Abstract
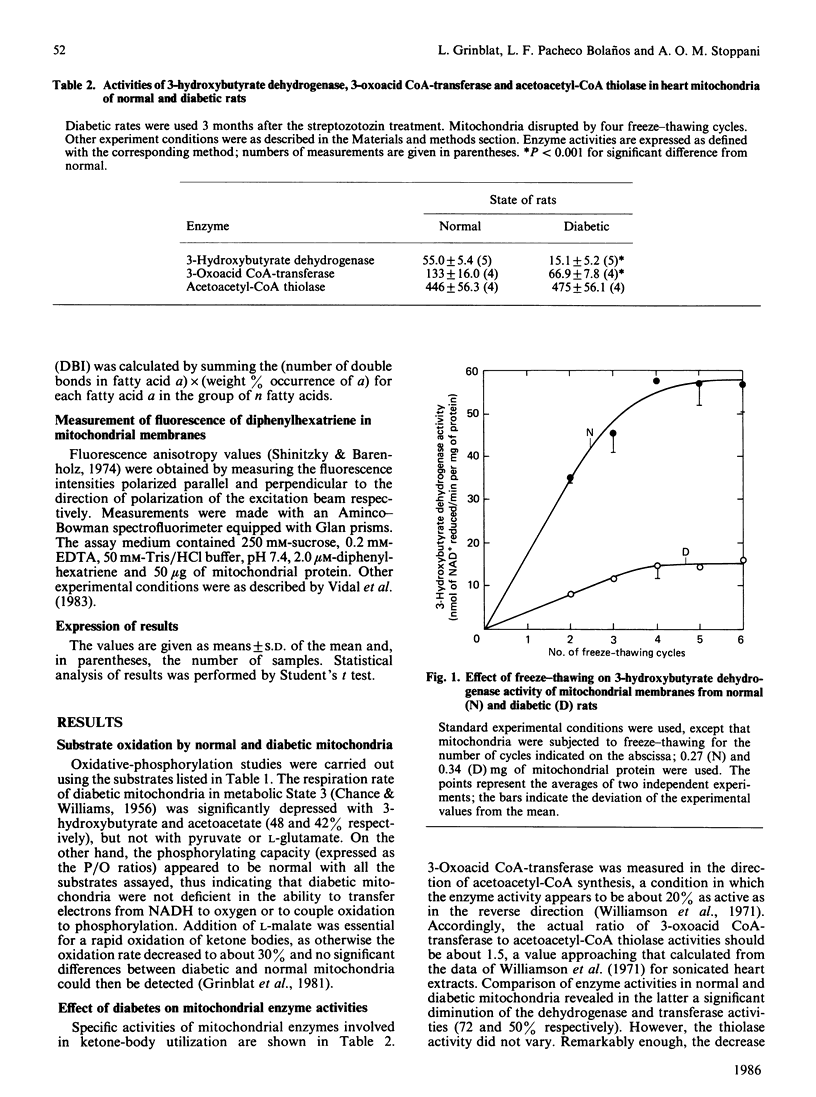
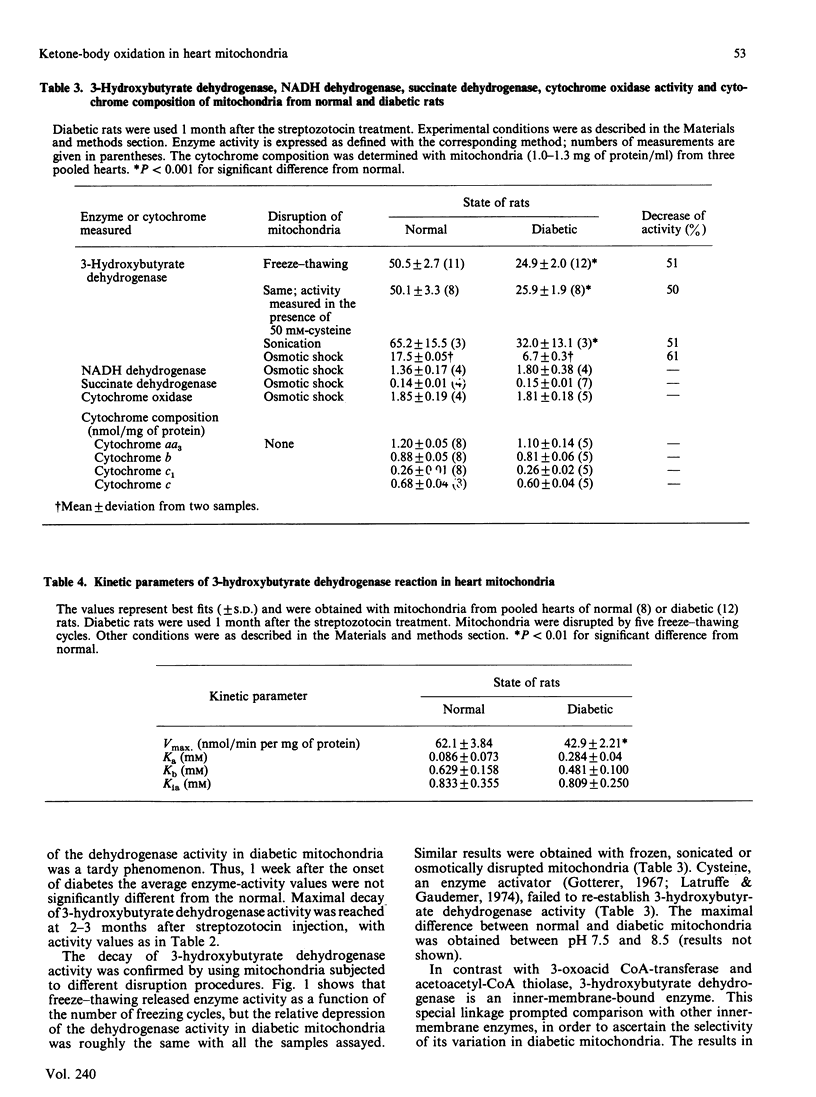
Heart mitochondria from chronically diabetic rats ('diabetic mitochondria'), in metabolic State 3, oxidized 3-hydroxybutyrate and acetoacetate at a relatively slow rate, as compared with mitochondria from normal rats ('normal mitochondria'). No significant differences were observed, however, with pyruvate or L-glutamate plus L-malate as substrates. Diabetic mitochondria also showed decreased 3-hydroxybutyrate dehydrogenase and succinyl-CoA: 3-oxoacid CoA-transferase activities, but cytochrome content and NADH-dehydrogenase, succinate dehydrogenase, cytochrome oxidase and acetoacetyl-CoA thiolase activities proved normal. The decrease of 3-hydroxybutyrate dehydrogenase activity was observed in diabetic mitochondria subjected to different disruption procedures, namely freeze-thawing, sonication or hypoosmotic treatment, between pH 7.5 and 8.5, at temperatures in the range 6-36 degrees C, and in the presence of L-cysteine. Determination of the kinetic parameters of the enzyme reaction in diabetic mitochondria revealed diminution of maximal velocity (Vmax) as its outstanding feature. The decrease in 3-hydroxybutyrate dehydrogenase in diabetic mitochondria was a slow-developing effect, which reached full expression 2-3 months after the onset of diabetes; 1 week after onset, no significant difference between enzyme activity in diabetic and normal mitochondria could be established. Insulin administration to chronically diabetic rats for 2 weeks resulted in limited recovery of enzyme activity. G.l.c. analysis of fatty acid composition and measurement of diphenylhexatriene fluorescence anisotropy failed to reveal significant differences between diabetic and normal mitochondria. The Arrhenius-plot characteristics for 3-hydroxybutyrate dehydrogenase in membranes of diabetic and normal mitochondria were similar. It is assumed that the variation of the assayed enzymes in diabetic mitochondria results from a slow adaptation to the metabolic conditions resulting from diabetes, rather than to insulin deficiency itself.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Kearney E. B., Singer T. P. Mammalian succinate dehydrogenase. Methods Enzymol. 1978;53:466–483. doi: 10.1016/s0076-6879(78)53050-4. [DOI] [PubMed] [Google Scholar]
- Armstrong R. B., Ianuzzo C. D. Decay of succinate dehydrogenase activity in rat skeletal muscle following streptozotocin injection. Horm Metab Res. 1976 Sep;8(5):392–394. doi: 10.1055/s-0028-1093621. [DOI] [PubMed] [Google Scholar]
