Abstract
Rat models of human T-cell leukemia virus type 1 (HTLV-1)-related diseases such as adult T-cell leukemia and HTLV-1-associated myelopathy/tropical spastic paraparesis have been reported. However, these models do not completely reproduce human diseases partly because HTLV-1 replicates poorly in rats. We investigated here the possible reason for this. We found that the activity of Rex in rat cells is quite low compared to that in human cells. As Rex function depends largely on the CRM1 protein, whose human type (human CRM1 [hCRM1]) directly binds to Rex and exports it from the nucleus to the cytoplasm, we assessed whether rat CRM1 (rCRM1) could act as well as hCRM1 as a cofactor for Rex activity. We first cloned a cDNA encoding rCRM1 and found that both rCRM1 and hCRM1 could bind to and export Rex protein to the cytoplasm with similar efficiencies. However, unlike hCRM1, rCRM1 could hardly support Rex function because of its poor ability in inducing the Rex-Rex interaction required for RNA export into the cytoplasm. These observations suggest that the poor ability of rCRM1 to act as a cofactor for Rex function may be responsible for the poor replication of HTLV-1 in rats.
Human T-cell leukemia virus type 1 (HTLV-1) is a causative agent of both adult T-cell leukemia (ATL), which is an aggressive malignancy of T cells (17, 23), and the chronic neurodegenerative disorder HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (22, 38). HTLV-1 belongs to a group of complex retroviruses that encode transactivator proteins for their gene expression. In HTLV-1, these transactivator proteins include Tax protein, which activates its own viral and cellular transcription (51), and Rex protein, a posttranscriptional regulator that is required for the expression of unspliced and incompletely spliced viral mRNAs that encode Gag and Env proteins, respectively (6, 15, 20). These proteins are therefore essential for viral replication.
Rex is a 27-kDa phosphoprotein that shuttles between the nucleus and the cytoplasm (3, 26). In the nucleus, Rex binds directly and specifically to the highly structured cis-acting Rex response element (RxRE), which is encoded by sequences within the 3′ long terminal repeat of HTLV-1 (1, 42). The arginine-rich basic domain comprising amino acids (aa) 1 to 19 of the 189-aa Rex protein has been shown to mediate its binding to the RxRE (1, 4, 10, 12). This region also serves as a nuclear/nucleolar localization signal (5, 29, 36, 41, 44).
Another important Rex domain is a leucine-rich region spanning aa 81 to 94 (3, 26), which functions as a nuclear export signal (NES). This domain binds directly to human CRM1 (hCRM1; exportin 1, XPO1) (11), a member of the importin β family, in cooperation with a GTP-bound form of small G-protein Ran (Ran-GTP). It is well known that Ran-GTP is located in the nucleus, while a GDP-bound form of Ran is in the cytoplasm. This gradient of Ran across the nuclear envelope is a key in the decision of directionality of the transport machine. hCRM1 has been shown to be a component of the cellular machinery involved in the protein export of various leucine-rich NES-bearing proteins (7, 9, 30, 35, 39, 45). The interaction of Rex, hCRM1, Ran-GTP, and the Rex target, viral mRNA, generates the export complex that is then transported to the cytoplasm.
Rex has a third domain that maps to aa 57 to 66 and 106 to 124 and that mediates Rex multimerization (2, 14, 50). Multimerization of Rex on the target viral mRNA is critical for Rex-mediated mRNA transport (1, 4, 10, 12) although the Rex protein itself does not require multimerization to exit the nucleus (14). It has been generally agreed that the ability of the Rex protein to form a homodimer, at least, is required for Rex multimerization on its cognate RNA (2). Our previous data showed that hCRM1 is involved in the homodimer formation of Rex (11). Thus, hCRM1 participates both in the export of the target viral mRNA complex and the multimerization of Rex. hCRM1 could therefore be considered to be the most critical cofactor guiding Rex function.
