Abstract
This study assessed the impacts of climatic parameters (rainfall and temperature) and environmental variables (transparency, depth, chloride, TS, TSS, TOC) on the abundance of commercially important fish species in Esa-Odo. The research question was to find out if climatic and environmental factors influence the abundance of fish species in an afro-tropical reservoir. Fish species were collected on a monthly basis for two annual cycles covering both dry and rainy seasons. GIS-based climatic data was used to determine the relationship between climatic conditions and fish species abundance. Results showed that the highest abundance of fish was recorded during the rainy season, with Oreochromis niloticus being the most dominant species throughout the sampling period. Trend analysis revealed that variations in climate and environmental parameters influenced the abundance of different fish species in the reservoir. Mann-Kendal analysis indicated that an increase in rainfall led to an increase in reservoir depth, a decrease in transparency levels, and a reduction in temperature, with a Sen’s slope value of -38. Additionally, CCA and correlation matrix results demonstrated that climate and environmental parameters significantly influenced fish species abundance. The study emphasized the importance of climatic and environmental factors in the abundance of fish species in the reservoir, providing valuable information for future research on fishery resources. Governments and stakeholders were urged to prioritize the conservation and management of the reservoir’s fish population to prevent declines.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75181-y.
Keywords: Abundance, Fish fauna, Coptodon Zillii, Esa-Odo, Marcusenius senegalensis
Subject terms: Ecology, Zoology, Limnology
Introduction
There is an increasing rise in the temperature of the earth, which was recorded to have risen by 0.74 °C over the last decade, with an expectation to increase from 1.1 °C to 1.6 °C by the end of the century as a result of climate change1. These changes can affect both lentic and lotic ecosystems, and in some cases, lake productivity could be altered by the influx of rainfall from the tributaries of the reservoir and evapotranspiration due to significant changes in climate-induced temperature2,3. The balance between rainfall influx, water loss from outflows, and evapotranspiration is crucial for aquatic ecosystems at various levels of biological organization4. Thus, the impoundment of a reservoir gives rise to a series of changes in the chemical, physical, and biological features5, leading to significant effects on habitats, obstruction of fish migration and reproduction due to fluctuations in the limnological characteristics of the environment, and to some extent, loss of aquatic biodiversity6,7. The resultant effects could also lead to a shift in water classifications from lotic to lentic habitats, both in lacustrine and riverine environments of the reservoir8,9. The alterations in the river water system also affect fish assemblages and diversity; some fish species might find it difficult to survive in the reservoir, resulting in extinction or reduction in their population10–13.
In aquatic habitats, fish constitute an essential component of the biodiversity. They play a crucial role in sustaining the ecosystem functions that benefit human communities14,15. As such, fish are vital in sustaining the overall health and balance of aquatic environments, and their importance cannot be overstated. By preserving and protecting fish populations, we can ensure that these crucial ecosystem services continue to support human livelihoods and well-being. Freshwater ecosystems, such as rivers, lakes, and swamps, provide habitat for a vast array of fish species, with around 11,952 species, or approximately 43% of all known fish species, found in these environments16,17. Freshwater fish are of great importance to the livelihoods of people, particularly those in developing countries18–20. They provide a valuable source of food and income for many communities that rely on fishing as a primary economic activity. Despite the significance of freshwater environments and the fish populations they support, the water in which these fish live is in high demand by multiple sectors, including agriculture, energy, industries, and households21,22. This has resulted in a decline in freshwater fish diversity, as these ecosystems and their associated fish populations become increasingly degraded. The complex interplay of factors that contribute to this situation underscores the need for a comprehensive approach to water management that balances competing demands and ensures the long-term sustainability of freshwater ecosystems and their biodiversity23,24. Industrial, agricultural, and domestic activities are a major source of pollution, and this pollution has harmful effects on both marine and freshwater fish25. The impacts of pollution can be detrimental to fish populations, with pollutants affecting their growth, reproduction, and survival. The protection of fish and their aquatic habitats from the harmful effects of pollution is therefore crucial for maintaining the health and resilience of aquatic ecosystems. River systems are among the most vulnerable environments on earth due to a range of human activities, including the construction of dams26,27. Dams can have important effects on the natural flow of rivers, altering the hydrology of entire river systems and affecting the habitats and populations of aquatic species, including fish. These impacts can be particularly severe for migratory fish species that depend on rivers that are free to flow in order to complete their life cycles. As such, the building of dams and other forms of river modification must be approached with caution and careful consideration of their ecological impacts to minimize harm to riverine ecosystems and the species they support28,29.
Since the impoundment of the Esa-Odo reservoir for domestic and industrial purposes, there is currently no available information on the impacts of climatic and environmental parameters on the abundance of fish in the waterbody. The impounded Esa-Odo reservoir presents an opportunity to investigate the ichthyofaunal community, assess fishery potentials, and support subsistence fishing activities. The objective of this study is to examine whether changes in climatic and environmental factors, particularly rainfall patterns, affect fish abundance in a lentic ecosystem. The data collected will serve as foundational knowledge for managing the fishery resources of the waterbody and will aid government officials and policymakers in implementing interventions to uphold healthy fish populations and sustainable fishing practices.
Specific objectives of the study
The specific objectives of the study were to:
-
(i)
Determine the distribution and abundance of fish species.
-
(ii)
Determine the seasonal variation in climatic and environmental parameters of the reservoir,
-
(iii)
Correlate climatic and environmental parameters with fish abundance of the reservoir.
Materials and methods
Study site
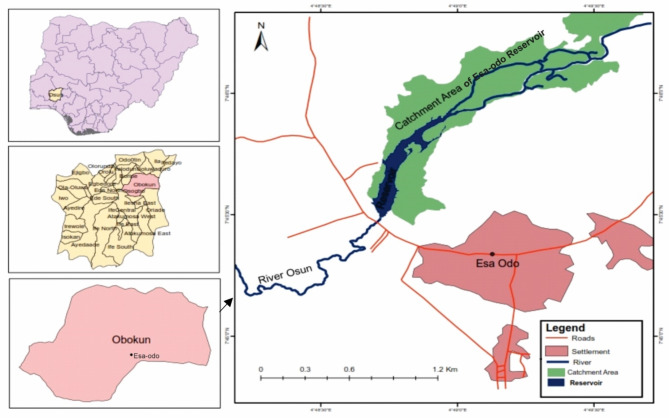
The Esa-Odo Reservoir is one of the largest reservoirs at impoundment and is located in the small community of Esa-Odo in Osun State (Fig. 1). It is situated in the humid tropics with high temperatures and rainfall, at Latitude 007°45’0’ N to 007°47’18’ N and Longitude 04°49’0’ E to 04°50’12’ E (Table 1). The Esa-Odo area lies on the southern foothills of the Yoruba Hills and Ranges in the area south of the Imesi-Ile Massif, with an undulating to rolling topography and an average elevation of 400 m above sea level. The reservoir was impounded in 1973 with a volume of 8.2 cubic meters of water.
Fig. 1.
Map of Esa-Odo reservoir. Software ArcGIS Desktop 10.8 http://my.esri.com.
Table 1.
Morphometric Parameters of Esa-Odo reservoir.
| Study area Esa-Odo | Esa-Odo |
|---|---|
| Location Coordinates | Latitude 007°45’0’ N - 007°47’18’ N |
| Longitude 04°49’0’ E - 04°50’12’ E | |
| Altitude | 458 m asl |
| Reservoir surface area | 50.2 hectares |
| Maximum depth | 4.23 m |
| Minimum depth | 1.54 m |
| Crest length | 677 m |
| Crest height | 11.3 m |
| Volume | <8.2 million m3 |
| Catchment area | 120 km2 |
The study area’s climate is characterized by two seasons: the wet and dry seasons. It falls under the koppen’s AfHumid Tropical climate type, with a short dry season from November to February and a rainy season from March to October, featuring bimodal rainfall distribution. The mean annual rainfall is around 1500 mm, with approximately 120 rainy days per year. The mean maximum temperature during the dry season is 31 °C, while in the rainy season, it is 28 °C (Climate–Data.org).
Climatic and environmental parameters
The data for both climatic and environmental parameters were collected from both primary and secondary sources. Water was sampled on a monthly basis for two annual cycles from the reservoir at six evenly distributed different sampling points covering three zones (riverine, transition, and dam site zones) using standard sampling polyethylene bottles. The grid coordinates of each station were measured and recorded using Global Positioning System (GPS). Environmental parameters such as depth, transparency, and temperature were measured in the field using a Secchi disc and a mercury-in–glass thermometer, respectively, while chloride, total suspended solids, total solids, and total organic carbon were determined in the laboratory based on standard protocols30. The data for rainfall were retrieved from the NASA Langley Research Center (LaRC) POWER Project funded through the NASA Earth Science/Applied Science Program (www.power.larc.nasa.gov).
Fish sampling
Fish collections were conducted on a monthly basis for two annual cycles, encompassing both wet and dry seasons, with the assistance of local fishermen. Fish were captured using cast nets, gill nets measuring 80 m in length and 2.5 m in depth, with a mesh size of 50 mm, and traps at various depths of the reservoir from October 2017 to September 2019. The fish were randomly collected early in the morning from different zones of the reservoir (riverine, transition, and dam site zones), stored in an ice-chest box, and transported to the Department of Zoology. They were then identified using standard keys31, and processed on the same day of capture.
