-19, is an infectious disease caused by the SARS-CoV-2 virus. It primarily spreads through respiratory droplets from coughs, sneezes, or talk. COVID-19 symptoms can range from mild to severe and include fever, cough, and difficulty breathing. Severe cases can lead to pneumonia, multi-organ failure, and even death, particularly in older adults and those with underlying health conditions1. The first case of COVID-19 pneumonia was reported on December 16th, 2019, in Wuhan, China, followed by the first wave of outbreaks across China until May 7th, 2020, when the Chinese CDC issued a document changing China’s prevention and control efforts from emergency status to normalization, and there are also studies suggested the first wave of the novel coronavirus outbreak in China was from December 2019 to April 20202. The first wave of COVID-19 was highly contagious and pathogenic with high mortality rate, and there was little experience with the treatment of this newly discovered virus at that time. COVID-19 patients in the first wave of outbreaks were withstand huge tests both in physical and psychological station, and their prognosis could be especially representative. Yet there are few studies on the short-term and long-term prognosis of patients discharged from this first wave of COVID-19. As time develops, SARS-CoV-2 continues to generate new mutant strains3 and caused several waves of outbreak, and severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) infections emerge to date persistently with great impact on society. People have been inevitably exposed to SARS-CoV-2, according to World Health Organization (WHO) statistics, the number of people infected with SARS-CoV-2 worldwide has exceeded 700 million, with more than 6 million deaths. The U.S. has experienced over 100 million confirmed cases and 1.1 million deaths, making it one of the most affected countries4. Brazil reported over 36 million cases and 700,000 deaths, with a notably high mortality rate, particularly among vulnerable populations5.
Many COVID-19 survivors complained about the remaining physical and mental discomfort though they had been cured and discharged. There is a concept of “long COVID-19” referring that some COVID-19 patients still have long-term sequelae after testing for nucleic acid has turned negative6. A large number of COVID-19 survivors complained of mental health problems, such as depression, anxiety, PTSD, sleep disorders7,8. The mental health impact of COVID-19 varied by age group: children and adolescents experienced increased anxiety and depression due to disrupted routines; young adults faced heightened stress from uncertainties about education, employment, and social life; middle-aged adults often struggled with burnout from balancing work and caregiving responsibilities; and older adults were particularly vulnerable to loneliness and anxiety, although some showed resilience9–12. COVID-19 has significantly exacerbated mental health problems through several interconnected factors. Social isolation and lockdown measures led to increased loneliness and a sense of abandonment, impacting emotional well-being13. Additionally, increased screen time and digital overload, along with difficulties in accessing mental health services and existing stigma, have complicated the situation14. Studies have also suggested physical sequelae in COVID-19 survivors, such as abnormalities in the respiratory, digestive, cardiovascular and neurological systems8. A study conducted at Jinyintan Hospital in Wuhan showed that as COVID-19 patients were discharged 1 year later, the proportion of patients with at least one sequelae symptom decreased when compared to 6 months after discharge, but the proportion of depression and anxiety did not decrease15. Another study showed that COVID-19 survivors couldn’t return to the previous health status even 2 years after acute infection16. The above studies suggest that mental disorders and physical problems are commonly reported from COVID-19 discharged patients, and although the incidence rate of COVID-19 sequelae varies under different times of discharge and may decrease with time, these health problems are arresting and could be long-term. In terms of correlation between physical and psychological conditions, it has been shown that long-term COVID-19 symptoms were associated with abnormal mental health16.
This study differs from previous research by providing a more objective and comprehensive evaluation of COVID-19 sequelae. While earlier studies largely relied on patients’ subjective reports for assessing cardiovascular and digestive system function, with only the respiratory system being objectively measured through pulmonary function tests and chest CT, this study aims to fill the gap by offering a more complete and long-term analysis based on objective data across multiple systems. Nevertheless, there are studies also shown an increased risk of certain clinical sequelae (e.g., mental health diagnoses) regardless of age and preexisting conditions17. Up to now, there are only a few studies on risk factors for COVID-19 sequelae, some studies have shown that women are prone to occur long-term COVID-1916, such as depression, anxiety, post-traumatic stress disorder, or sleep disorder sequelae16,17. Women may be more prone to long-term COVID-19 due to stronger immune responses, hormonal influences, and genetic factors that affect disease progression18. To this end, we asked the following questions: Is there any difference in the distribution of sequelae under different times in discharged COVID-19 patients? Is there a relationship between psychological and physical conditions? What kind of patients are more likely to develop long-term mental health or physical health abnormalities?
Methods
Study population
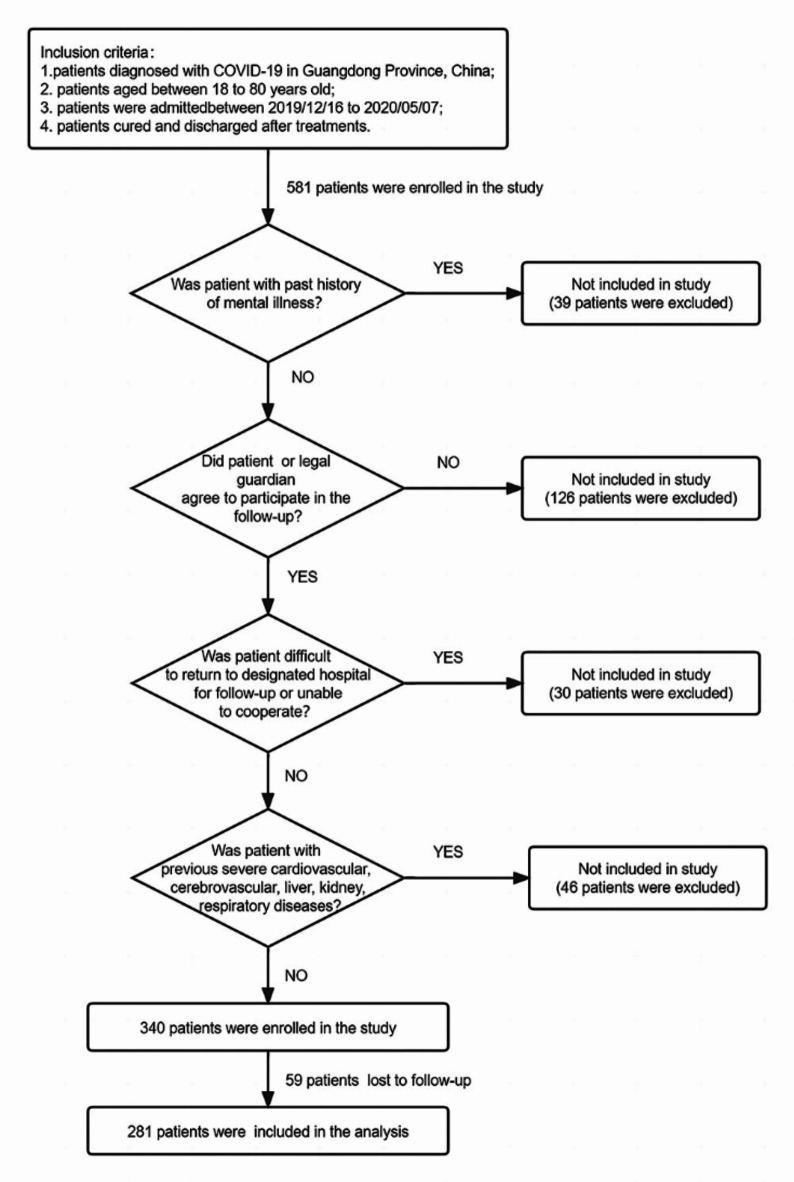
A total of 581 patients diagnosed and admitted to designated hospitals for COVID-19 infection from 2019/12/16 to 2020/05/07 in Guangzhou and Shenzhen city, Guangdong Province, China, met the inclusion criteria were recruited to the study. Among them, 39 patients had a previous history of mental illness; 126 patients and/or their families did not agree to participate in the subsequent follow-up; 30 patients had difficulty returning to the designated hospital for follow-up and/or were unable to cooperate; 46 patients had a previous history of severe cardiovascular, cerebrovascular, liver, kidney, respiratory diseases, during the 1-year follow-up period, 59 patients were completely lost to follow-up, and these patients were excluded from this study. Eventually, 281 patients became the research subjects of this study. (Fig. 1)
Fig. 1.