- BRADLEY L. B., JACOB M., JACOBS E. E., SANADI D. R. Uncoupling of oxidative phosphorylation by cadmium ion. J Biol Chem. 1956 Nov;223(1):147–156. [PubMed] [Google Scholar]
- Boveris A. A., Cattaneo de Peralta Ram, Stoppani A. O., Foglia V. G. Phosphorylation, oxidation, and ubiquinone content in diabetic mitochondria. Proc Soc Exp Biol Med. 1969 Oct;132(1):171–174. doi: 10.3181/00379727-132-34174. [DOI] [PubMed] [Google Scholar]
- Brignone J. A., Campos de Brignone C. M., Rodriguez R. R., Badano B. N., Stoppani A. O. Modified oscillation behavior and decreased D-3-hydroxybutyrate dehydrogenase activity in diabetic rat liver mitochondria. Arch Biochem Biophys. 1982 Apr 1;214(2):581–588. doi: 10.1016/0003-9861(82)90063-7. [DOI] [PubMed] [Google Scholar]
- Bässler K. H., Ackermann R. H., Wagner K., Schönerstedt B. Enzymatic changes associated with ketosis in long standing diabetes and prolonged starvation of rats. Hoppe Seylers Z Physiol Chem. 1973 Jan;354(1):48–52. doi: 10.1515/bchm2.1973.354.1.48. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., PERRY S. V. Biochemical and osmotic properties of skeletal muscle mitochondria. Nature. 1954 Jun 5;173(4414):1094–1095. doi: 10.1038/1731094a0. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Chen V., Ianuzzo C. D., Fong B. C., Spitzer J. J. The effects of acute and chronic diabetes on myocardial metabolism in rats. Diabetes. 1984 Nov;33(11):1078–1084. doi: 10.2337/diab.33.11.1078. [DOI] [PubMed] [Google Scholar]
- Chen V., Ianuzzo C. D. Metabolic alterations in skeletal muscle of chronically streptozotocin-diabetic rats. Arch Biochem Biophys. 1982 Aug;217(1):131–138. doi: 10.1016/0003-9861(82)90486-6. [DOI] [PubMed] [Google Scholar]
- Churchill P., McIntyre J. O., Eibl H., Fleischer S. Activation of D-beta-hydroxybutyrate apodehydrogenase using molecular species of mixed fatty acyl phospholipids. J Biol Chem. 1983 Jan 10;258(1):208–214. [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Dow D. S. The isolation from thyrotoxic and diabetic rats of skeletal muscle mitochondria showing tight coupling, high respiratory indices, and normal adenosine triphosphatase activities. Biochemistry. 1967 Nov;6(11):3350–3355. doi: 10.1021/bi00863a003. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GORANSON E. S., ERULKAR S. D. The effect of insulin on the aerobic phosphorylation of creatine in tissues from alloxan-diabetic rats. Arch Biochem. 1949 Nov;24(1):40–48. [PubMed] [Google Scholar]
- Galante Y. M., Hatefi Y. Resolution of complex I and isolation of NADH dehydrogenase and an iron--sulfur protein. Methods Enzymol. 1978;53:15–21. doi: 10.1016/s0076-6879(78)53007-3. [DOI] [PubMed] [Google Scholar]