Catch per unit effort
The catch per unit effort of total fish sampled per month over the study period was determined using the number of fish individuals and the sampling efforts using the formula:32
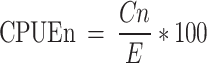 |
Cn = Number of fish caught.
E = Sampling efforts (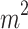 of net by hours of sampling)
of net by hours of sampling)
In this study, sampling effort was 90 m2 of net in 15 h32.
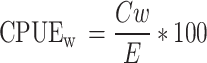 |
Cn = Weight of fish caught.
Statistical analysis
The Palaeontological Statistics PAST, version 3.22, was utilized for the multivariate analysis. Calculations such as mean values, percentages, and graph preparations were performed using MS Excel 2013. An independent T-test was conducted to assess the significant differences between the two seasons based on each parameter. Canonical correspondence analysis was employed to ascertain the influence of various environmental and climatic parameters on the abundance of fish species in the reservoir. The position of fish species on CCA indicates their preference for specific environmental or climatic parameters in the aquatic environment. Additionally, Pearson correlation coefficients were used to examine the relationships between water quality parameters, rainfall, and fish species abundance33,34. The statistical software package IBM SPSS 26.0 (SPSS, USA) (https://www.ibm.com/products/spss-statistics) was utilized to analyze fish species abundance by seasons. ANOVA was applied to determine significance, while tukey’s test was employed to differentiate means, with significance set at a probability level of 0.05. GLMM was also used to assess how climatic and environmental variables affect the abundance of fish species in the reservoir.
Ethical approval
Animal ethics approval was not required for this current study since fish specimens were collected from resident fishermen.
Results
Climatic and environmental variables
Table 2 shows the seasonal changes in climatic and environmental parameters of the reservoir. The mean values of temperature, depth, TSS, TS, TOC, and rainfall were significantly higher during the rainy season compared to the dry season. Additionally, the results of an independent t-test indicated a significant difference (p < 0.05) between the mean values of temperature, transparency, and rainfall during the rainy and dry seasons. The time series variation of rainfall is illustrated in Suppl. 1, ranging from 0 mm to 537.89 mm, with annual rainfall fluctuating between 2409.96 mm and 2531.25 mm. The highest annual rainfall was recorded in 2017, while the lowest rainfall was observed in 2018.
Table 2.
Seasonal variation in climate and environmental parameters of the reservoir.
| Parameters | Rainy season | Dry season | t | p | ||
|---|---|---|---|---|---|---|
| Min – Max | Mean ± SE | Min – Max | Mean ± SE | |||
| Temperature (°C) | 24.0–27.2 | 25.5 ± 0.28 | 25.7–26.5 | 26.1 ± 0.06 | 2.56 | 0.018* |
| Depth (m) | 2.4–3.5 | 2.8 ± 0.08 | 2.5–3.10 | 2.6 ± 0.08 | 1.16 | 0.26 |
| Transparency (m) | 0.6–0.9 | 0.8 ± 0.03 | 0.8–1.2 | 1.0 ± 0.04 | −5.08 | 0.00* |
| Chloride (mg/L) | 4.5–11.4 | 7.7 ± 0.65 | 5.7–12.6 | 7.9 ± 0.66 | −0.29 | 0.77 |
| Total suspended solids (mg/L) | 22.2–41.3 | 29.8 ± 1.39 | 24.2–31.2 | 28.2 ± 0.80 | 0.86 | 0.39 |
| Total solids (mg/L) | 95.7–126.5 | 110.0 ± 2.87 | 100.0–117.1 | 109.3 ± 1.49 | 0.19 | 0.39 |
| Total organic carbon (mg/L) | 2.5–7.8 | 5.0 ± 0.50 | 1.6–8.0 | 4.7 ± 0.77 | 0.42 | 0.68 |
| Rainfall (mm) | 26.4–490.4 | 284.8 ± 35.02 | 0.00–163.5 | 68.0 ± 20.45 | 4.80 | 0.00* |
*Significant (p < 0.05).
Fish abundance and composition of the esa-Odo reservoir
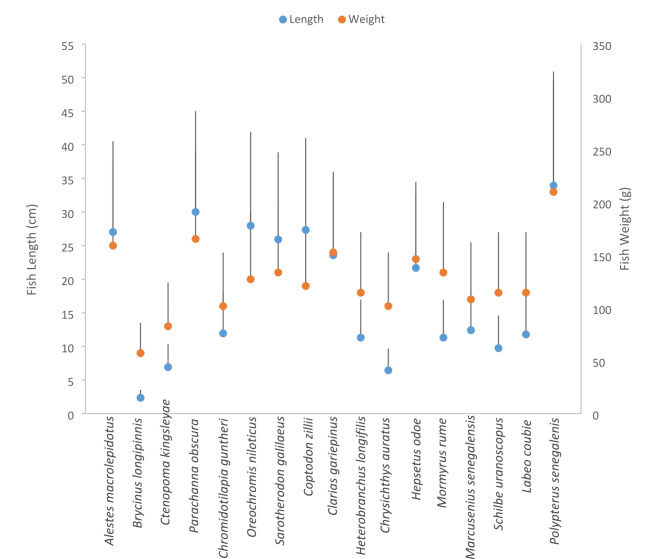
The checklist of fish species from the Esa-Odo reservoir is shown in Suppl. 2. A total of 1043 fish samples comprising seventeen species and eleven families were caught during the sampling period (Suppl. 3). The study revealed that O. niloticus was the most abundant fish species, accounting for 21.9% of the total catch, followed closely by C. zillii (19.9%), M. senegalensis (18.7%), C. gariepinus (14.1%), P. obscura (11.7%), C. kingslayae (3.0%), Schilbe uranoscopus (2.6%), and S. galilaeus (2.2%). Other species included H. odoe (1.88%), C. guntheri (1.1%), (A) macrolepitedus (0.8%), L. coubie (0.8%), and (B) longipinnis (0.4%), while H. longipinnis, M. rume, and P. senegalensis each accounted for 0.1% (Suppl. 3). The mean total length and weight ranges of the fishes caught in the Esa-Odo reservoir are depicted in Fig. 2, with mean total lengths ranging from 9.2 cm (Brycinus longipinnis) to 25.5 cm (Parachanna obscura), and fish weights ranging between 15.3 g (Brycinus longipinnis) and 270 g (Sarotherodon galilaeus).
Fig. 2.
Mean total length and weight of fish species caught in Esa-Odo Reservoir.
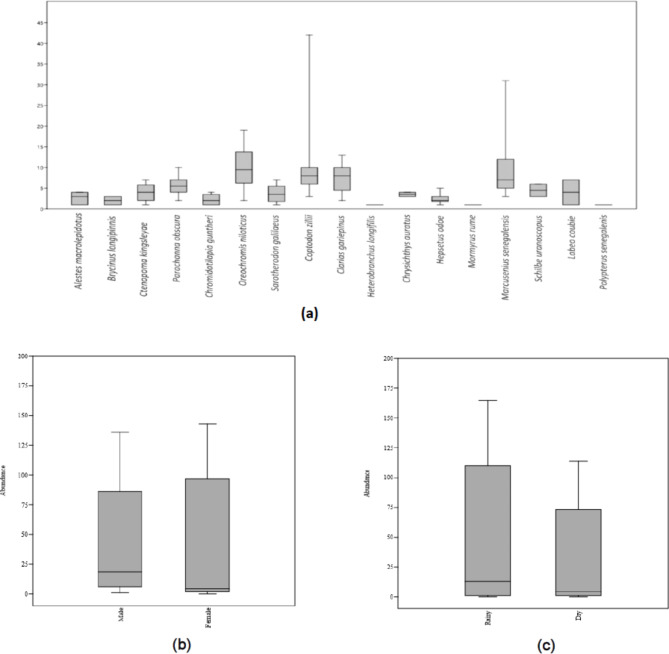
Also, the monthly distribution of the fish showed that the month of July 2018 and 2019 recorded the highest fish catch, while the lowest fish catch was observed in May 2018 (Suppl. 3). Statistically, there was a significant difference (p < 0.05) in the population of P. obscura and C. zillii in both rainy and dry seasons (Suppl. 4). Figure 3a showed the mean abundance of fish species in Esa-Odo reservoir. Also, the mean fish abundance (47 ± 16.2) was higher in female fish populations compared to the male fish populations (39 ± 11.6) with a significant difference (p < 0.05) between the two fish populations (Fig. 3b). Furthermore, the mean fish abundance (54 ± 17.2) was significantly higher during the rainy season compared to the dry season populations (31 ± 10.2) with a significant difference (p < 0.05) between the two seasons (Fig. 3c).
Fig. 3.
(a) Mean Fish Abundance (± SE) of Esa-Odo Reservoir. (b) Mean Fish Abundance (± SE) of Esa-Odo Reservoir based on Sex. (c) Mean Fish Abundance (± SE) of Esa-Odo Reservoir based on Season.