Selection of discharged COVID-19 patients for inclusion in the study.
Inclusion criteria: (1) patients diagnosed with COVID-19 in Guangzhou and Shenzhen city, Guangdong Province, China; (2) patients aged between 18 and 80 years old; (3) patients were admitted to hospital for COVID-19 infection between 2019/12/16 to 2020/05/07; (4) patients cured and discharged after treatments. Exclusion criteria were as follows: (1) patients with past history of mental illness; (2) patients or legal guardian who did not agree to participate in follow-up; 3.patients who were difficult to return to the designated hospital for follow-up or unable to cooperate; 4. patients with previous severe cardiovascular, cerebrovascular, liver, kidney, respiratory diseases (according to clinical guideline, severe cardiovascular disease was defined as NYHA class II; severe cerebrovascular disease was defined as hemiplegia and verbal cognitive dysfunction after stroke; severe liver disease was defined as Alanine Aminotransferase (ALT)>80U/L or Aspartate Aminotransferase (AST)>80U/L or Total Bilirubin (T-bil)>34.2µmol/L; severe kidney disease was defined as stage 3 chronic kidney disease according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines; severe respiratory disease was defined as the presence of respiratory failure).
Study design
In this study, COVID-19 pneumonia patients discharged from Guangzhou and Shenzhen city, Guangdong Province, China, were systematically followed for 12 months to assess both their psychological and physical health. The diagnosis of COVID-19 for all patients included in the study was confirmed under standard clinical procedures, and each patient received standardized treatments as per the relevant guidelines19,20.Referring to our previous research and other study, COVID-19 cases can be categorized into mild, moderate, severe, and critical based on the severity of symptoms and clinical findings19,20. Mild cases involve symptoms such as fever, cough, sore throat, fatigue, headache, muscle pain, nausea, vomiting, diarrhea, and loss of taste or smell, without evidence of pneumonia or hypoxia. Moderate cases are characterized by clinical or imaging evidence of lower respiratory tract involvement, with oxygen saturation (SpO2) ≥ 94% on room air. Severe cases are identified by severe pneumonia symptoms, including a respiratory rate >30 breaths per minute, severe respiratory distress, SpO2 <94% on room air, or lung infiltrates covering more than 50% of the lung fields on imaging. Critical cases involve life-threatening conditions such as respiratory failure requiring mechanical ventilation, septic shock, or multiple organ dysfunction or failure.
The study involved follow-up visits at five specific time points post-discharge: 2 weeks, 4 weeks, 12 weeks, 24 weeks, and 48 weeks. During these visits, patients underwent comprehensive assessments that included both psychological and physical evaluations.
For the psychological assessments, patients were asked to self-administer several standardized questionnaires designed to measure various aspects of their mental health. These questionnaires are widely recognized and validated tools in clinical research and practice21–34. These questionnaires included the PHQ-9 (Patient Health Questionnaire-9), which evaluates the severity of depressive symptoms through a 9-item scale with scores ranging from 0 to 2735; the GAD-7 (Generalized Anxiety Disorder-7), a 7-item scale used to assess the severity of anxiety symptoms, with scores ranging from 0 to 2136; the PCL-5 (Posttraumatic Stress Disorder Checklist for DSM-5), a 20-item checklist used to determine the presence and severity of PTSD symptoms, with scores ranging from 0 to 8026; and the PSQI (Pittsburgh Sleep Quality Index), which assesses sleep quality over the past month through 19 self-rated questions that produce a global score ranging from 0 to 2137. Higher scores on these scales indicate greater severity of the respective psychological conditions38–42. After patients completed these questionnaires, the scores were collected, verified, and entered into a database by specialized medical staff to ensure accuracy and consistency in the data collection process.
In parallel with the psychological assessments, the physical health of the patients was closely monitored through a series of laboratory tests and imaging studies. Blood samples were taken to evaluate various organ functions: heart function was assessed through markers such as Creatine Kinase (CK) and Creatine Kinase Isoenzyme-MB (CK-MB)); liver function was evaluated using ALT, AST, and T-bil; and kidney function was measured through serum creatinine levels and Blood Urea Nitrogen (BUN). In addition to these laboratory tests, patients underwent chest CT scans to monitor lung recovery and to identify any lasting pulmonary damage resulting from COVID-19. Pulmonary function tests were also conducted to assess lung capacity and efficiency, including measurements of Forced Vital Capacity (FVC) and Forced Expiratory Volume in 1 s (FEV1).
