Abstract
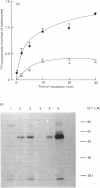
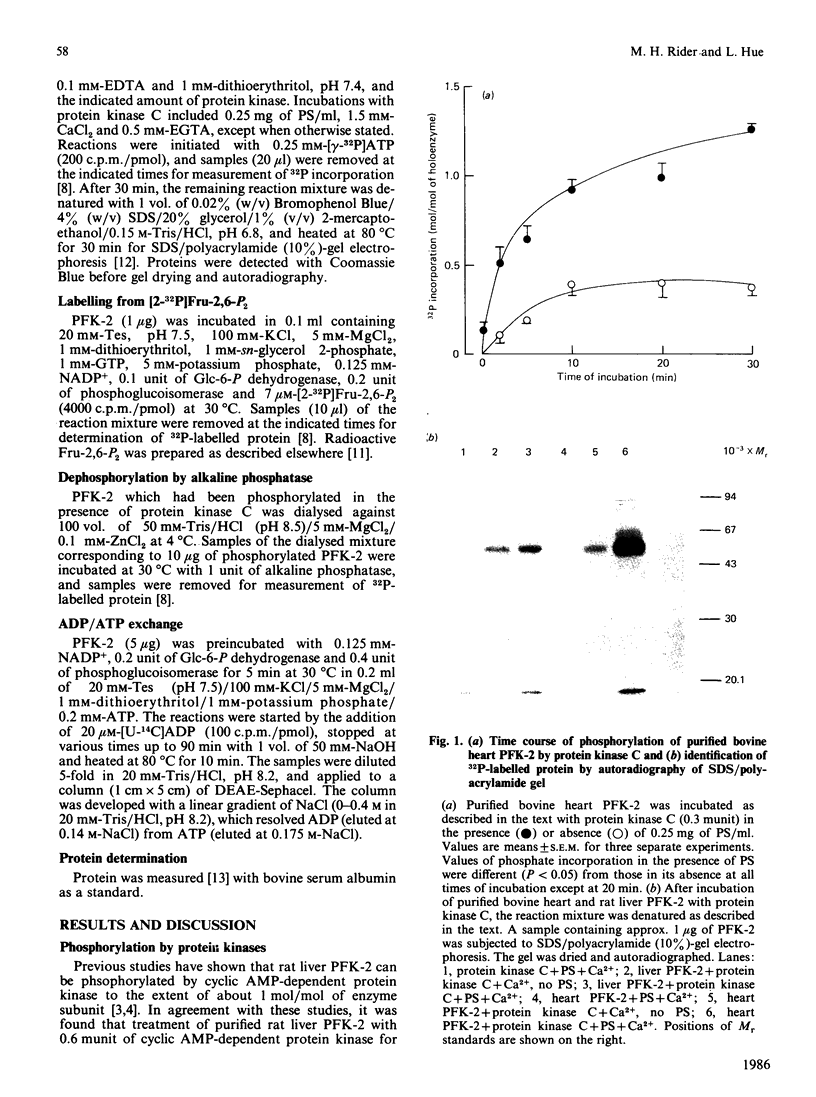
Purified bovine heart 6-phosphofructo-2-kinase can be phosphorylated in the presence of protein kinase C and dephosphorylated by alkaline phosphatase; changes in phosphorylation state have no effect on enzyme activity. By contrast, the rat liver enzyme is a poor substrate for protein kinase C. Unlike the liver enzyme, which is bifunctional and is phosphorylated by fructose 2,6-[2-32P]bisphosphate, the heart enzyme contains 10 times less fructose 2,6-bisphosphatase activity and is phosphorylated at a slower rate and to a lesser extent than the liver enzyme. Both rat liver and bovine heart enzymes catalyse a similar exchange reaction between [U-14C]ADP and ATP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- El-Maghrabi M. R., Claus T. H., Pilkis J., Fox E., Pilkis S. J. Regulation of rat liver fructose 2,6-bisphosphatase. J Biol Chem. 1982 Jul 10;257(13):7603–7607. [PubMed] [Google Scholar]
- El-Maghrabi M. R., Pate T. M., Murray K. J., Pilkis S. J. Differential effects of proteolysis and protein modification on the activities of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Biol Chem. 1984 Nov 10;259(21):13096–13103. [PubMed] [Google Scholar]
- El-Maghrabi M. R., Pate T. M., Pilkis S. J. Characterization of the exchange reactions of rat liver 6-phosphofructo 2-kinase/fructose 2,6-bisphosphatase. Biochem Biophys Res Commun. 1984 Sep 17;123(2):749–756. doi: 10.1016/0006-291x(84)90293-6. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Johnsen D. E., Campanile C. P. Evidence for the role of phosphorylase kinase, protein kinase C, and other Ca2+-sensitive protein kinases in the response of hepatocytes to angiotensin II and vasopressin. J Biol Chem. 1984 Mar 10;259(5):3283–3292. [PubMed] [Google Scholar]
- Hue L., Blackmore P. F., Shikama H., Robinson-Steiner A., Exton J. H. Regulation of fructose-2,6-bisphosphate content in rat hepatocytes, perfused hearts, and perfused hindlimbs. J Biol Chem. 1982 Apr 25;257(8):4308–4313. [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kitajima S., Sakakibara R., Uyeda K. Kinetic studies of fructose 6-phosphate,2-kinase and fructose 2,6-bisphosphatase. J Biol Chem. 1984 Jun 10;259(11):6896–6903. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murray K. J., El-Maghrabi M. R., Kountz P. D., Lukas T. J., Soderling T. R., Pilkis S. J. Amino acid sequence of the phosphorylation site of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Biol Chem. 1984 Jun 25;259(12):7673–7681. [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkis S. J., Chrisman T. D., El-Maghrabi M. R., Colosia A., Fox E., Pilkis J., Claus T. H. The action of insulin on hepatic fructose 2,6-bisphosphate metabolism. J Biol Chem. 1983 Feb 10;258(3):1495–1503. [PubMed] [Google Scholar]
- Pilkis S. J., Regen D. M., Stewart H. B., Pilkis J., Pate T. M., El-Maghrabi M. R. Evidence for two catalytic sites on 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase. Dynamics of substrate exchange and phosphoryl enzyme formation. J Biol Chem. 1984 Jan 25;259(2):949–958. [PubMed] [Google Scholar]
- Reimann E. M., Beham R. A. Catalytic subunit of cAMP-dependent protein kinase. Methods Enzymol. 1983;99:51–55. doi: 10.1016/0076-6879(83)99039-0. [DOI] [PubMed] [Google Scholar]
- Rider M. H., Foret D., Hue L. Comparison of purified bovine heart and rat liver 6-phosphofructo-2-kinase. Evidence for distinct isoenzymes. Biochem J. 1985 Oct 1;231(1):193–196. doi: 10.1042/bj2310193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider M. H., Hue L. Activation of rat heart phosphofructokinase-2 by insulin in vivo. FEBS Lett. 1984 Oct 29;176(2):484–488. doi: 10.1016/0014-5793(84)81223-5. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Sakakibara R., Kitajima S., Uyeda K. Differences in kinetic properties of phospho and dephospho forms of fructose-6-phosphate, 2-kinase and fructose 2,6-bisphosphatase. J Biol Chem. 1984 Jan 10;259(1):41–46. [PubMed] [Google Scholar]
- Stewart H. B., el-Maghrabi M. R., Pilkis S. J. Evidence for a phosphoenzyme intermediate in the reaction pathway of rat hepatic fructose-2,6-bisphosphatase. J Biol Chem. 1985 Oct 25;260(24):12935–12941. [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Phosphofructokinase 2: the enzyme that forms fructose 2,6-bisphosphate from fructose 6-phosphate and ATP. Biochem Biophys Res Commun. 1981 Aug 14;101(3):1078–1084. doi: 10.1016/0006-291x(81)91859-3. [DOI] [PubMed] [Google Scholar]
- van Schaftingen E., Davies D. R., Hers H. G. Fructose-2,6-bisphosphatase from rat liver. Eur J Biochem. 1982 May;124(1):143–149. doi: 10.1111/j.1432-1033.1982.tb05917.x. [DOI] [PubMed] [Google Scholar]