- Gazzotti P., Bock H., Fleischer S. Interaction of D-beta-hydroxybutyrate apodehydrogenase with phospholipids. J Biol Chem. 1975 Aug 10;250(15):5782–5790. [PubMed] [Google Scholar]
- Gotterer G. S. Rat liver D-beta-hydroxybutyrate dehydrogenase. I. Partial purification and general properties. Biochemistry. 1967 Jul;6(7):2139–2152. doi: 10.1021/bi00859a035. [DOI] [PubMed] [Google Scholar]
- Grinblat L., Pacheco L. F., Stoppani A. O. Alteraciones mitocondriales en el miocardio de ratas diabeticas. Medicina (B Aires) 1981;41(3):309–320. [PubMed] [Google Scholar]
- HALL J. C., SORDAHL L. A., STEFKO P. L. The effect of insulin on oxidative phosphorylation in normal and diabetic mitochondria. J Biol Chem. 1960 May;235:1536–1539. [PubMed] [Google Scholar]
- HAUGAARD E. S., HAUGAARD N. DIABETIC METABOLISM. I. CARBOHYDRATE UTILIZATION AND HIGH ENERGY PHOSPHATE FORMATION IN HEART HOMOGENATES FROM NORMAL AND ALLOXAN-DIABETIC RATS. J Biol Chem. 1964 Mar;239:705–709. [PubMed] [Google Scholar]
- HYVARINEN A., NIKKILA E. A. Specific determination of blood glucose with o-toluidine. Clin Chim Acta. 1962 Jan;7:140–143. doi: 10.1016/0009-8981(62)90133-x. [DOI] [PubMed] [Google Scholar]
- Hall S. E., Wastney M. E., Bolton T. M., Braaten J. T., Berman M. Ketone body kinetics in humans: the effects of insulin-dependent diabetes, obesity, and starvation. J Lipid Res. 1984 Nov;25(11):1184–1194. [PubMed] [Google Scholar]
- Harano Y., DePalma R. G., Lavine L., Miller M. Fatty acid oxidation, oxidative phosphorylation and ultrastructure of mitochondria in the diabetic rat liver. Hepatic factors in diabetic ketosis. Diabetes. 1972 May;21(5):257–270. doi: 10.2337/diab.21.5.257. [DOI] [PubMed] [Google Scholar]
- Harano Y., DePalma R. G., Miller M. Fatty acid oxidation, citric acid cycle activity, and morphology of mitochondria in diabetic rat liver. Proc Soc Exp Biol Med. 1969 Jul;131(3):913–917. doi: 10.3181/00379727-131-34008. [DOI] [PubMed] [Google Scholar]
- Latruffe N., Gaudemer Y. Propriétés et mécanisme cinétique de la D(minus)beta-hydroxybutyrique déshydrogénase de particules sous-mitochondriales de foie de rat. Effets comparés de différents agents thiols. Biochimie. 1974;56(3):435–444. doi: 10.1016/s0300-9084(74)80152-5. [DOI] [PubMed] [Google Scholar]
- Lerner E., Shug A. L., Elson C., Shrago E. Reversible inhibition of adenine nucleotide translocation by long chain fatty acyl coenzyme A esters in liver mitochondria of diabetic and hibernating animals. J Biol Chem. 1972 Mar 10;247(5):1513–1519. [PubMed] [Google Scholar]