To facilitate the analysis, the five follow-up time points were categorized into two stages: the early stage, which included the 2-week, 4-week, and 12-week visits, and the late stage, which included the 24-week and 48-week visits. If a patient exhibited an abnormal result at any time point within a stage, this abnormality was recorded as representative of that entire stage. The comprehensive data collected from these assessments, along with patients’ demographic and clinical information (such as gender, age, comorbidities, severity of COVID-19, and duration of hospitalization), were systematically recorded and analyzed to provide insights into the long-term effects of COVID-19 and the recovery process. (The scores and related meanings of each psychological scale are shown in Table 1, and the indexes and normal ranges/recorded results of each organ function are shown in Table 2)
Table 1.
The scores of each psychological scale and the related meaning.
| Psychological Scale | Scores | Meaning |
|---|---|---|
| PHQ-9/GAD-7 | 0–4 | No Depression/Anxiety |
| 5–9 | Mild Depression/Anxiety | |
| 10–14 | Moderate Depression/Anxiety | |
| ≥ 15 | Severe Depression/Anxiety | |
| PCL-5 | <33 | No PTSD |
| ≥ 33 | PTSD | |
| PSQI | 0–5 | Good Sleep Quality |
| >5 | Poor Sleep Quality |
Table 2.
The functional indexes and normal ranges/recorded results of each organ.
| Organ | Index | Normal range/recorded results | Unit | |
|---|---|---|---|---|
| Liver | Alanine Aminotransferase (ALT) | 0–40 | U/L | |
| Aspartate Aminotransferase (AST) | 0–40 | U/L | ||
| Total Bilirubin (T-bil) | 3.4–17.1 | µmol/L | ||
| Heart | Creatine Phosphokinase (CK) | Male | 15–174 | U/L |
| Female | 3-140 | U/L | ||
| Serum Creatine Kinase Isoenzyme (CK-MB) | 0–25 | U/L | ||
| Kidney | Urea Nitrogen (BUN) | 3.2–7.1 | mmol/L | |
| Blood Creatinine (Scr) | Male | 53–106 | µmol/L | |
| Female | 44–97 | µmol/L | ||
| Lung | Pulmonary Function Test | Abnormal/Normal | ||
| Chest CT Results | Lesion Increase | Abnormal | ||
| Lesion No Change | Normal | |||
| Lesion More Previous Absorption | Normal |
As for the lung, whether the pulmonary function test was normal or not was recorded based on the findings reported by each designated hospital.
Data source
All data were obtained from the telemedicine platform for COVID-19 in Guangdong Province with the authorization of the COVID-19 prevention and control group of the Guangdong Provincial Health Commission.
Ethics approval and consent to participate
We did not contact any individual subjects. This follow-up study is an investigator initiated multi-center clinical observation study, which is authorized by COVID-19 prevention and control group of the Guangdong Provincial Health Commission, so as to provide scientific basis for further pandemic prevention and control in Guangdong Province. No intervention with medication or medical devices was involved, and patients’ personal identifiable information was concealed in this study. Written informed consent was waived due to the use of deidentified data for the purpose of public health surveillance by the Ethics Review Committee of Guangdong Provincial People’s Hospital. This study was approved by the Ethics Review Committee of Guangdong Provincial People’s Hospital (NO.KY-Q-2021-241-01). And we confirmed that all experiments were performed in accordance with relevant guidelines and regulations.
Statistical analysis
SPSS 27.0 software was used for statistical analysis. We used chi-square test or Fisher’s exact test and binary logistic regression to analyze the related factors for different outcomes under the two stages, and the main outcomes included the risk of having depression, anxiety, PTSD, sleep disorder, and abnormal heart, liver, kidney, and lung organ function. We need to exclude the effect of comorbidity on organ function, so the effect of that comorbidity on the prognosis of that organ is not discussed if the organ itself has a comorbidity. And patients with comorbidity of each organ were excluded before the analysis was performed. We analyzed the relationship between psychological and physical states using Chi-square or Fisher’s exact tests, based on sample size. Additionally, the Phi coefficient was calculated to quantify the association’s strength between the variables.
Results
General characteristics of COVID-19 discharged patients
Among the 281 COVID-19 discharged patients, there was little difference between the proportion of males and females (52.3% vs.47.7%), 69.4% of the recruited patients are concentrated under the age of 50 years old, 65.5% of the patients with a hospitalization duration of more than 14 days and 73% with no previous underlying medical history. Only a small proportion of the patients had underlying diseases previously like diabetes (6.8%), hypertension (12.1%), coronary heart disease (2.1%), chronic liver disease (6.0%), chronic kidney disease (2.8%) and chronic obstructive pulmonary disease (COPD) (3.6%). None of the 281 discharged patients reported a documented history of psychiatric systemic disorders. Most patients were moderate cases (76.5%), followed by the mild cases (12.1%), severe cases (9.3%) and a few critical cases (1.4%) (Table 3).
Table 3.
General characteristics of discharged COVID-19 patients(N = 281).
| Characteristics | N (%) |
|---|---|
| Gender | |
| Female | 134 (47.7) |
| Male | 147 (52.3) |
| Age(yr) | |
| ≤ 50 | 195 (69.4) |
| >50 | 86 (30.6) |
| Hospitalization time(day) | |
| ≤ 14 | 95 (33.8) |
| >14 | 184 (65.5) |
| Comorbidity | |
| Any | 76 (27) |
| Diabetes | 19 (6.8) |
| Hypertension | 34 (12.1) |
| Coronary heart disease | 6 (2.1) |
| Stroke | 3 (1.1) |
| Chronic renal disease | 8 (2.8) |
| COPD | 10 (3.6) |
| Chronic liver disease | 17 (6) |
| Tumor | 4 (1.4) |
| Severity of COVID-19 | |
| Mild | 34 (12.1) |
| Moderate | 215 (76.5) |
| Severe | 26 (9.3) |
| Critical | 4 (1.4) |
Psychological and physical Follow-Up results of COVID-19 discharged patients under two stages
The following results show the percentage of abnormal scores on psychological scales and abnormal laboratory testing results of heart, liver, kidney, and lung function in the early and late stages. Compared to the early stage, the proportions of patients with depression (early stage 14.6%, late stage 4.6%), anxiety (early stage 8.9%, late stage 5.3%), PTSD(early stage 3.6%, late stage 0.7%), abnormal liver function (early stage 24.6%, late stage 11.0%), abnormal cardiac function (early stage 10.0%, late stage 7.8%), abnormal renal function (early stage 20.6%, late stage 11.0%) and abnormal pulmonary function (early stage 40.9%, late stage 13.5%) were significantly reduced in the late stage. The proportion of sleep disorders did not change in both stages (Figs. 2 and 3; see in Additional file 1 and Additional file 2).