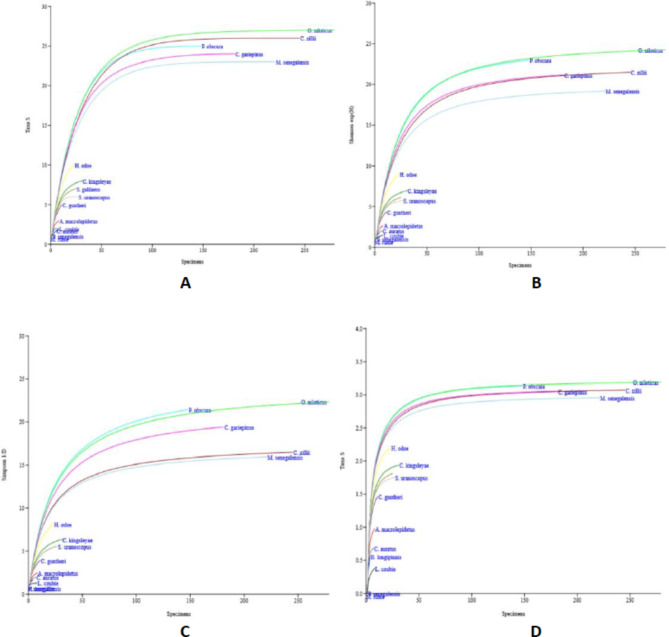
The results of individual rarefaction curves showed that Oreochromis niloticus had increased richness, Shannon exp, Simpson, and Shannon all year round. Other fish species that showed an increase in individual rarefactions are Coptodon zillii, Parachanna obscura, Marcusenius senegalensis, and Clarias gariepinus (Fig. 4). In summary, the reservoir experienced an abundance of Oreochromis niloticus throughout the sampling period.
Fig. 4.
Rarefaction Curves for Fish Species in Esa-Odo Reservoir. (A) Richness (S), (B) Shannon exp (H), (C) Simpson 1/D, (D) Shannon.
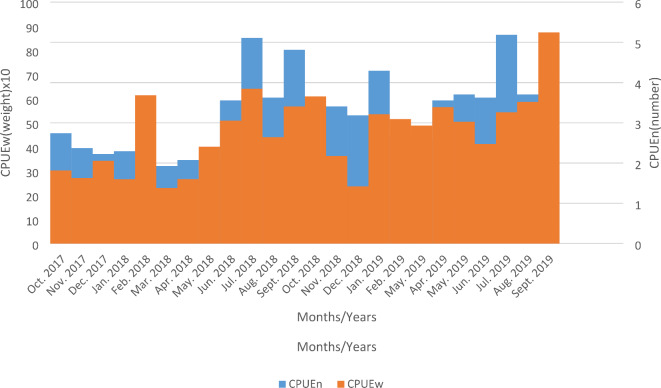
Catch per unit effort (CPUE)
The results of Catch per unit effort in Esa-Odo reservoir showed that there was a gradual increase in the CPUEn during the 1st annual cycle from the early dry season (October 2017 – December 2017) and reaching its peak in the late rainy season (July 2018 – September 2018). A similar pattern was also observed in the 2nd annual cycle with the early dry season showing an increase in catch abundance, with the highest catch effort recorded in July 2019 – September 2019 (Late rainy season) (Fig. 5). Of the most dominant fish species in the reservoir, only C. zillii had the highest CPUEn in July 2016, while other fish species such as M. senegalensis had its CPUEn peak in September 2018, and O. niloticus in July 2019. During the sampling period, CPUEw was maximum in September 2019, while the least was recorded in December 2018. Additionally, CPUEw showed significantly higher variation when compared with CPUEn.
Fig. 5.
Catch per unit effort (CPUE) in number and weight of fishes caught in Esa-Odo reservoir.
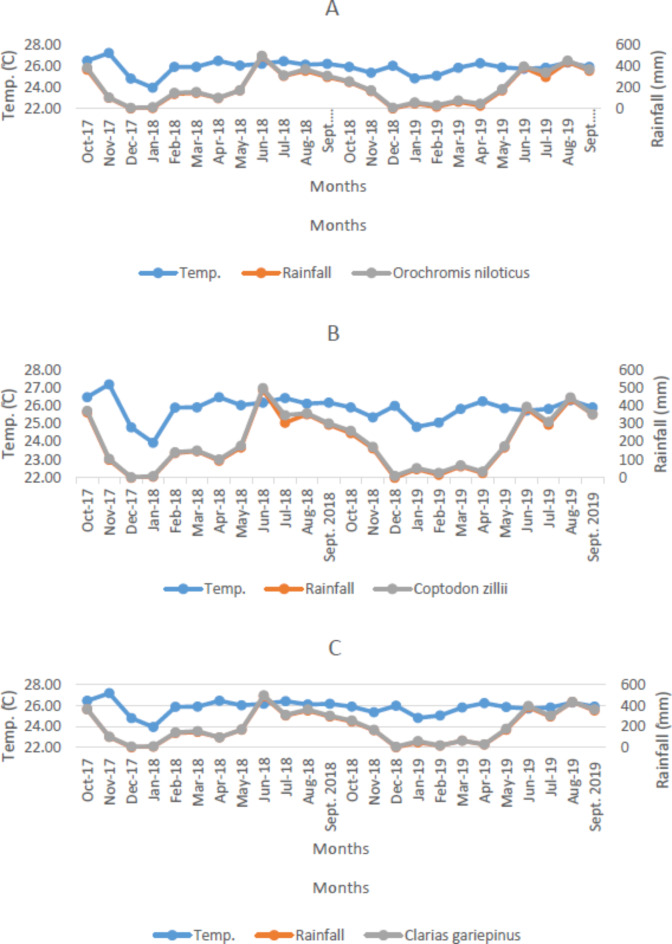
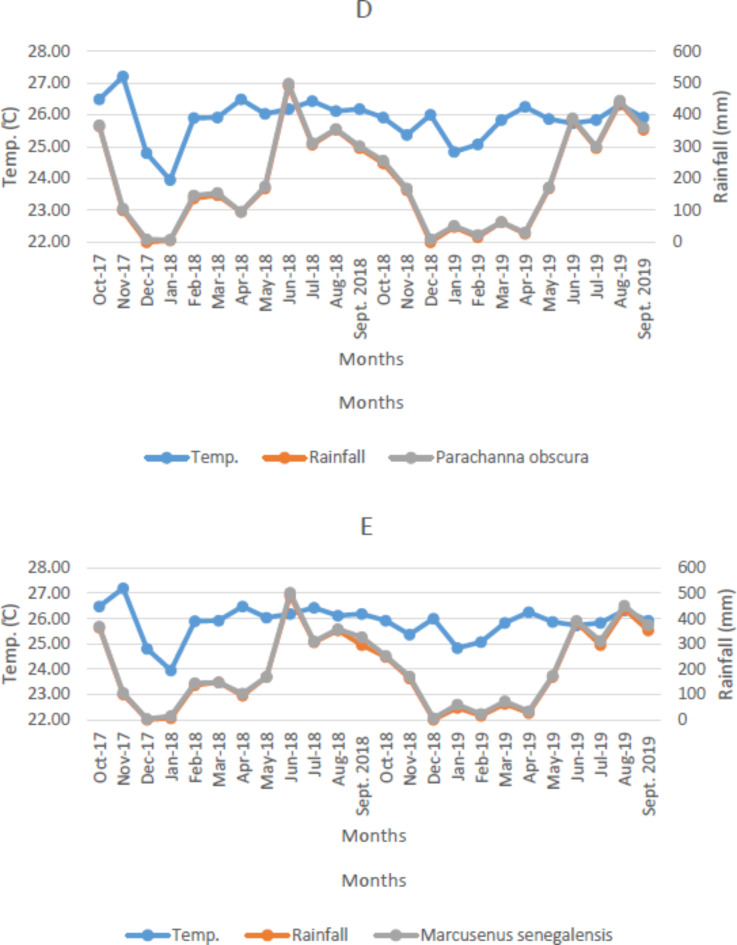
Trend analysis in temperature, rainfall and fish species
The graph of the monthly temperature, rainfall, and fish species is as shown in Fig. 6A-E. It was observed that July 2018 recorded the key period of fish abundance in relation to the rainfall and temperature, while the month of December 2017 and 2017 recorded the least abundance of fish. The mean temperature and rainfall for each month exhibited significant changes in transparency, TOC, and Oreochromis niloticus at p < 0.05. Rainfall increased significantly, as shown by the value of Sen’s slope estimator in Table 3. Additionally, rainfall, depth, TSS, TS, TOC, Oreochromis niloticus Catch, Coptodon zillii Catch, Clarias gariepinus Catch, and Marcusenius senegalensis Catch showed positive trends, while temperature, transparency, chloride, and Parachanna obscura Catch exhibited negative trends during the sampling period. The climatic factor (rainfall) that increases reservoir depth decreases the level of transparency, resulting in a reduction in temperature, as shown by the value of Sen’s slope (-38). These factors also contributed to the increase in fish species catch, except for P. obscura, as revealed by the values of Sen’s slope.
Fig. 6.
(A–E) Distribution Pattern in Temperature, Rainfall and Fish Species Catch in Esa-Odo Reservoir. (A) Oreochromis niloticus, (B) Coptodon zillii, (C) Clarias gariepinus, (D) Parachanna obscura, (E) Marcusenius senegalensis.
Table 3.