- Mackerer C. R., Paquet R. J., Mehlman M. A., Tobin R. B. Oxidation and phosphorylation in live mitochondria from alloxan and steptozotocin diabetic rats. Proc Soc Exp Biol Med. 1971 Jul;137(3):992–995. doi: 10.3181/00379727-137-35712. [DOI] [PubMed] [Google Scholar]
- Malmström K., Carafoli E. The effect of Ca2+ on the oxidation of beta-hydroxybutyric acid by heart mitochondria. Biochem Biophys Res Commun. 1976 Apr 5;69(3):658–664. doi: 10.1016/0006-291x(76)90926-8. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Tochino Y. Depression of respiratory activities in the liver mitochondria of diabetic rats and the restorative action of insulin. J Biochem. 1969 Sep;66(3):397–404. doi: 10.1093/oxfordjournals.jbchem.a129158. [DOI] [PubMed] [Google Scholar]
- McIntyre J. O., Bock H. G., Fleischer S. The orientation of D-beta-hydroxybutyrate dehydrogenase in the mitochondrial inner membrane. Biochim Biophys Acta. 1978 Nov 2;513(2):255–267. doi: 10.1016/0005-2736(78)90178-5. [DOI] [PubMed] [Google Scholar]
- McMurchie E. J., Gibson R. A., Charnock J. S., McIntosh G. H. A comparison of mitochondrial respiration and membrane lipid composition in the rat and marmoset following dietary lipid supplementation. Comp Biochem Physiol B. 1984;78(4):817–826. doi: 10.1016/0305-0491(84)90193-7. [DOI] [PubMed] [Google Scholar]
- Menzel H. M., Hammes G. G. Purification and characterization of a lecithin-D(-)- -hydroxybutyrate dehydrogenase complex. J Biol Chem. 1973 Jul 25;248(14):4885–4889. [PubMed] [Google Scholar]
- Mercuri O., De Tomás M. E. Regulation of the delta9 desaturation. Adv Exp Med Biol. 1977;83:75–83. doi: 10.1007/978-1-4684-3276-3_7. [DOI] [PubMed] [Google Scholar]
- Nielsen N. C., Zahler W. L., Fleischer S. Mitochondrial D- -hydroxybutyrate dehydrogenase. IV. Kinetic analysis of reaction mechanism. J Biol Chem. 1973 Apr 10;248(7):2556–2562. [PubMed] [Google Scholar]
- PARKS R. E., Jr, ADLER J., COPENHAVER J. H., Jr The efficiency of oxidative phosphorylation in mitochondria from diabetic rats. J Biol Chem. 1955 Jun;214(2):693–698. [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980 Jan;60(1):143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- Roldan A. G., Del Castillo E. J., Boveris A., Garaza Pereira A. M., Stoppani A. O. Decreased activity of 3-hydroxybutyrate dehydrogenase in diabetic liver mitochondria. Proc Soc Exp Biol Med. 1971 Jul;137(3):791–793. doi: 10.3181/00379727-137-35668. [DOI] [PubMed] [Google Scholar]
- Royce S. M., Holmes R. P. The saturation and isomerization of dietary fatty acids and the respiratory properties of rat heart mitochondria. Biochim Biophys Acta. 1984 Mar 7;792(3):371–375. doi: 10.1016/0005-2760(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Rösen P., Senger W., Feuerstein J., Grote H., Reinauer H., Schrör K. Influence of streptozotocin diabetes on myocardial lipids and prostaglandin release by the rat heart. Biochem Med. 1983 Aug;30(1):19–33. doi: 10.1016/0006-2944(83)90004-2. [DOI] [PubMed] [Google Scholar]