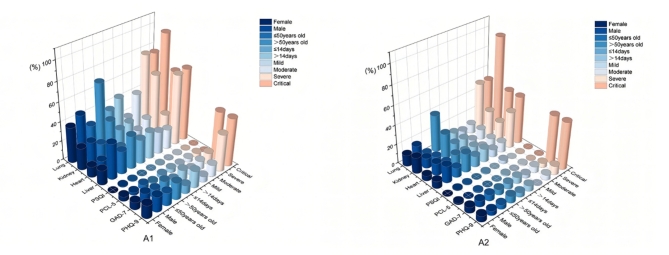
Fig. 2.
Characteristics of abnormal psychological and physical results in different groups during the two stages. As shown in A1, in early stage the proportion of abnormal liver and lung in females was lower than that in males; the proportion of abnormal liver, heart, kidney and lung of people aged > 50 years was higher than that of people aged ≤ 50 years; the proportion of abnormal PHQ-9 and lung in patients with hospitalization time > 14 days was higher than that in patients with hospitalization time ≤ 14 days; the proportion of abnormal PHQ-9 in mild patients was lower than that in moderate, severe and critical patients; the proportion of abnormal GAD-7 in critical patients was higher than the other three; the proportion of abnormal heart in critical patients was higher than the other three; the proportion of abnormal kidney in critical patients was higher than the other three; the proportion of abnormal liver and lung in severe and critical patients was significantly higher than the other two. As shown in A2, in late stage the proportion of abnormal liver and heart in females was lower than that in males, and the proportion of abnormal kidney in females was higher than that in males; the proportion of abnormal liver, kidney and lung of people aged > 50 years was higher than that of people aged ≤ 50 years; the proportion of lung abnormalities in patients with hospitalization time > 14 days was higher than the other; the proportion of patients with abnormal PHQ-9, GAD-7, liver, heart and kidney was higher than that of patients with the other three; the proportion of severe and critical patients with abnormal lung was higher than the other two.
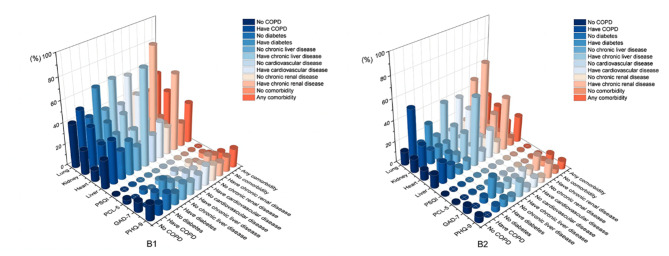
Fig. 3.
Characteristics of abnormal psychological and physical results in patients in two stages with different diseases. As shown in B1, in early stage the proportion of abnormal liver, kidney and lung in patients with comorbidity was higher than patients without comorbidity, while the proportion of PTSD in patients without comorbidity was higher than patients with comorbidity; the proportion of abnormal GAD-7, liver, kidney and lung in patients with diabetes was higher than patients without diabetes; the proportion of abnormal heart, kidney, and lung in patients with chronic liver disease was higher than patients without chronic liver disease; the proportion of abnormal liver, kidney and lung in patients with cardiovascular disease was higher than patients without cardiovascular disease; the proportion of abnormal liver, heart and lung in patients with chronic renal disease was higher than patients without chronic renal disease; the proportion of abnormal liver, heart and kidney in patients with COPD was higher than patients without COPD, and the proportion of abnormal GAD-7 in patients with COPD was lower than patients without COPD. As shown in B2, in late stage the proportion of abnormal liver, heart, kidney and lung in patients with comorbidity was higher than patients without comorbidity; the proportion of abnormal GAD-7 and lung in patients with diabetes was higher than patients without diabetes; the proportion of abnormal PHQ-9, heart, kidney and lung in patients with chronic liver disease was higher than the other; the proportion of abnormal liver, kidney and lung in patients with cardiovascular disease was higher than patients without cardiovascular disease; the proportion of abnormal PHQ-9, GAD-7, liver, heart and lung in patients with chronic renal disease was higher than the other; the proportion of abnormal liver and kidney in patients with COPD was higher than the other, and the proportion of abnormal PHQ-9, GAD-7 and heart in patients with COPD was lower than the other.
Comparison of Follow-Up results of COVID-19 discharged patients in different populations in the early stage
For psychological aspects, patients with hospitalization > 14 days (17.4% vs. 8.4%, p = 0.043) and severe clinical condition (50% vs. 38.5% vs. 12.1% vs. 5.9%, p = 0.043) were prone to present depression. Critical cases were prone to present depression and anxiety. Patients with no comorbidity (4.9%, p = 0.067) were prone to get PTSD.
For liver function, patients in male gender (37%, p = 0.043), severe clinical condition (75% vs. 73.1% vs. 20.5% vs. 23.1%, p<0.001), comorbidities such as chronic kidney disease (71.4%, p = 0.017)were prone to show abnormality.
For cardiovascular function, patients in critical cases (66.7%, p = 0.002) and with chronic liver disease (28.6%, p<0.001) and COPD (30%, p = 0.090) were prone to show abnormality.
For renal function, patients with age > 50 years old (36%, p<0.001), critical cases (100%, p<0.001), comorbidities (38.9%, p<0.001) such as cardiovascular disease (48.7%, p<0.001) were prone to show abnormality.
For lung function, patients with age > 50 years old (68.6%, p<0.001), severe cases (76.9%, p<0.001), critical cases (75%, p<0.001), comorbidities (51.3%, p<0.001) such as diabetes (63.2%, p<0.001), chronic liver disease (64.7%, p<0.001) and cardiovascular disease (61.5%, p<0.001) were prone to show abnormality. (Figures 2 and 3; see in Additional file 1 and Additional file 3)
Comparison of Follow-Up outcomes of COVID-19 discharged patients in different populations in late stage
For psychological aspects, critical cases (50%, p = 0.016) were prone to present depression. Patients with diabetes (20%, p = 0.054) were prone to present anxiety.
For liver function, patients in male gender (17.7%, p = 0.019), age > 50 years old (19.5%, p = 0.041), critical cases (50%, p<0.001), comorbidities like chronic kidney disease (50%, p = 0.011) were prone to show abnormality.
For cardiovascular function, patients in male gender (12.6%. p = 0.044), critical cases (50%, p = 0.001) and with chronic liver disease (25%, p = 0.044) were prone to show abnormality.
For renal function, patients in female gender (16.7%, p = 0.160), age > 50 years old (25.9%, p<0.001), critical cases (100%, p<0.001), comorbidities (23.5%, p = 0.003) such as cardiovascular disease (34.3%, p<0.001) were prone to show abnormality.