Appropriate animal models of HTLV-1 infection would allow us to analyze the pathogenesis and oncogenesis of HTLV-1-associated diseases, which could lead to the development of therapeutic and preventative measures. HTLV-1 has been known to be able to infect experimental animals such as monkeys, rabbits, and rats. Monkey and rabbit models have been used in vaccine development and to study mother-to-child transmission caused by breast feeding and in some cases ATL (19, 43, 48, 49). The utility of these models is limited, however, because of the difficulty in dealing with a number of these animals and the lack of inbred strains. Consequently, it would be more convenient to use small-animal models, in particular mouse and rat models, because their inbred strains are well characterized and can be genetically manipulated. With regard to mouse models, although attempts to infect mice with HTLV-1 have been reported, the infection was limited and could be detected only with PCR that measured the integrated genomes of HTLV-1 (6). Rat models, on the other hand, have been extensively developed to study HTLV-1-associated diseases. For example, the HAM/TSP-like disease models for rats of the WKA strain have been used to analyze the pathogenic mechanisms of this disease (21, 32), although further study is required to determine its similarity to the corresponding human disease. Recently, in attempts to establish rat models for lymphoproliferative disease and the seronegative HTLV-1 carrier state, various syngeneic HTLV-1-immortalized cell lines and administration routes in immunocompetent and T-cell-deficient nude rats were examined. The nude rats receiving syngeneic HTLV-1-producing cells developed ATL-like disease (25, 28, 37). However, although these rat models have certainly allowed us to understand HTLV-1-associated diseases better, the ATL-like disease in these models could be induced in only T-cell-deficient nude rats. This is unlike the human situation, in which patients with ATL have competent immune systems prior to disease development. Thus, to better understand the mechanism by which ATL and HAM/TSP develop, a better rat model is required. Developing such models has, however, been hampered by the poor replication of HTLV-1 in rats.
HTLV-1 has been reported to be able to infect a number of rat cells, which indicates that the rat cells possess the receptors for viral attachment and penetration into the cells (33, 46, 47). Thus, the blocking of viral propagation must be occurring at subsequent steps within the rat cells. Identification of the blocking step and the responsible host factor(s) could lead to the construction of transgenic rats that express the critical human factor(s) and that are highly susceptible to HTLV-1 infection. Such transgenic rats might be expected to develop ATL- and HAM/TSP-like diseases that more closely resemble the human diseases.
The first hint of a blocking step was a report that showed that the viral mRNAs encoding Gag and Env proteins are produced at low levels in rat cells despite the fact that the mRNA for the Tax/Rex protein is abundant (28). This observation led us to hypothesize that Rex may function poorly in rat cells. In this study, we have assessed this hypothesis and found that rat CRM1 (rCRM1) is a major cause of the poor activity of Rex in rat cells. Our results indicate that, unlike hCRM1, rCRM1 does not support Rex function efficiently because of its poor ability to induce Rex dimer formation. Both rCRM1 and hCRM1, however, bind to Rex NES and support the export of the Rex protein with similar efficiencies. These findings suggest that rCRM1 is a poor cofactor for assisting Rex multimerization and that this may be responsible for the poor replication of HTLV-1 in rat cells.
MATERIALS AND METHODS
Cloning and plasmid construction.
To construct pSRαrCRM1, full-length rCRM1 cDNA was amplified from a rat heart marathon cDNA library (Clontech) by PCR using primers 5′-AAG AAG GAG CAG TTG GTT CAA TCT CTG GTA A-3′ and 5′-CGG GGT ACC CCC AGC CAC AAA AAT GGG CAT GAA G-3′. The PCR product was blunt-ended by Pfu polymerase, digested with KpnI, and cloned into pSRα296 (47), which had been digested with PstI, blunt-ended, and then digested with KpnI. The insert sequence of the resulting construct was confirmed by DNA sequencing using an Applied Biosystems 377 automated DNA sequencer.
To construct pGALrCRM1, the rcrm1 coding region was amplified from pSRαrCRM1 by PCR with primer pair 5′-GGA AGA TCT ATG CCA GCA ATT ATG ACA ATG TTA G-3′ and 5′-AGG ACA AAC GCT GCA CAG GGA AA-3′ and then blunt-ended by Pfu polymerase treatment, digested with BglII, and cloned in-frame downstream of the GAL4 DNA-binding domain in pSGGALVP (8), which had been digested with BamHI, blunt-ended, and digested with BglII.