Mann-Kendal trend analysis for fish species catch, climate and environmental parameters of the reservoir.
| Mann-Kendal | p-value | Sen’s slope |
|---|---|---|
| Rainfall | 0.263 | 46 |
| Temperature | 0.358 | −38 |
| Depth | 0.691 | 17 |
| Transparency | 0.006* | −122 |
| Chloride | 0.784 | −12 |
| Total suspended solids | 0.487 | 29 |
| Total solids | 0.823 | 10 |
| Total organic carbon | 0.006* | 111 |
| Oreochromis niloticus Catch | 0.010* | 104 |
| Coptodon zillii Catch | 0.094 | 68 |
| Clarias gariepinus Catch | 0.306 | 42 |
| Parachanna obscura Catch | 0.900 | -6 |
| Marcusenius senegalensis Catch | 0.018* | 96 |
*Significant (p < 0.05).
Correlates between fish abundance, climatic and environmental variables
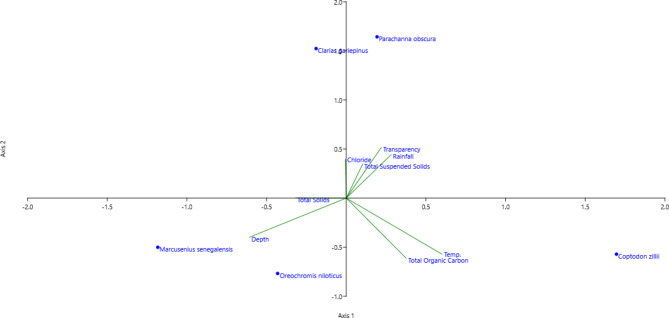
Principal components and canonical correspondence analyses
Canonical Correspondence Analysis, a direct statistical technique used to determine patterns in aquatic species data described by environmental variables (Alvarez et al. 2017; Palanivel et al., 2019), was employed in this study. Eight climatic and environmental parameters, including temperature, depth, transparency, chloride, TSS, TS, TOC, and rainfall, were selected for the CCA analysis. A CCA graph (see Fig. 7) was generated to explore the relationship between these environmental parameters and fish species in the reservoir. The eigenvalue for axis 1 was 0.06, while for axis 2, it was 0.02. The vector length indicated positive correlations for transparency, chloride, rainfall, and TSS, while TS and depth showed negative correlations. This suggests that water quality parameters play a significant role in influencing the overall abundance of fish species.
Fig. 7.
Canonical Correspondence Analysis of the Fish Species Abundance, Environmental and Climatic Variable.
Axis 1 indicated that Parachanna obscura exhibited a positive correlation with transparency, chloride, rainfall, and TSS, while axis 2 showed that Marcusenius senegalensis had a negative correlation with TS and depth. The water quality parameters displayed significant interactions concerning the abundance of fish species in the habitat. The relationship between TOC and temperature was linked to the abundance of C. zillii in comparison to C. gariepinus. The PCA loadings of the climate and environmental parameters of the reservoir revealed that PC1 and PC2 had the most substantial contributions, with temperature, rainfall, depth, transparency, and TOC influencing the abundance of fish species in the waterbody (Table 4).
Table 4.
Principal components loadings of the climate and environmental parameters of the Reservoir.
| Parameters | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | PC 7 | PC 8 |
|---|---|---|---|---|---|---|---|---|
| Temperature | 0.5948 | 0.1463 | 0.0838 | −0.1686 | −0.0315 | 0.064 | 0.1371 | 0.752 |
| Rainfall | 0.9999 | −0.0098 | 0.012 | −0.0012 | −0.0039 | −0.0022 | 0.0004 | −0.0003 |
| Depth | 0.3894 | −0.1535 | −0.473 | −0.4421 | 0.2072 | 0.5477 | −0.2505 | 0.0003 |
| Transparency | 0.3965 | 0.2301 | −0.3513 | 0.4858 | 0.6521 | 0.0547 | 0.0464 | 0.0024 |
| Chloride | −0.1231 | -0.1448 | 0.9688 | −0.0877 | 0.1308 | −0.0133 | −0.0169 | −0.0003 |
| Total Suspended Solids | −0.0597 | 0.0747 | 0.6659 | 0.6194 | −0.3265 | 0.2027 | −0.1268 | 0.0044 |
| Total Solids | −0.0179 | 0.0014 | 0.5322 | −0.0748 | −0.1631 | 0.5564 | 0.6119 | −0.0165 |
| Total Organic Carbon | 0.0411 | 0.9978 | 0.0475 | −0.0204 | −0.0019 | 0.0011 | −0.0028 | −0.0004 |
Correlation matrix and general linear mixed model
Table 5 presents the relationship between fish species catch and climatic and environmental variables as per the correlation analysis. A highly significant (p ≤ 0.01) relationship was observed between rainfall and TSS (0.585), TOC (0.909), and the catch of Parachanna obscura (0.836). Additionally, temperature exhibited a very highly significant relationship with chloride (0.959), the catch of Oreochromis niloticus (0.990), the catch of Parachanna obscura (0.904), and the catch of Marcusenius senegalensis (0.971). Similarly, depth demonstrated a highly significant relationship with TS (0.635), the catch of Coptodon zillii (0.894), the catch of Clarias gariepinus (0.625), and the catch of Parachanna obscura (0.673). Furthermore, transparency showed a highly significant relationship with TSS (0.642) and TS (0.577). Chloride exhibited a positive significant relationship between TOC (0.718) and the catch of Parachanna obscura (0.723). The findings also revealed that TSS had a highly significant relationship with TOC (0.718), the catch of Clarias gariepinus (0.870), and the catch of Marcusenius senegalensis (0.816), while TS showed a positive correlation with TOC (0.686). Moreover, TOC displayed a positive relationship with the catch of Oreochromis niloticus (0.630), the catch of Clarias gariepinus (0.741), the catch of Parachanna obscura (0.583), and the catch of Marcusenius senegalensis (0.581).
Table 5.
Correlation Matrix table showing relationship between Fish species catch, climatic and Environmental Variables.
| Rainfall | Temp. | Depth | Transp. | Chloride | TSS | TS | TOC | Oreochromis niloticus Catch | Coptodon zillii Catch | Clarias gariepinus Catch | Parachanna Obscura Catch |
Marcusenius Senegalensis Catch |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainfall | 1 | ||||||||||||
| Temp. | 0.033 | 1 | |||||||||||
| Depth | 0.032 | 0.283 | 1 | ||||||||||
| Transp. | 0.001 | 0.027 | 0.195 | 1 | |||||||||
| Chloride | 0.494* | 0.959*** | 0.052 | 0.145 | 1 | ||||||||
| TSS | 0.586** | 0.835** | 0.009 | 0.642** | 0.013 | 1 | |||||||
| TS | 0.239 | 0.447 | 0.635** | 0.577** | 0.017 | 0.038 | 1 | ||||||
| TOC | 0.909** | 0.771** | 0.337 | 0.025 | 0.718** | 0.686** | 0.916** | 1 | |||||
| Oreochromis niloticus Catch | 0.400 | 0.990*** | 0.094 | 0.089 | 0.135 | 0.207 | 0.332 | 0.630** | 1 | ||||
| Coptodon zillii Catch | 0.332 | 0.291 | 0.894** | 0.313 | 0.141 | 0.412 | 0.052 | 0.078 | 0.824** | 1 | |||
| Clarias gariepinus Catch | 0.144 | 0.212 | 0.625** | 0.243 | 0.182 | 0.870** | 0.416 | 0.741** | 0.758** | 0.925*** | 1 | ||
| Parachanna obscura Catch | 0.836** | 0.904*** | 0.673** | 0.999*** | 0.723** | 0.443 | 0.316 | 0.583** | 0.564** | 0.945*** | 0.274 | 1 | |
|
Marcusenius senegalensis Catch |
0.143 | 0.971*** | 0.054 | 0.047 | 0.197 | 0.816** | 0.978*** | 0.581** | 0.073 | 0.482* | 0.566** | 0.895** | 1 |
Transp. Transparency, TSS Total Suspended Solids, TS Total Solids, TOC Total Organic Carbon, Temp. Temperature.
***Very highly significant (p ≤ 0.001).
**Highly significant (p ≤ 0.01).
*Significant (p ≤ 0.05).
The GLMM results for the impact of rainfall on environmental variables and fish species indicated significant effects on transparency, chloride, temperature, and Clarias gariepinus, as presented in Table 6. Additionally, temperature was significantly influenced by transparency, total solids, rainfall, Coptodon zillii, Clarias gariepinus, and marcusenius senegalensis, as shown in Table 7.
Table 6.