For lung function, patients with age > 50 years old (38.8%, p<0.001), severe cases (50% p<0.001), critical cases (50% p<0.001), comorbidities (28.4%, p<0.001) such as diabetes (27.8%, p = 0.149), chronic liver disease (41.2%, p = 0.004), cardiovascular disease (39.5%, p<0.001) and chronic kidney disease (50%. P = 0.016) were prone to show abnormality. (Figures 2 and 3, see in Additional file 2 and Additional file 3)
The correlation between psychological and physical conditions
In the early stage, there were correlations between liver function abnormality and depression (P = 0.0007, φ = 0.167), results also observed correlations between pulmonary function abnormality and depression (P = 0.036, φ = 0.125), pulmonary function abnormality and anxiety (P = 0.046, φ = 0.120), and pulmonary function abnormality and PTSD (P = 0.018, φ = 0.152), all φ values less than 0.2, which showed their correlations were weak. And in the late stage, there was no correlation between psychological and physical conditions. (Table 4).
Table 4.
The relationship between physical and psychological conditions in two stages (N = 281).
| Time | PHQ-9 | GAD-7 | PCL-5 | PSQI | |
|---|---|---|---|---|---|
| P-Value | (Phi coefficient) | ||||
| Early Stage | Liver | 0.007 (0.167) * | 0.459 (0.046) | 0.706 (0.027) | 1 (-0.038) |
| Heart | 0.391 (-0.075) | 0.487 (-0.070) | 0.603 (-0.069) | 1 (-0.023) | |
| Kidney | 0.547 (0.038) | 0.549 (0.038) | 0.123 (-0.112) | 0.232 (0.115) | |
| Lung | 0.036 (0.125) * | 0.046 (0.120) * | 0.018 (0.152) * | 1 (-0.050) | |
| Late Stage | Liver | 0.695 (0.012) | 0.698 (-0.051) | 1 (-0.039) | 1 (-0.028) |
| Heart | 1 (-0.020) | 1 (-0.026) | 1 (-0.033) | 1 (-0.023) | |
| Kidney | 0.222 (0.080) | 1 (0.004) | 1 (-0.038) | 1 (-0.027) | |
| Lung | 0.402 (0.063) | 0.238 (0.095) | 1 (-0.037) | 1 (-0.026) | |
Comparing psychological and physical conditions in pairs, using the chi-square test, or Fisher’s exact test, as appropriate. * A two-sided α of < 0.1 was considered statistically significant. The Phi(φ) coefficient reflects the strength of association between the two.
Factors associated with the prognosis of discharged COVID-19 patients
The results suggest that gender, age, duration of hospitalization, severity of COVID-19, and comorbidities (e.g., chronic liver disease, cardiovascular disease, chronic kidney disease and COPD were associated with psychological and physical conditions (see in Additional file 4), and for further validation of the results, we included the above related factors in the logistic regression model.
As shown in Additional file 4, in early stage, the results of the binary logistic regression model analysis demonstrate as below. When compared to mild and moderate cases, severe (OR = 14.101, 95% CI 2.311–86.026, P = 0.004) and critical case (OR = 67.539, 95% CI 2.561-1781.459, P = 0.012) were related factors for depression. Severe case was also a related factor for liver (OR = 21.256, 95% CI 4.111-109.903, P<0.001), heart (OR = 8.331, 95% CI 1.168–59.420, P = 0.034), renal (OR = 9.332, 95% CI 1.947–44.715, P = 0.005) and pulmonary (OR = 9.622, 95% CI 2.391–38.726, P = 0.001) function abnormality.
Medical history of chronic liver disease (OR = 11.666, 95% CI 1.350-100.776, P = 0.026) and COPD (OR = 17.827, 95% CI 1.561-203.555, P = 0.02) were related factors for heart function abnormality, age > 50 years old (OR = 4.446, 95% CI 2.376–8.317, P = < 0.001) was related factor for pulmonary function abnormality.
In late stage, female gender (OR = 3.489, 95% CI 1.257–9.681, P = 0.016) and age > 50 years (OR = 3.508, 95% CI 1.299–9.469, P = 0.013) were related factors for renal function abnormality, age > 50 years (OR = 20.357, 95% CI 6.521–63.556, P < 0.001) and medical history of cardiovascular disease (OR = 12.878, 95% CI 1.384–119.800, P = 0.025) were related factors for pulmonary abnormality. Male gender is more likely to develop liver function both in early and late stage.
Discussion
The COVID-19 epidemic has been ongoing since the WHO declared the pandemic on March 11, 2020, and a highly transmissible Delta variant was identified in late May 2021 in Guangdong Province, China45. Studies have shown that inactivated vaccines effectively reduce Delta transmission, with no deaths reported in Guangdong until June 18, 2021, emphasizing the importance of widespread vaccination46. Vaccines significantly reduce the spread of the virus and associated risks of infection and hospitalization47. Although the new variants like Omicron continue to emerge, they pose less threat, being more transmissible but resulting in fewer hospitalizations and deaths compared to previous waves48. In contrast, the initial outbreak was devastating, causing severe physical and psychological harm due to a lack of vaccines and clinical guidelines, with a high mortality rate among severe cases in China’s first wave49. Therefore, the first wave of pandemic can particularly represent the novel coronavirus pneumonia, research on patients suffered from the first wave of pandemic could especially help us understand the psychological and physiological prognosis of COVID-19. This study focused on the short-term and long-term psychological and physiological prognosis of patients discharged from the first wave of the epidemic.
Unlike previous studies of which the assessment of patients’ physical functions relied mostly on the description of patients’ subjective symptoms, our study collected laboratory test results of organ function to systematically and objectively assess the physical functions of the first wave discharged patients.
The results demonstrated that discharged COVID-19 patients had persistent psychological and physical sequelae in both stages, including depression, anxiety, PTSD, sleep disorder, and abnormal cardiac, liver, renal and pulmonary functions. It is evident that psychological and physical sequelae do persist in patients after the infection of COVID-1950–54, and our study even confirmed the occurrence of various organ function abnormalities from objective perspective.
Some studies have shown that patients with residual symptoms tend to reduce over time in a long-term follow-up16,17,55–57. From our results, we noticed that only a few of patients would leave over partial sequelae after 6 to 12 months’ rehabilitation. The proportion of psychological and physical sequelae in discharged COVID-19 patients under the two stages also differed, with a gradual decrease over time. It indicates the prognosis of COVID-19 patients is promising and most patients could recover from sequelae.