- SMITH L. A study of some oxidative enzymes of baker's yeast. Arch Biochem Biophys. 1954 Jun;50(2):285–298. doi: 10.1016/0003-9861(54)90044-2. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem. 1974 Apr 25;249(8):2652–2657. [PubMed] [Google Scholar]
- Tahin Q. S., Blum M., Carafoli E. The fatty acid composition of subcellular membranes of rat liver, heart, and brain: diet-induced modifications. Eur J Biochem. 1981 Dec;121(1):5–13. doi: 10.1111/j.1432-1033.1981.tb06421.x. [DOI] [PubMed] [Google Scholar]
- Tucker G. A., Dawson A. P. The kinetics of rat liver and heart mitochondrial beta-hydroxybutyrate dehydrogenase. Biochem J. 1979 Jun 1;179(3):579–581. doi: 10.1042/bj1790579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VESTER J. W., STADIE W. C. Studies of oxidative phosphorylation by hepatic mitochondria from the diabetic cat. J Biol Chem. 1957 Aug;227(2):669–676. [PubMed] [Google Scholar]
- Vidal J. C., Guglielmucci E. A., Stoppani A. O. 3-D-(-) Hydroxybutyrate dehydrogenase from rat liver mitochondria--purification and interaction with phospholipids. Adv Exp Med Biol. 1977;83:203–217. doi: 10.1007/978-1-4684-3276-3_18. [DOI] [PubMed] [Google Scholar]
- Vidal J. C., Guglielmucci E., Stoppani A. O. Interaction of rat liver 3-D-(-)-hydroxybutyrate aopdehydrogenase with phospholipids. Arch Biochem Biophys. 1978 Apr 15;187(1):138–152. doi: 10.1016/0003-9861(78)90016-4. [DOI] [PubMed] [Google Scholar]
- Vidal J. C., McIntyre J. O., Churchill P., Andrew J. A., Péhuet M., Fleischer S. Influence of diabetes on rat liver mitochondria: decreased unsaturation of phospholipid and D-beta-hydroxybutyrate dehydrogenase activity. Arch Biochem Biophys. 1983 Jul 15;224(2):643–658. doi: 10.1016/0003-9861(83)90252-7. [DOI] [PubMed] [Google Scholar]
- WEBER G., STAMM N. B., FISHER E. A. INSULIN: INDUCER OF PYRUVATE KINASE. Science. 1965 Jul 2;149(3679):65–67. doi: 10.1126/science.149.3679.65. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J. N., Jr A METHOD FOR THE SIMULTANEOUS QUANTITATIVE ESTIMATION OF CYTOCHROMES A, B, C1, AND C IN MITOCHONDRIA. Arch Biochem Biophys. 1964 Sep;107:537–543. doi: 10.1016/0003-9861(64)90313-3. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Favelukes S. S., de Tarlovsky M. S., Bedetti C. D., Stoppani A. O. Influence of the hypophysis on mitochondrial protein synthesis. Acta Physiol Lat Am. 1975;25(2):107–111. [PubMed] [Google Scholar]