General Linear Mixed Modelling for Climatic Factor (rainfall) influence on environmental variables and fish abundance.
| Value | SE | Z | p | |
|---|---|---|---|---|
| Residual effect | ||||
| Variance | 0.273 | 0.045 | 5.996 | 0.000 |
| Fixed effect | ||||
| Intercept | −25.833 | 5.418 | −4.768 | 0.000 |
| Depth | 0.326 | 0.214 | 1.522 | 0.134 |
| Transparency | −1.249 | 0.624 | −2.002 | 0.051 |
| CL | 0.064 | 0.031 | 2.056 | 0.045 |
| TSS | 0.012 | 0.011 | 0.994 | 0.325 |
| TS | −0.009 | 0.008 | −1.190 | 0.240 |
| TOC | −0.039 | 0.038 | −1.011 | 0.317 |
| Temperature | 1.168 | 0.219 | 5.336 | 0.000 |
| Oreochromis niloticus | 0.003 | 0.006 | 0.479 | 0.634 |
| Coptodon zillii | 0.007 | 0.008 | 0.827 | 0.412 |
| Clarias gariepinus | 0.119 | 0.020 | 6.068 | 0.000 |
| Parachanna obscura | 0.021 | 0.022 | 0.951 | 0.346 |
| Marcusenus senegalensis | 0.006 | 0.009 | 0.700 | 0.487 |
Numbers in Bold are Significant at p < 0.05.
Table 7.
General Linear Mixed Modelling for Climatic Factor (temperature) influence on environmental variables and fish abundance.
| Value | SE | Z | p | |
|---|---|---|---|---|
| Residual effect | ||||
| Variance | 0.273 | 0.045 | 5.996 | 0.000 |
| Fixed effect | ||||
| Intercept | 3.166 | 0.0410 | 77.134 | 0.000 |
| Depth | 0.003 | 0.0055 | 0.600 | 0.551 |
| Transparency | −0.042 | 0.0123 | −3.390 | 0.001 |
| CL | 0.000 | 0.0009 | −0.518 | 0.607 |
| TSS | 0.000 | 0.0005 | −0.305 | 0.762 |
| TS | 0.001 | 0.0003 | 3.944 | 0.000 |
| TOC | 0.000 | 0.0010 | 0.037 | 0.971 |
| Rainfall | 0.000 | −0.000 | 6.271 | 0.000 |
| Oreochromis niloticus | −0.000 | 0.0002 | −0.438 | 0.663 |
| Coptodon zillii | 0.001 | 0.0002 | 3.071 | 0.003 |
| Clarias gariepinus | −0.004 | 0.0007 | −5.083 | 0.000 |
| Parachanna obscura | 0.002 | 0.0008 | 1.921 | 0.060 |
| Marcusenus senegalensis | −0.001 | 0.0002 | −2.772 | 0.008 |
Numbers in Bold are Significant at p < 0.05.
Discussion
Water quality
In this study, we investigated the influence of climatic and environmental factors on the fish abundance of an afro-tropical reservoir. Our study addresses the potential impacts of rainfall and water quality parameters on fish species abundance in a lentic ecosystem. Continuous monitoring of water quality parameters is crucial to assess water quality, as changes in climatic and environmental factors can affect the distribution, abundance, and composition of aquatic animals, particularly fishes35–37. Our findings indicate that the environmental parameters of the reservoir are comparable to other tropical regions. During the dry season, the temperature was higher compared to the wet season, with a significant difference (p < 0.05) between the two seasons. Changes in atmospheric temperature are influenced by seasonal variations and play a vital role in determining waterbody gas activities38–40. The temperatures recorded in our study support fish growth by providing a suitable habitat41,42. The rainy season experienced a higher volume of rainfall, impacting the reservoir’s depth, a common feature in afro-tropical regions. Additionally, variations in reservoir transparency were noted. Rainfall in the study area exhibited a fluctuating pattern, with the highest mean rainfall in 2017 (2695 mm), slightly lower in 2018 (2407 mm), and an increase in 2019 (2531 mm). Recent global phenomena may have influenced rainfall amounts in the tropics, with many researchers attributing it to climate change43–45.
The mean value of Chloride in this study was higher than the reports of46 in Agodi reservoir; however, it was lower than the values recorded by47 in Eleyele reservoir. Additionally, the mean chloride value of the reservoir indicated good water quality and is adequate for the survival of the fish. Total Suspended Solids in the reservoir ranged between 20.00 mg/L and 46.00 mg/L. The mean values recorded in both the rainy (29.76 ± 6.33) and dry (28.02 ± 3.95) seasons are less than48 standard for drinking water. These values were also lower than the value recorded by49 in Iju River, with a mean value of 240.00 mg/L. The Total Solids values of 93.15 mg/L to 131.20 mg/L recorded during this study are higher than the values reported by50 in Opa reservoir, who reported a mean total solids value of 190.00 mg/L. The mean values recorded in the rainy season were slightly higher than the dry season, possibly due to the movement of organic and inorganic materials during the rainy season. Total Organic Carbon values ranging from 0.17 mg/L to 10.70 mg/L, with an overall mean of 4.87 ± 2.25 mg/L, were observed in Esa-Odo reservoir during this study. This value was lower than the mean TSS value of 31.54 mg/L recorded upstream and the 12.55 mg/L of TSS reported downstream in the Inland Reservoir in South China.
Fish abundance and composition
In this study, the abundance of fish collected from Esa-Odo reservoir is relatively high when compared with other water bodies. The fish in the reservoir consist of seventeen species and eleven families, aligning with similar records from other inland water bodies. This finding is consistent with Erinle reservoir, which documented nineteen species from ten families51, and Osinmo reservoir, which reported fourteen species from eight families52,53. noted seventeen species from ten families, while Owalla reservoir documented eighteen fish species. It can be inferred that habitat characteristics and ecological interactions may play a significant role in the number of fish species in this study compared to other water bodies. The reservoir exhibited a high abundance of fish samples compared to other afro-tropical reservoirs, with a total of 1221 fishes collected over two annual cycles. Similar observations were made by54 in Jebba Hydroelectric Power Dam, Jebba. The abundance of fishes in the reservoir could be attributed to the high plasticity of the fish in the water body55–57. In this study, we observed fluctuations in the monthly abundance of fish, likely influenced by environmental factors of the reservoir.
The Cichlidae family in this study recorded the highest numerical percentage of fish captured in the reservoir at 45.87%. The high numerical percentage of the cichlids could be attributed to their fecund ability and good parental care in the reservoir58–60. This study aligns with previous findings61 in Opa reservoir51, in Erinle reservoir, and62 in Asejire dam, representing the lower course of the Osun River. Various studies have shown that freshwater bodies in Nigeria and afro-tropical inland water bodies are dominated by Cichlids63–66. This dominance was evident in the fish species caught during both rainy and dry seasons. In this study, O. niloticus was the most abundant species, comprising 22.9% of the population, followed closely by C. zillii (20%). Tilapine species have thrived in most afro-tropical reservoirs due to their high fecundity, enabling them to colonize these water bodies67,68. O. niloticus, in particular, is known for its highly invasive behavior, adaptability to various niches, rapid growth rate, and impressive reproductive capacity69–71. These fish species, valued for their commercial importance, are targeted by resident fishermen in the study area.
In this study, the fish population consists of several fish species such as Chromidotilapia guntheri, Oreochromis niloticus, Sarotherodon galilaeus, and Coptodon zillii. Others include Alestes macrolepidotus, Brycinus longipinnis, clarias gariepinus, Heterobranchus longifilis, Mormyrus rume, and Marcusenius senegalensis. Additionally, Ctenopoma kingsleyae, Parachanna obscura, Chrysichthys auratus, Hepsetus odoe, Schilbe uranoscopus, Labeo coubie, and Polypterus senegalensis were caught during the sampling period. The fish species encountered in our study were similar to observations made by authors who worked on checklists of fish species in afro-tropical reservoirs69,72–74. The similarity in geographical location, habitat characteristics, and human activities such as land use could have been the major reasons for the similar fish community checklists.
There was an abundance of M. senegalensis in the reservoir, especially during the flooding months of September 2018 and 2019. The high abundance of M. senegalensis during the flooding period could be attributed to this fish species being flooded from the tributaries into the reservoir, potentially boosting the population and impacting the ecosystem75–77. Additionally, there is a high abundance of P. obscura and C. gariepinus in the reservoir. The muddy nature of the reservoir may have supported the abundance of these benthic fish species, known for feeding on smaller fishes in the water body78–80. A significant number of predatory fish species like S. uranoscopus, H. odoe, and C. auratus indicate a suitable environment for these species. Predatory fishes are known to play crucial roles in fish communities and ecosystem functioning81,82. The morphometric parameters, such as length and weight of the fishes, varied among different fish species. Similar observations in these morphometric measurements were reported by10 in Tugwi-Mukosi Reservoir. Differences in these morphometric parameters could be influenced by the environmental conditions of the various reservoirs.
Correlates of climatic and environmental parameters on fish abundance
Pattern in temperature, rainfall, and fish species catch in Esa-Odo reservoir showed that different fish species were influenced by the investigated water quality parameters. This study indicated that temperature increases have an influence on the abundance of O. niloticus, P. obscura, and M. senegalensis in the reservoir. It has been predicted that warming of the aquatic environment, based on the temperature-size rule, could increase fish growth rates leading to an abundance of fish in the aquatic ecosystem83,84. Moreover, rainfall had a positive correlation with P. obscura, while depth had a similar correlation with Coptodon zillii catch, Clarias gariepinus catch, and Parachanna obscura catch. With the depth of the reservoir ranging from 2.4 to 3.1 m, this shows that the fish species expanded their depth ranges over time and increased in abundance85,86. Also, floods resulting from intense rainfall and allochthonous materials might have led to an increase in the population of P. obscura in the reservoir.