Due to the lack of reports investigating the incidence of PTSD in the general population in China, we refer to a survey from other countries which reported the incidence of PTSD in general population ranged from 1–14%58. The results of our study found that patients without any previous comorbidities had a higher chance of developing PTSD (4.9%), and no significant difference showed between gender. For patients without any previous comorbidities, the sudden appearance of novel coronavirus pneumonia symptoms could bring huge impact to them, they are more likely to induce PTSD.
The results suggested that depression and anxiety of discharged COVID-19 patients were correlated with severity of COVID-19, which is consist of other studies19,59. The more severe the patient’s clinical condition is, the more complications, more possible invasive operations, more discomfortable symptoms and longer duration of hospitalization would be, patients would generate more worries, leading to a higher incidence rate of anxiety and depression. A national survey published in 2021 indicated that the prevalence of depression among Chinese adults ranges between 6% and 7%60. In early stage, our study did observe that patients who were hospitalized > 14 days prone to develop depression (17.4% vs. 8.4%).
One study reported that the lifetime prevalence of anxiety disorder among Chinese adults is 7.6%, and multiple studies have found that anxiety disorder occurs more frequently in women than in men60. In the late stage, we found that patients with diabetes (20% vs. 5.2%) were prone to be anxious. Patients with diabetes develop more activated immunity and inflammatory response, inflammatory factors are significantly elevated, increasing the oxidative stress in the brain and inducing psychiatric disorders, leading to an increased incidence rate of anxiety61–65.
However, the results of this study found no significant difference in the incidence rate of anxiety between different genders, and incidence rate of sleep disorder was especially low, this remains to be further investigate.
Our results suggest that gender is associated with cardiac function in late stage and that chronic kidney disease is associated with liver function in both stages. These results can be understood from the following studies. Studies have shown that gender differences in immunology contribute to male susceptibility to cardiovascular diseases and COVID-1960,66. And evidence suggests a link between non-alcoholic fatty liver disease (NAFLD) and chronic kidney disease, and the two can influence each other, with NAFLD being the most common chronic liver disease67.
Patients with COPD and chronic liver disease are prone to heart function abnormality in the early stage. As we know that Angiotensin-converting enzyme 2 (ACE2) is the main target for SARS-CoV-2 to enter host cells, which is highly expressed in the lung of COPD patient68,69, and the systemic inflammatory response associated with COPD is associated with an increased risk of myocardial injury70. Besides, there is a statement about the heart-liver axis, which mentions the mutual interaction between the liver and the heart71. This evidences above support our results.
Notably, the present data showed that liver function abnormality was more likely to occur in the male in both stages, we hypothesized it was related to more alcohol consumption in the male, but data support is lacking.
Renal and pulmonary function abnormalities were the most common physical sequelae. Especially for lung, more than 1/3 of the patients showed pulmonary function abnormality in the early stage, which may be the reason that infection of SARS-CoV-2 mainly manifest with respiratory symptoms, and respiratory abnormality is more likely to remained. Studies have shown that advanced age is an independent risk factor for Acute kidney injury (AKI) during COVID-1972, while our results showed that age > 50 years is a related factor for abnormal renal function in late stage. Some studies reported that the incidence of COVID-19 renal injury was as high as 28-34%, and even higher in ICU patients, among which male patients with comorbidities were even possible to develop renal injury73,74. The renal injury could not only induce by the direct damage to renal podocytes and proximal tubular cells by SARS-CoV-2, but also the virus-induced inflammatory response which causing endothelial cell damage and microvascular thrombosis73,74. The incidence rate of liver and cardiac function abnormalities was less likely to occur.
In addition, the results didn’t show effective correlation between psychological and physical conditions in discharged COVID-19 patients in both stages. A study reported that psychological condition was associated with the presence of COVID-19 symptoms at the time of COVID-19 infection16. This seems inconsistent with our findings, but in fact, the physical conditions in our study mainly referred to the organ function condition but not the subjective symptoms described by the patients, and secondly, we did not analyze each pulmonary function index separately, but assessed overall pulmonary function.
Analysis of the composition of populations in our study showed that the critical cases were male gender with comorbidities, most of them aged > 50 years old, these were related factors for physical prognosis, especially for renal and pulmonary function. A lot of studies have shown that male gender, elder age, and comorbidities were risk factors for progression and mortality in patients with COVID-1920,75–78. It could be seen that the related factors affecting physical prognosis screened by our findings were consistent with the study above78, indicating that the above-mentioned population was not only susceptible to COVID-19 in the uninfected stage, but also prone to develop severe cases in the infected stage and to leave long-term sequelae in the recovered stage, so clinicians need to devote more medical resources to them in order to improve their prognosis.
Nevertheless, there are limitations in our study, most of the patients recruited in this study are diagnosed in the first wave of COIVD-19 infection, of which SARS-CoV-2 RNA variant these patients got infected was unknow, neither we could obtain. These would need further study to explore, whether different variants could cause different impacts on short-term and long-term sequelae in patients. At the same time, we were unable to determine whether the discharged patients had received the COVID-19 vaccine or this behavior had an impact on their physical and psychological conditions.
Conclusion
In a 1-year-long longitudinal study conducted on COVID-19 patients admitted to hospital in the first wave of pandemic, we concluded that (1) a range of sequelae existed in COVID-19 discharged patients, psychological sequelae included depression, anxiety, PTSD, sleep disorders, and physical sequelae included abnormal liver, heart, kidney, and lung function; (2) psychological and physical sequelae decreased as COVID-19 patients discharged from the hospital, and the correlation between them was not significant; (3) the elderly, male, severe clinical condition of COVID-19, and comorbidity were related factors that significantly affect the prognosis of COVID-19 discharged patients. However, our study has certain limitations. Firstly, we used self-reported psychological scales so we could only evaluate mental health and could not clinically confirm the diagnosis of psychiatric disorders. Secondly, we could only determine that the patient had an abnormality in that organ among the organ indicators we included, and could not confirm that the patient had an irreversible lesion in that organ. Finally, the psychological and physical conditions observed at our follow-up cannot be directly attributed to COVID-19 due to the lack of baseline data and the fact that people cannot be separated from the various contingencies of social life.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Guangdong Center for Disease Control and Prevention and all the members of the designated hospital for COVID-19 treatment for their contributions to the prevention and control of COVID-19, treatment of COVID-19 patients, data collection, and follow-up of discharged patients.