To construct pDM128RxRE, the RxRE of the HTLV-1 genome (42) was amplified by PCR using primers 5′-AAA CCG CTC GAG CAC GCA TAT GGC TCA ATA AAC-3′ and 5′-AAA CCG CTC GAG GGC GCA GAA CAG AAA A-3′, digested with XhoI, and cloned into XhoI-digested pDM128, whose expression of the chloramphenicol acetyltransferase (CAT) protein is dependent on the activity of human immunodeficiency virus type 1 Rev (18). The amount of CAT protein expressed from pDM128RxRE reflects Rex activity.
Plasmids pSRαRex, pSRαTAgRexM64, pCDMβ-galactosidase (pCDMβ-gal), pGAL-, pGALRex, pRexVP, pSRαhCRM1, and pGALhCRM1 have all been reported previously (11, 24).
Reporter plasmid for protein-protein interaction pG5BLuc, which harbors the luciferase gene downstream of GAL4 binding sites, was kindly donated by Y. Komoda (JAPAN TOBACCO, Inc.). This reporter plasmid expresses the luciferase protein when the GAL-fused protein interacts with the VP-fused protein.
Recombinant protein expression plasmids pET3dRanQ69L, pET3ahCRM1, and pHisRex(g10) were kindly given by Y. Yoneda (16), D. Görlich (40), and J. Katahira.
Cell culture and transfection.
HeLa cells and rat REF52 cells were maintained in a 5% CO2 atmosphere at 37°C in Dulbecco modified Eagle medium that was supplemented with 10% fetal bovine serum. Transfection was carried out either with DOTAP (Boehringer Mannheim) or Lipofectamine Plus (GIBCO-BRL Life Technologies) reagents according to the respective manufacturer's instructions. Leptomycin B (LMB) (31) was administered 8 h posttransfection by replacing the medium with fresh medium containing LMB at the proper concentration. The cells were harvested 24 h posttransfection. To normalize the transfection efficiency, 0.1 μg of pCDMβ-gal was cotransfected in all samples and the total amount of DNA in each experiment was kept constant by adding pSRα296. All transfection experiments were performed in duplicate and were done at least three times.
Measurement of Rex activity.
Various amounts of pSRαRex were transfected along with 0.5 μg of pDM128RxRE and 0.1 μg of pCDMβ-gal into HeLa or REF52 cells. In some experiments, pSRαhCRM1 or pSRαrCRM1 was cotransfected with or without LMB treatment. At 24 h posttransfection, the cells were lysed and the amount of CAT was quantified using a CAT enzyme-linked immunosorbent assay kit (Boehringer Mannheim) according to the manufacturer's instructions. The β-galactosidase (β-Gal) activity was measured by standard colorimetric methods. The ratio of CAT/β-Gal was calculated for each sample.
In vivo assay of protein-protein interaction.
Protein-protein interactions in mammalian cells were analyzed using the mammalian two-hybrid system (11). The cells were transfected with 0.2 μg of the plasmid expressing the GAL fusion protein, 0.2 μg of the plasmid expressing the Rex-VP fusion protein, 0.2 μg of pG5BLuc as a reporter, and 0.1 μg of pCDMβ-gal. In some experiments, pSRαhCRM1 or pSRαrCRM1 was cotransfected with or without LMB treatment. Luciferase (Luc) activity was quantified using the Steady-Glo Luc assay system (Promega) and the Wallac 1420 ARVOsx system. The ratio of Luc/β-Gal was calculated for each sample. The activity of β-Gal in all samples was more than 3.0 × 10−3 U.
Immunofluorescence microscopy.
HeLa and REF52 cells were transfected with 0.1 μg of pSRαRex along with 0.25 μg of pSRαhCRM1, pSRαrCRM1, or pSRα296. At 24 h posttransfection, the cells were fixed with 2% paraformaldehyde and dissolved in phosphate-buffered saline (PBS) for 15 min. After perforation with 0.1% NP-40 for 5 min, the cells were incubated with a rabbit anti-Rex C-terminal antibody for 1 h at room temperature. After being washed with PBS, the cells were stained with Cy3- or rhodamine-B-conjugated goat anti-rabbit immunoglobulin G antibody for 1 h (Jackson ImmunoResearch or Biosource) as previously described (11). The stained cells were observed with an Axiovert 135 system (Carl Zeiss, Inc.).
Western blot analysis.