Chloride also showed a positive correlation with transparency, while TSS, TS, and TOC exhibited a positive correlation with M. senegalensis. Water quality parameters play a significant role in influencing the growth, survival, and reproduction of fishes in water bodies, thereby contributing to the abundance of different fish species36,87,88. The behavioral effects of TSS conditions on the predators of M. senegalensis could have influenced the abundance of the fish, given the positive correlation between TSS and M. senegalensis89–91. Additionally, the range of TSS values in the reservoir during the sampling period was within the recommended limits for the survival of the fish92,93. However, the positive correlation between TSS and C. gariepinus could be due to the tolerant and microhabitat nature of the fish that favored the biological functioning of the benthic fish94–97. The abundance of M. senegalensis and C. gariepinus with TSS was also associated with the rainy period in the study area as demonstrated by the rainfall pattern.
Evidence from this study also showed that environmental variables affect the abundance of different fish species, especially their high affinity to rainfall and temperature. Other environmental parameters also influence fish species abundance either negatively or positively based on the CCA analysis. Rainfall has been observed to increase the reservoir volume, thereby impacting the abundance of fish species98–100. The study also provided insights into the role of climatic and environmental variables in explaining fish abundance in the reservoir. This further highlights that rainfall and temperature are good predictors of seasonal changes in the aquatic environment. The association between climatic, environmental factors, and fish abundance could be a result of seasonal changes in allochthonous materials (food items) and habitat preferences of the fish.
Conclusion and future of Esa-Odo reservoir
The information recorded in this study is essential for the management of the fisheries resources of the Esa-Odo reservoir. This study has provided the first record of the fish community in an afro-tropical reservoir post-impoundment. The findings indicate a diverse range of fish species in the reservoir, with climatic and environmental variables supporting their growth and development. Further research is needed to assess the fish community in the small rivers and streams that flow into the reservoir. It is crucial for stakeholders, particularly the government, to document the fish community of rivers before impoundment. Additionally, we recommend conducting continuous surveys of the fish community to monitor any changes in the fish population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors’ acknowledge the assistance of Mr Blessing, Mr Ochuko and Mr Samuel, the fishermen who assisted in capturing of the fishes.
Author contributions
Obayemi, O.E.: designed the study, wrote the manuscript, collected fish samples and analysed the dataKomolafe, O.O.: designed the study and read the manuscriptAyodeji, O.A.: collected fish samples, analysed the dataAjayi, O.: read the manuscript and analysed the dataAdewumi, P.O.: collected fish samplesAdeniran, I.I.: collected fish samplesOlalekan, K.O.: read the manuscript and analysed the dataOladimeji, T.K.: read the manuscript and analysed the data.
Data availability
All data generated during this study are included in this published article (and its supplementary information files).
Declarations
Competing interests
The authors declare no competing interests.
Consent for publication
The authors declared that the paper is satisfied for publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IPCC. Fifth Assessment Report Synthesis Report 16 th Annual Symposium of the French Renewable Energy Association (2014).
- 2.Knouft, J. H. & Anthony, M. M. Climate and local abundance in freshwater fishes. R Soc. Open. Sci.3(6). 10.1098/rsos.160093 (2016). [DOI] [PMC free article] [PubMed]
- 3.Ficke, A. D., Myrick, C. A. & Hansen, L. J. Potential impacts of global climate change on freshwater fisheries. 17(4), (2007).
- 4.Ipinloju, J. K., Magawata, I. & Shinkafi, B. A. Fisheries and aquatic science. J. Fish. Aquat. Sci.9(5), 338–344 (2014). [Google Scholar]
- 5.Alhassan, E. H., Ofori-Danson, P. K. & Nunoo, F. K. E. Fish abundance and diversity during the pre-and post-impoundment periods of the Black Volta at Bui, Ghana Fish abundance and diversity during the pre- and post-impoundment periods of the Black Volta at Bui, Ghana. J. Appl. Sci. Res.11(5), 42–49 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Dudgeon, D. Large-scale hydrological changes in Tropical Asia: prospects for Riverine Biodiversity, 50, 9, (2000).
- 7.McLaughlin, R. L. et al. Effects of low-head barriers on stream fishes: taxonomic affiliations and morphological correlates of sensitive species. Can. J. Fish. Aquat. Sci.63(4), 766–779. 10.1139/f05-256 (2006). [Google Scholar]
- 8.Turgeon, K., Turpin, C., Gregory-Eaves, I. Dams have varying impacts on fish communities across latitudes: a quantitative synthesis. Ecol. Lett.22, 1501–1516. 10.1111/ele.13283 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Cantonati, M. et al. Characteristics, main impacts, and stewardship of natural and artificial freshwater environments: consequences for biodiversity conservation. Water (Switzerland). 12(1). 10.3390/w12010260 (2020).
- 10.Madzivanzira, T. C. et al. impoundment study of the fish communities of Mukosi Reservoir, Zimbabwe, no. October 2020, pp. 1–10, doi: 10.1111/lre.12393 (2022).
- 11.Šmejkal, M. et al. Living on the edge: reservoirs facilitate enhanced interactions among generalist and rheophilic fish species in tributaries. Front. Environ. Sci.11. 10.3389/fenvs.2023.1099030 (2023).
- 12.Agostinho, A. A., Gomes, L. C., Santos, N. C. L., Ortega, J. C. G. & Pelicice, F. M. Fish assemblages in Neotropical reservoirs: colonization patterns, impacts and management. Fish. Res.173, 26–36. 10.1016/j.fishres.2015.04.006 (2016). [Google Scholar]
- 13.Liu, X. et al. Biodiversity pattern of fish assemblages in Poyang Lake Basin: threat and conservation. Ecol. Evol.9(20), 11672–11683. 10.1002/ece3.5661 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arthington, A. H., Dulvy, N. K., Gladstone, W. & Winfield, I. J. Fish conservation in freshwater and marine realms: status, threats and management. Aquat. Conserv. Mar. Freshw. Ecosyst.26(5), 838–857. 10.1002/aqc.2712 (2016). [Google Scholar]
- 15.Summers, J. K., Smith, L. M., Fulford, R. S. & Crespo, R. J. The role of ecosystem services in community well-being. Ecosyst. Serv. Glob Ecol.10.5772/intechopen.74068 (2018). [Google Scholar]
- 16.Nelson, J. S., Grande, T. C. & Wilson, M. V. H. Fishes of the World. (2016).
- 17.Kottelat, M. The Raffles Bulletin of Zoology 2013, no. 27. (2013).
- 18.Welcomme, R. L. et al. Inland capture fisheries. Philos. Trans. R Soc. B Biol. Sci.365(1554), 2881–2896. 10.1098/rstb.2010.0168 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayer, C. A. et al. The importance of freshwater species to livelihoods in the Lake Victoria Basin, Freshw. Biodivers. Lake Victoria Basin Guid. species Conserv. site Prot. Clim. Resil. Sustain. livelihoods, no. May, pp. 136–151, doi: 10.2305/IUCN.CH.2018.RA.2.en (2018).
- 20.UNCTP. Harnessing Fishery Resources for Socioeconomic Development, United Nations Publ. New York Geneva, 2022. (2022).
- 21.Bhagwat, V. R. Safety of water used in food production, Food Saf. Hum. Heal., no. January, pp. 219–247, doi: 10.1016/B978-0-12-816333-7.00009-6 (2019).
- 22.FAO, A. O. F. and The State of World Fisheries and Aquaculture 5. (2016).
- 23.Oberdorff, T. Freshwater ecosystems under threats with global change. Arch. Ouvert HAL 1–22, (2022).
- 24.Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol.29(19), R960–R967. 10.1016/j.cub.2019.08.002 (2019). [DOI] [PubMed]
- 25.Ganguly, S. Water Pollution from various sources and human infringements: an Editorial. Ind. J. Sci. Res. Tech.1(1), 54–55. http://www.indjsrt.com (2013). [Google Scholar]
- 26.Schneider, A. et al. Global-scale river network extraction based on high-resolution topography and constrained by lithology, climate, slope, and observed drainage density. Geophys. Res. Lett.44(6), 2773–2781. 10.1002/2016GL071844 (2017). [Google Scholar]
- 27.Darwall, W. et al. The Alliance for Freshwater Life: a global call to unite efforts for freshwater biodiversity science and conservation. Aquat. Conserv. Mar. Freshw. Ecosyst.28(4), 1015–1022. 10.1002/aqc.2958 (2018). [Google Scholar]
- 28.Liermann, C. R., Nilsson, C., Robertson, J. & Ng, R. Y. Implications of dam obstruction for global freshwater fish diversity, 62, 6, pp. 539–548, doi: 10.1525/bio.2012.62.6.5 (2012).
- 29.Mano, K. et al. Fish assemblages in the Upper part of the Volta River, Burkina Faso: a link analysis towards fisheries management and conservation. Int. J. Biol. Chem. Sci.13(6), 2546. 10.4314/ijbcs.v13i6.11 (2020). [Google Scholar]
- 30.APHA. Standard methods for the examination of water and wastewater. Am. Public. Heal Assoc.20, 2671 (1999). [Google Scholar]
- 31.Paugy, D., Leveque, C. & Teugels, G. G. The Fresh and Brackish Water Fishes of West Africa, 843. (Publications Scientifiques du Museum Diffusion, 2003).