Abbreviations
- COVID-19
Coronavirus disease 2019
- PHQ-9
Patient Health Questionnaire-9
- GAD-7
7-item Generalized Anxiety Disorder Questionnaire
- PCL-5
The Posttraumatic Stress Disorder Checklist for DSM-5
- PSQI
Pittsburgh Sleep Quality Index
- CT
Computed Tomography
- PTSD
Post-Traumatic Stress Disorder
- SARS-CoV-2
Severe acute respiratory distress syndrome coronavirus 2
- WHO
World Health Organization
- ALT
Alanine Aminotransferase
- AST
Aspartate Aminotransferase
- T-bil
Total Bilirubin
- KDIGO
Kidney disease improving global outcomes
- CK
Creatine Kinase
- CK-MB
Creatine Kinase Isoenzyme-MB
- BUN
Blood Urea Nitrogen
- FVC
Forced Vital Capacity
- FEV1
Forced Expiratory Volume in 1 s
- COPD
chronic obstructive pulmonary disease
- NAFLD
non-alcoholic fatty liver disease
- ACE2
Angiotensin-converting enzyme 2
- AKI
Acute kidney injury
Author contributions
M.F. contributed to conception of the study, P.L., C.X. and Z.J. contributed to methodology, Y.D. helped perform the analysis with constructive discussion, M.F. contributed to supervision. All authors contributed to writing the original draft, reviewing, and editing.
Funding
This study was supported by the Health Commission of Guangdong Province, the Department of Science and Technology of Guangdong Province, and the Special Project on Emergency Response to Control of Novel Coronavirus Infection of Guangdong Province (No. 2020B1111330006).
Data availability
The data involved in this study contain privacy and sensitive information. According to the regulations of the COVID-19 prevention and control group of the Guangdong Provincial Health Commission, the data cannot be publicly shared. If readers have any questions or need further discussion regarding this study, please contact the corresponding author: Ming Fang, Email: drfangming@163.com.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pei-hong Li and Hui Xu contributed equally to this work.
References
- 1.(WHO), W.H.O. Coronavirus disease (COVID-19): Overview. [cited 2024 17/8/2024]; (2023). https://www.who.int/health-topics/coronavirus
- 2.Su, X. et al. Brief report: a cross-sectional study of anxiety levels and concerns of Chinese families of children with special Educational needs and disabilities post-first-wave of COVID-19. Front. Psychiatry. 12, 708465 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao, K. et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 22 (12), 757–773 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamana, T. K. et al. The impact of COVID-19 vaccination in the US: averted burden of SARS-COV-2-related cases, hospitalizations and deaths. PLoS One. 18 (4), e0275699 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berra, T. Z. et al. The COVID-19 pandemic in Brazil: space-time approach of cases, deaths, and vaccination coverage (February 2020 - April 2024). BMC Infect. Dis. 24 (1), 704 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan, Z., Yang, M. & Lai, C. L. Long COVID-19 syndrome: a Comprehensive Review of its effect on various Organ systems and recommendation on Rehabilitation Plans. Biomedicines, 9(8), 996 (2021). [DOI] [PMC free article] [PubMed]
- 7.Tanriverdi, A. et al. Extrapulmonary features of post-COVID-19 patients: muscle function, physical activity, mood, and sleep quality. Ir. J. Med. Sci. 191 (3), 969–975 (2021). 1971 -). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 594 (7862), 259–264 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Meherali, S. et al. Mental Health of children and adolescents amidst COVID-19 and Past Pandemics: a Rapid systematic review. Int. J. Environ. Res. Public. Health, 18(7), 3432 (2021). [DOI] [PMC free article] [PubMed]
- 10.Xiong, J. et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J. Affect. Disord. 277, 55–64 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vindegaard, N. & Benros, M. E. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav. Immun. 89, 531–542 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parlapani, E. et al. A review on the COVID-19-related psychological impact on older adults: vulnerable or not? Aging Clin. Exp. Res. 33 (6), 1729–1743 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfefferbaum, B. & North, C. S. Mental Health and the Covid-19 pandemic. N. Engl. J. Med. 383(6), 510–512 (2020). [DOI] [PubMed]
- 14.Torales, J. et al. The outbreak of COVID-19 coronavirus and its impact on global mental h ealth. Int. J. Soc. Psychiatry 66(4), 317–320 (2020). [DOI] [PubMed]
- 15.Huang, L. et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 398 (10302), 747–758 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, L. et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 10 (9), 863–876 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugherty, S. E. et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 373, n1098 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi, T. et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 588 (7837), 315–320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu, H. et al. Demographic, virological characteristics and prognosis of asymptomatic COVID-19 patients in South China. Front. Med. (Lausanne). 9, 830942 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu, Z. & McGoogan, J. M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA, 323(13): pp. 1239–1242. (2020). [DOI] [PubMed]
- 21.Choi, E. P. H., Hui, B. P. H. & Wan, E. Y. F. Depression and anxiety in Hong Kong during COVID-19. Int. J. Environ. Res. Public. Health, 17(10), 3740(2020). [DOI] [PMC free article] [PubMed]
- 22.Kocalevent, R. D., Hinz, A. & Brahler, E. Standardization of the depression screener patient health questionnaire (PHQ-9) in the general population. Gen. Hosp. Psychiatry. 35 (5), 551–555 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Wang, W. et al. Reliability and validity of the Chinese version of the Patient Health Questionnaire (PHQ-9) in the general population. Gen. Hosp. Psychiatry. 36 (5), 539–544 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Kertz, S., Bigda-Peyton, J. & Bjorgvinsson, T. Validity of the generalized anxiety Disorder-7 scale in an acute psychiatric sample. Clin. Psychol. Psychother. 20 (5), 456–464 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Kroenke, K. et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann. Intern. Med. 146 (5), 317–325 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Blevins, C. A. et al. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J. Trauma. Stress. 28 (6), 489–498 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Liu, D., Kahathuduwa, C. & Vazsonyi, A. T. The Pittsburgh Sleep Quality Index (PSQI): psychometric and clinical risk score applications among college students. Psychol. Assess. 33 (9), 816–826 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Mollayeva, T. et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep. Med. Rev. 25, 52–73 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Yan, D. Q. et al. Application of the Chinese Version of the Pittsburgh Sleep Quality Index in people living with HIV: preliminary reliability and validity. Front. Psychiatry. 12, 676022 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, C. et al. Reliability, validity, and Measurement Invariance of the General anxiety disorder scale among Chinese Medical University students. Front. Psychiatry. 12, 648755 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong, X. et al. Validation of the generalized anxiety Disorder-7 (GAD-7) among Chinese people with epilepsy. Epilepsy Res. 120, 31–36 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Li, G. et al. Analysis of risk factors and construction of a prediction model for posttraumatic stress disorder among Chinese college students during the COVID-19 pandemic. J. Affect. Disord. 362, 230–236 (2024). [DOI] [PubMed] [Google Scholar]