The proteins resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were transferred to a nitrocellulose filter. A mouse anti-GAL4 monoclonal antibody (Santa Cruz Biotechnology) and a rabbit anti-Rex C terminus antibody (24) were used as primary antibodies to detect GAL-fused proteins and the Rex protein, respectively. To compare the expression levels of endogenous CRM1 in REF52 and HeLa cells, approximately 20 μg of cell lysates was subjected to SDS-PAGE. The blots were incubated with either of several primary antibodies, namely, rabbit anti-CRM1 antiserum generated by immunization with the peptide with the amino acid sequence 1024EFAGEDTSDLFLEEREIALR1043 (30), chicken anti-hCRM1 antiserum (specific for 1041ALRQADEEKHKRQMSVPG1058), or rabbit anti-rCRM1 (specific for 1040TALRQAQEEKHKLQMSVP1057). Horseradish peroxidase- or alkaline phosphatase-conjugated anti-immunoglobulin G antibodies (Promega) were used as secondary antibodies. Immunoreactive bands were visualized using ECL+plus (Amersham Pharmacia Biotech) followed by the LAS-1000 Plus system (Fujifilm) or BCIP (5-bromo-4-chloro-3-indolyl-phosphate)-nitroblue tetrazolium (NBT) solution.
Expression and purification of recombinant proteins.
The recombinant RanQ69L protein was expressed in Escherichia coli, purified, and then charged with GTP according to the method previously reported by Hieda et al. (16) with slight modification. The MonoQ column (Amersham Pharmacia Biotech) was used instead of a Fractogel EMDSO3-650(s) column.
Plasmid pHisRex(g10) expresses recombinant Rex protein with an NH2-terminal His tag and a COOH-terminal gene10 peptide of λ phage. Recombinant HisRex(g10) protein was expressed by 1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) for 15 h at 20oC in E. coli strain BL21 (DE3) Gold (Stratagene). The E. coli cells were lysed in buffer A (50 mM Tris-HCl [pH 8.0], 50 mM NaCl, 0.1 mg of pefabloc/ml, 1 μg each of aprotinin, leupeptin, and pepstatin/ml) containing 10 mM imidazole by sonication after two freeze-thaw cycles and clarified by centrifugation (100,000 × g, 1 h). The supernatant was applied to a His-Trap Ni+-chelating column (Amersham Pharmacia Biotech). The recombinant protein, which was trapped in a chelating column, was eluted with buffer A with 300 to 500 mM imidazole. The pooled fraction containing the HisRex(g10) protein was subjected to chromatography on a Hi-Trap Q column in a fast protein liquid chromatography system (Amersham Pharmacia Biotech) equilibrated with buffer A with 10 mM imidazole and separated with linear gradient of buffer A containing 50 to 500 mM NaCl. The purified fraction was desalted with a PD10 column (Amersham Pharmacia Biotech) equilibrated with transport buffer (20 mM HEPES [pH 7.3], 110 mM potassium acetate, 5 mM sodium acetate, 2 mM magnesium acetate, 0.5 mM EGTA, 2 mM dithiothreitol, 1 μg each of aprotinin, leupeptin, and pepstatin/ml) followed by concentration by ultrafiltration using Centricon YM 10 (Amicon).
Recombinant hCRM1 protein was expressed in E. coli strain BL21 (DE3) Gold without addition of IPTG at 30°C. The E. coli cells were lysed in buffer B (50 mM Tris-HCl [pH 8.0], 50 mM NaCl, 1 mM MgCL2, 1 mM dithiothreitol, 1 μg each of aprotinin, leupeptin, and pepstatin/ml) by freezing-thawing and sonication. After centrifugation (100,000 × g, 1 h), the clarified lysate was applied to a Hi-Trap Q column equilibrated with buffer B and separated with a linear gradient of buffer B containing 50 to 500 mM NaCl. The fractions containing recombinant hCRM1 protein were applied to Superdex 200 (Amersham Pharmacia Biotech) equilibrated with the transport buffer and then concentrated by ultrafiltration using Centricon YM 10.
Pull-down assay.