- 32.Sá-Oliveira, J. C., Hawes, J. E., Isaac-Nahum, V. J. & Peres, C. A. Upstream and downstream responses of fish assemblages to an eastern amazonian hydroelectric dam. Freshw. Biol.60(10), 2037–2050. 10.1111/fwb.12628 (2015). [Google Scholar]
- 33.Trokhymets, V. et al. Species composition, distribution and relative abundance of the inshore fish community off the Argentine Islands, Bellingshausen Sea. Polar Biol.45(5), 845–855. 10.1007/s00300-022-03040-5 (2022). [Google Scholar]
- 34.Chen, C., Guo, T. & Sun, D. Relationships between fish community structure and environmental factors in the Nearshore Waters of Hainan. Diversity15, 1–17 (2023). [Google Scholar]
- 35.Arunachalam, M. Assemblage structure of stream fishes in the western ghats (India). Hydrobiologia430, 1–3. 10.1023/a:1004080829388 (2000). [Google Scholar]
- 36.Rosette, L., Mbuya Nina, P., Bakaki, F. & Yusuf Muhammad Munir, A. The influence of water quality parameters on fish species abundance and distribution near shoreline of Lake Victoria. Afr. J. Environ. Nat. Sci. Res.3(2), 1–12 (2020). [Google Scholar]
- 37.Bhateria, R. & Jain, D. Water quality assessment of lake water: a review. Sustain. Water Resour. Manag,2(2), 161–173. 10.1007/s40899-015-0014-7 (2016). [Google Scholar]
- 38.Mohammad, M. J., Krishna, P. V., Lamma, O. A. & Khan, S. Analysis of Water Quality using Limnological Studies of Wyra Reservoir, Khammam District, Telangana, India, Int.J.Curr.Microbiol.App.Sci, vol. 4, no. 2, pp. 880–895. http://www.ijcmas.com (2015).
- 39.Coffey, R., Paul, M. J., Stamp, J., Hamilton, A. & Johnson, T. A review of water quality responses to air temperature and precipitation changes 2: nutrients, Algal blooms, sediment, pathogens. J. Am. Water Resour. Assoc.55(4), 844–868. 10.1111/1752-1688.12711 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akhtar, N., Izzuddin, M., Ishak, S., Bhawani, S. A. & Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation. A Review, (2021).
- 41.Reeder, W. J. et al. Some (fish might) like it hot: Habitat quality and fish growth from past to future climates. Sci. Total Environ.787, 147532. 10.1016/j.scitotenv.2021.147532 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lourenço, S. et al. Short and long-term temperature variations drive recruitment variability in marine and estuarine juvenile fishes. Mar. Pollut Bull.192, 115093. 10.1016/j.marpolbul.2023.115093 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Dore, M. H. I. Climate change and changes in global precipitation patterns: What do we know? Environ. Int., vol. 31, no. 8, pp. 1167–1181, doi: 10.1016/j.envint.2005.03.004 (2005). [DOI] [PubMed]
- 44.Trenberth, K. E. Changes in precipitation with climate change. Clim. Res.47(47), 123–138. 10.3354/cr00953 (2011). [Google Scholar]
- 45.Abbass, K. et al. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut Res.29(28), 42539–42559. 10.1007/s11356-022-19718-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogungbile, P., Akande, J., Sridhar, M. & Ogunbode, T. Heavy metal and chemical uptake patterns of water and plant (Ipomoea Aquatica) obtained from Agodi Reservoir in Ibadan. MOJ Ecol. Environ. Sci.5(2), 70–78. 10.15406/mojes.2020.05.00178 (2020). [Google Scholar]
- 47.Olanrewaju, A., Ajani, E. & Kareem, O. Physico-chemical status of Eleyele reservoir, Ibadan, Nigeria. J. Aquac Res. Dev.08(09), 1–8. 10.4172/2155-9546.1000512 (2017). [Google Scholar]
- 48.WHO. Guidelines for Drinking–water Quality. (2017).
- 49.Chinedu, S. N., Nwinyi, O. C., Oluwadamisi, A. Y. & Eze, V. N. Assessment of water quality in Canaanland, Ota, Southwest Nigeria. Agric. Biol. J. North. Am.2 (4), 577–583. 10.5251/abjna.2011.2.4.577.583 (2011). [Google Scholar]
- 50.Adesakin, A. A., Adedeji, A. I., Aduwo & Taiwo, Y. F. Effect of discharges from re-channeled rivers and municipal runoff on water quality of Opa reservoir, Ile-Ife, Southwest Nigeria. Afr. J. Environ. Sci. Technol.11(1), 56–70. 10.5897/ajest2016.2086 (2017). [Google Scholar]
- 51.Komolafe, O. O. & Arawomo, G. A. O. Observations on the composition, physiological condition and fisheries in erinle lake, osun state, Nigeria. West. Afr. J. Appl. Ecol.18, 71–78. 10.4314/wajae.v18i1.70317 (2011). [Google Scholar]
- 52.Komolafe, O. O., Arawomo, G. A. O., Idowu, E. O. & Adedeji, A. A. Status and economic impact of the fisheries of Osinmo Reservoir, Ejigbo, Nigeria. Ife J. Sci.18(1), 309–317 (2014). [Google Scholar]
- 53.Taiwo, Y. F. Fish diversity in two reservoirs in southwest Nigeria. Fish. Soc. Niger1(1), 258–265 (2010). [Google Scholar]
- 54.Oladipo, S. O. et al. Patterns of ichthyofaunal diversity and distribution across Jebba Hydro-Electric Power (HEP) dam, Jebba, north-central Nigeria, 81, 2, pp. 258–267, (2021). [DOI] [PubMed]
- 55.Vidotto-Magnoni, A. P. & Carvalho, E. D. Composition and structure of fish community in a stretch of the Santa Bárbara River influenced by Nova Avanhandava Reservoir (low Tietê River, São Paulo State, Brazil). Acta Sci. - Biol. Sci.31(1), 56–63 (2009). [Google Scholar]
- 56.Muhammed, H. M., Ibrahim, B. U., Balogu, D. O., Isah, M. C. & Musa, A. I. Biodiversity of fishes of Tagwai reservoir, Minna, Niger state, Nigeria. Cogent Biol.5(1), 1564525. 10.1080/23312025.2018.1564525 (2019). [Google Scholar]
- 57.Morgan, R. et al. Reduced physiological plasticity in a fish adapted to stable temperatures, Proc. Natl. Acad. Sci. U. S. A., vol. 119, no. 22, doi: 10.1073/pnas.2201919119 (2022). [DOI] [PMC free article] [PubMed]
- 58.Komolafe, O. O., Olofinmehinti, M. O., Adedeji, A. A. & Adewole, H. A. The distribution, composition and abundance of fish species in two abandoned gold mine reservoirs, Igun, Osun State, Nigeria. Ife J. Sci.18(1), 95–102 (2016). [Google Scholar]
- 59.Sidi Imorou, R., Adite, A., Arame, H. & Sonon, P. S. Aspects of life history patterns of the cichlid fish Hemichromis fasciatus Peters, 1857 from Okpara Stream, Northern Benin, West Africa. Aquat. Res.3(2), 110–123. 10.3153/ar20010 (2020). [Google Scholar]
- 60.Roshni, K. & Renjithkumar, C. R. Reproductive ecology of an invasive cichlid fish Oreochromis mossambicus. Indian J. Ecol.47(4), 1180–1184 (2020). [Google Scholar]
- 61.Komolafe, O. O. Some aspects of the Biology of Tilapia Zillii (Gervais) (Pisces: Cichlidae) in Opa Reservoir, Ile-Ife, Nigeria. J. Sci. Technol.28(1), 49–56. 10.4314/just.v28i1.33077 (2008). [Google Scholar]
- 62.Ipinmoroti, M. O., Iyiola, A. O., Akanmu, O. A., Orisasona, O. & Fawole, N. Diversity and distribution of fish species in lake asejire, Adv. Sci. Technol. Innov., no. December, pp. 1447–1448, doi: 10.1007/978-3-319-70548-4_422 (2018).
- 63.Ajagbe, R. O. et al. Diversity and abundance of cichlids in Ikere Gorge Reservoir, Iseyin, Oyo State, Nigeria. Zool18, 52–56 (2020). [Google Scholar]
- 64.Olopade, O. A. & Dienye, H. E. Distribution and abundance of cichlids in the New Calabar River Distribution and abundance of cichlids in the New Calabar River, Nigeria. No June. 10.17017/jfish.v6i2.2018.318 (2018). [Google Scholar]
- 65.Mohamed, A. R. M. Population dynamics and management of two cichlid species in the Shatt Al - Arab River, Iraq, 12, 2, pp. 261–269, (2020).
- 66.Nazeef, S., Ja’afar, A., Abubakar, K. A. & Kabiru, M. Fish species biodiversity of Dadin-Kowa reservoir: current status. World J. Adv. Res. Rev.11(2), 100–111. 10.30574/wjarr.2021.11.2.0370 (2021). [Google Scholar]
- 67.Lowe-McConnell, R. H. The roles of tilapias in ecosystems. Tilapias Biol. Exploit. 129–162. 10.1007/978-94-011-4008-9_5 (2000).