- 33.Cheng, P. et al. Psychometric property study of the posttraumatic stress disorder checklist for DSM-5 (PCL-5) in Chinese healthcare workers during the outbreak of corona virus disease 2019. J. Affect. Disord. 277, 368–374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong, Y. et al. Validation of the 7-item generalized anxiety disorder scale (GAD-7) as a screening tool for anxiety among pregnant Chinese women. J. Affect. Disord. 282, 98–103 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16 (9), 606–613 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitzer, R. L. et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 166 (10), 1092–1097 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Buysse, D. J. et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28 (2), 193–213 (1989). [DOI] [PubMed] [Google Scholar]
- 38.Bovin, M. J. et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental disorders-Fifth Edition (PCL-5) in veterans. Psychol. Assess. 28 (11), 1379–1391 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Creamer, M., Bell, R. & Failla, S. Psychometric properties of the impact of event scale - revised. Behav. Res. Ther. 41 (12), 1489–1496 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Kruger-Gottschalk, A. et al. The German version of the posttraumatic stress disorder checklist for DSM-5 (PCL-5): psychometric properties and diagnostic utility. BMC Psychiatry. 17 (1), 379 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Backhaus, J. et al. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 53 (3), 737–740 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Fabbri, M. et al. Measuring subjective sleep quality: a review. Int. J. Environ. Res. Public Health. 18(3), 1082 (2021). [DOI] [PMC free article] [PubMed]
- 43.Kocalevent, R. D., Hinz, A. & Brähler, E. Standardization of the depression screener patient health questionnaire (PHQ-9) in the general population. Gen. Hosp. Psychiatry. 35 (5), 551–555 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Löwe, B. et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med. Care. 46 (3), 266–274 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Zhang, M. et al. Transmission dynamics of an outbreak of the COVID-19 Delta variant B.1.617.2 - Guangdong Province, China, May-June 2021. China CDC Wkly. 3 (27), 584–586 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang, M. et al. Transmission dynamics and epidemiological characteristics of SARS-CoV-2 Delta variant infections in Guangdong, China, May to June 2021. Euro. Surveill. 27(10), 2100815 (2022). [DOI] [PMC free article] [PubMed]
- 47.Mohamed, K. et al. COVID-19 vaccinations: the unknowns, challenges, and hopes. J. Med. Virol. 94 (4), 1336–1349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Araf, Y. et al. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 94 (5), 1825–1832 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, M. et al. A look back at the first wave of COVID-19 in China: a systematic review and meta-analysis of mortality and health care resource use among severe or critical patients. PLoS One. 17 (3), e0265117 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ceban, F. et al. Fatigue and cognitive impairment in Post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 101, 93–135 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrigues, E. et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 81 (6), e4–e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang, C. et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 397 (10270), 220–232 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendez, R. et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J. Intern. Med. 290 (3), 621–631 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Munblit, D. et al. Studying the post-COVID-19 condition: research challenges, strategies, and importance of Core Outcome Set development. BMC Med. 20 (1), 50 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, X. et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 9 (7), 747–754 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romero-Duarte, Á. et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 19 (1), 129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han, Q. et al. Long-term sequelae of COVID-19: a systematic review and Meta-analysis of one-year Follow-Up studies on Post-COVID symptoms. Pathogens. 11(2), 269 (2022). [DOI] [PMC free article] [PubMed]
- 58.Kessler, R. C. et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry. 52 (12), 1048–1060 (1995). [DOI] [PubMed] [Google Scholar]
- 59.Zandifar, A. et al. Prevalence and severity of depression, anxiety, stress and perceived stress in hospitalized patients with COVID-19. J. Diabetes Metab. Disord. 19 (2), 1431–1438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang, Y. et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 6 (3), 211–224 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Benedetti, F. et al. Neuroinflammation in Bipolar Depression. Front. Psychiatry. 11, 71 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller, B. J. et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 70 (7), 663–671 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moulton, C. D., Pickup, J. C. & Ismail, K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. 3 (6), 461–471 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Najjar, S. et al. Neuroinflammation and psychiatric illness. J. Neuroinflammation. 10, 43 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Renna, M. E. et al. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: a systematic review and meta-analysis. Depress. Anxiety. 35 (11), 1081–1094 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Viveiros, A. et al. Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. Am. J. Physiol. Heart Circ. Physiol. 320 (1), H296–H304 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Musso, G. et al. Emerging liver-kidney interactions in nonalcoholic fatty liver disease. Trends Mol. Med. 21 (10), 645–662 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Renu, K., Prasanna, P. L., Valsala, A. & Gopalakrishnan Coronaviruses pathogenesis, comorbidities and multi-organ damage - A review. Life Sci. 255, 117839 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bienvenu, L. A. et al. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc. Res. 116 (14), 2197–2206 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sin, D. D. & Man, S. F. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 107 (11), 1514–1519 (2003). [DOI] [PubMed] [Google Scholar]
- 71.El Hadi, H. et al. Relationship between Heart Disease and Liver Disease: a two-Way Street. Cells. 9(3), 567 (2020). [DOI] [PMC free article] [PubMed]
- 72.Lin, L. et al. Risk factors and prognosis for COVID-19-induced acute kidney injury: a meta-analysis. BMJ Open. 10 (11), e042573 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mir, S. Hantavirus Induced kidney disease. Front. Med. (Lausanne). 8, 795340 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schiffl, H. & Lang, S. M. Long-term interplay between COVID-19 and chronic kidney disease. Int. Urol. Nephrol. 55(8),1977–1984 (2023). [DOI] [PMC free article] [PubMed]
- 75.Chen, S. L. et al. Patterns of deterioration in moderate patients with COVID-19 from Jan 2020 to Mar 2020: a Multi-center, Retrospective Cohort Study in China. Front. Med. (Lausanne). 7, 567296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395 (10223), 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pijls, B. G. et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 11 (1), e044640 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, J. J. et al. Risk and protective factors for COVID-19 morbidity, severity, and Mortality. Clin. Rev. Allergy Immunol. 64 (1), 90–107 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data involved in this study contain privacy and sensitive information. According to the regulations of the COVID-19 prevention and control group of the Guangdong Provincial Health Commission, the data cannot be publicly shared. If readers have any questions or need further discussion regarding this study, please contact the corresponding author: Ming Fang, Email: drfangming@163.com.