- 68.Arantes, C. C., Fitzgerald, D. B., Hoeinghaus, D. J. & Winemiller, K. O. Impacts of hydroelectric dams on fishes and fisheries in tropical rivers through the lens of functional traits. Curr. Opin. Environ. Sustain.37, 28–40. 10.1016/j.cosust.2019.04.009 (2019). [Google Scholar]
- 69.Magqina, T. Fish diversity and composition of Tugwi Mukosi Dam, Zimbabwe ’ s largest inland reservoir post impoundment, no. June, pp. 75–83, doi: 10.1002/aff2.24 (2021).
- 70.Akian, D. D. et al. Reproductive behaviour of two tilapia species (Oreochromis niloticus, Linné, 1758; Sarotherodon melanotheron, Rüppel, 1852) in freshwater intra and interspecific pairing context, Appl. Anim. Behav. Sci., vol. 193, no. August, pp. 104–113, doi: 10.1016/j.applanim.2017.03.005 (2017).
- 71.Jemal, K. & Aemro, D. Age and growth of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758), from Koka Reservoir, Ethiopia. Asian Fish. Sci.35(4), 314–324. 10.33997/j.afs.2022.35.4.004 (2022). [Google Scholar]
- 72.Taiwo, Y. F., Adedeji, A. A. & Adesakin, T. A. The ecological balance of the Fish Community in an Aging Reservoir. Nat. Resour.09(09), 327–335. 10.4236/nr.2018.99020 (2018). [Google Scholar]
- 73.Englmaier, G. K. et al. Longitudinal river zonation in the tropics: examples of fish and caddisflies from the endorheic Awash River, Ethiopia. Hydrobiologia847, 4063–4090. 10.1007/s10750-020-04400-0 (2020). [Google Scholar]
- 74.Osure, G., Getabu, A., Aura, C., Omondi, R. & Basweti, E. Longitudinal fish species diversity and distribution in a medium-sized afro-tropical river in the Lake Victoria Basin. Pan Afr. Sci. J.2 (2), 246–274. 10.47787/pasj.v2i2.18 (2022). [Google Scholar]
- 75.Carlson, A. K. et al. Effects of historic flooding on fishes and aquatic habitats in a Missouri River delta. J. Freshw. Ecol.31(2), 271–288. 10.1080/02705060.2015.1128989 (2016). [Google Scholar]
- 76.Castello, L., Bayley, P. B., Fabré, N. N. & Batista, V. S. Flooding effects on abundance of an exploited, long-lived fish population in river-floodplains of the Amazon. Rev. Fish. Biol. Fish.29(2), 487–500. 10.1007/s11160-019-09559-x (2019). [Google Scholar]
- 77.Mohamad Radhi, A., Rohasliney, H. & Zarul, H. Fish Composition and Diversity in Perak, Galas and Kelantan Rivers (Malaysia) after the Major Flood of 2014. Transylv. Rev. Syst. Ecol. Res.19(3), 41–56. 10.1515/trser-2017-0020 (2017). [Google Scholar]
- 78.Kpogue, D. N. S., Mensah, G. A. & Fiogbe, E. D. A review of biology, ecology and prospect for aquaculture of Parachanna obscura, Rev Fish Biol Fish., no. March, pp. 1–13, doi: 10.1007/s11160-012-9281-7 (2012).
- 79.Oribhabor, B. J., Akpan, A. E. & David, G. S. The Food and Feeding Habits of Fishes of a Coastal, vol. VII, no. Ix, pp. 43–63, doi: 10.31364/SCIRJ/v7.i9.2019.P0919697 (2019).
- 80.Komolafe, O. O. & Arawomo, G. A. Food and diet relationships of Parachanna obscura (Gunther) and Clarias gariepinus (Burchell) in a newly impounded Osinmo reservoir, Ejigbo, Nigeria. Cameroon J. Exp. Biol.07(1), 9–15 (2011). [Google Scholar]
- 81.Holomuzki, J. R. & Stevenson, R. J. Role of predatory fish in community dynamics of an ephemeral stream. Can. J. Fish. Aquat. Sci.49(11), 2322–2330. 10.1139/f92-255 (1992). [Google Scholar]
- 82.Zhang, J., Yang, H., Ma, M., Pu, T. & Yin, X. Predator-mediated diversity of stream fish assemblages in a boreal river basin, China. Sci. Rep.13(1), 1–8. 10.1038/s41598-023-27854-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lindmark, M., Audzijonyte, A., Blanchard, J. L. & Gårdmark, A. Temperature impacts on fish physiology and resource abundance lead to faster growth but smaller fish sizes and yields under warming. Glob Chang. Biol.28(21), 6239–6253. 10.1111/gcb.16341 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahani, S. & Khorsandi, B. Effects of abundance, water temperature and light on the turbulence and mixing generated by fish schools of different sizes in quiescent water in a laboratory. Front. Mar. Sci.9, 1–13. 10.3389/fmars.2022.892249 (2022).35450130 [Google Scholar]
- 85.Chaikin, S. & Belmaker, J. Fish depth redistributions do not allow maintenance of abundance in a region of rapid change, Oikos vol. no. 8, pp. 1–15, 2023, doi: 10.1111/oik.09650 (2023).
- 86.Scott, M. E. et al. Variation in abundance, diversity and composition of coral reef fishes with increasing depth at a submerged shoal in the northern Great Barrier Reef. Rev. Fish. Biol. Fish.32(3), 941–962. 10.1007/s11160-022-09716-9 (2022). [Google Scholar]
- 87.Jepleting, H. et al. Influence of Water Quality on distribution patterns and Diversity of Enteromius Fish Species in Small Water bodies of Uasin Gishu County, Kenya. Afr. J. Educ. Sci. Technol.7(2), 1–20 (2022). [Google Scholar]
- 88.Rajanee, R. Role of water temperature and water quality on reproductive performance of fishes. Int. J. Fauna Biol. Stud.4(3), 139–142. 10.22271/23940522.2020.v7.i6a.773 (2017). [Google Scholar]
- 89.Kjelland, M. E., Woodley, C. M., Swannack, T. M. & Smith, D. L. A review of the potential effects of suspended sediment on fishes: potential dredging-related physiological, behavioral, and transgenerational implications. Environ. Syst. Decis.35(3), 334–350. 10.1007/s10669-015-9557-2 (2015). [Google Scholar]
- 90.Bax, N. J. The significance and prediction of predation in marine fisheries. ICES J. Mar. Sci.55(6), 997–1030. 10.1006/jmsc.1998.0350 (1998). [Google Scholar]
- 91.Scoulding, B. et al. Estimating abundance of fish associated with structured habitats by combining acoustics and optics. J. Appl. Ecol.60, 1274–1285. 10.1111/1365-2664.14412 (2023). [Google Scholar]
- 92.Nyanti, L. et al. Effects of water temperature, dissolved oxygen and total suspended solids on juvenile barbonymus schwanenfeldii (Bleeker, 1854) and Oreochromis Niloticus (Linnaeus, 1758). AACL Bioflux11(2), 394–406 (2018). [Google Scholar]
- 93.Chapman, P. M., Hayward, A. & Faithful, J. Total suspended solids effects on Freshwater Lake Biota Other than Fish. Bull. Environ. Contam. Toxicol.99(4), 423–427. 10.1007/s00128-017-2154-y (2017). [DOI] [PubMed] [Google Scholar]
- 94.Okyere, I. & Kobil, E. Occurrence and food habits of the bagrid catfish Chrysichthys nigrodigitatus in the Pra River Estuary, Ghana. J. Fish. Coast Manag2854 (0, p. 1, ). 10.5455/jfcom.20190315071516 (2020).
- 95.Cambray, J. A. The need for research and monitoring on the impacts of translocated sharptooth catfish, clarias gariepinus, in South Africa. Afr. J. Aquat. Sci.28(2), 191–195. 10.2989/16085910309503786 (2003). [Google Scholar]
- 96.Van Steenberge, M. W. et al. Unravelling the evolution of Africa’s drainage basins through a widespread freshwater fish, the African sharptooth catfish Clarias gariepinus. J. Biogeogr.47(8), 1739–1754. 10.1111/jbi.13858 (2020). [Google Scholar]
- 97.Bhandarkar, S. V. & CLARIUS GARIEPINUS: AFRICAN CATFISH EXOTIC SPECIES ENDANGERING NATIVE., vol. 9, no. 5, pp. 43–48, (2022).
- 98.Feng, Y., Shi, H., Hou, G., Zhao, H. & Dong, C. Relationships between environmental variables and spatial and temporal distribution of jack mackerel (Trachurus japonicus) in the Beibu Gulf, South China Sea. PeerJ9. 10.7717/peerj.12337 (2021). [DOI] [PMC free article] [PubMed]
- 99.Hong, X. et al. Impacts of climate events on life history parameters of major commercial fishes in the Beibu Gulf, South China Sea in the last 15 years. Front. Mar. Sci.10. 10.3389/fmars.2023.1234772 (2023).
- 100.Mohamed Observed impact of climate change on abundance and distribution of Nile fish in Sudan during the period (2001–2020). Int. J. Fish. Aquat. Stud.9(1), 322–327. 10.22271/fish.2021.v9.i1d.2419 (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are included in this published article (and its supplementary information files).