HeLa and REF52 cells (1.0 × 107) were scraped off, washed twice with 10 ml of ice-cold PBS, and lysed in 200 μl of buffer C (50 mM HEPES [pH 7.9], 200 mM KCl, 2 mM 2-mercaptoethanol, 0.4% Tween 20) containing 0.4% skim milk by sonication. After centrifugation at 20,000 × g for 10 min, the supernatants were transferred to new microcentrifuge tubes, and recombinant RanQ69L protein, GTP, and MgCl2 were added at final concentrations of 2 μM, 2 mM, and 5 mM, respectively. One-tenth volume of the supernatant was reserved as the input fraction. The remaining supernatants were then incubated at 4°C for 2 h with recombinant HisRex(g10) protein immobilized on chelating Sepharose. This conjugate was prepared by incubating 30 μl of Ni+-chelating Sepharose (reconstituted with 0.1 M NiSO4 solution and Chelating Sepharose Fast Flow; Amersham Pharmacia Biotech) with 3 μg of recombinant HisRex(g10) protein for 2 h at 4°C. The beads were washed three times with 1 ml of buffer C containing 5 mM MgCl2 by low-speed centrifugation, and then sample buffer was added to the beads (bound fraction). CRM1 proteins in the input and bound fractions were analyzed by SDS-PAGE followed by Western blotting.
Coimmunoprecipitation assay.
HeLa and REF52 cells (2.0 × 106) cultured on a 10-cm-diameter dish were transfected with GAL- and VP-fused protein expression plasmids. To adjust the expression level of fusion proteins, the following amounts of the expression plasmids were used: 1.5 μg of pGAL-Rex and pRex-VP (HeLa cells), 1.5 μg of pGAL-RexM90 and pRexM90-VP (HeLa cells), and 2 μg of pGAL-Rex and 4 μg of pRex-VP (REF52 cells). At 48 h posttransfection, the cells were lysed in 100 μl of buffer D (10 mM HEPES [pH 7.3], 150 mM NaCl, 3 mM MgCl2) by sonication. After centrifugation at 20,000 × g for 10 min, the supernatants were transferred to new microcentrifuge tubes and recombinant RanQ69L protein and GTP were added at final concentrations of 2 μM and 2 mM, respectively. Recombinant hCRM1 protein (at a final concentration of 100 nM) was also added in some samples. One-tenth volume of the supernatants was reserved as the input fraction. The remaining supernatants were then incubated with 2 μg of rabbit anti-VP antibody (Clontech) immobilized on 20 μl of protein G Sepharose at 4°C for 2 h. The beads were recovered by low-speed centrifugation and washed four times with 1 ml of buffer D, and then sample buffer was added to the beads (bound fraction). The fusion proteins in the input and bound fractions were analyzed by SDS-PAGE followed by Western blotting.
RESULTS
Poor activity of Rex in rat cells.
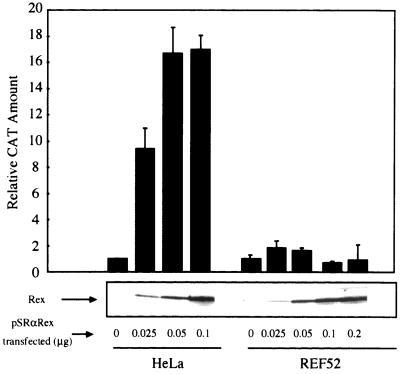
Unspliced and incompletely spliced viral mRNAs that, respectively, encode Gag and Env proteins are produced at much lower levels in HTLV-1-infected rat cells than in infected human cells despite the fact that Tax/Rex mRNA is abundantly produced in both cell types (28). On the basis of these observations, we hypothesized that Rex function may be impaired in rat cells. To test Rex function in rat and human cells, we cotransfected the rat REF52 and human HeLa cell lines with plasmids pDM128RxRE, a reporter, pSRαRex expressing the Rex protein, and pCDMβ-gal. Plasmid pDM128RxRE is a CAT-RxRE reporter construct that allows Rex activity to be quantitated in a transient expression system (11, 18) because this plasmid expresses the CAT protein in a manner that is dependent on Rex function. HeLa and REF52 were transfected with various amounts of the Rex expression plasmid, and the levels of CAT and Rex proteins in both cell types were evaluated by CAT enzyme-linked immunosorbent assay and Western blotting, respectively. Figure 1 shows that transfection of 0.05 μg of pSRαRex into HeLa cells enhanced production of CAT to a level about 17-fold more than that for the control sample without Rex. A greater amount of pSRαRex transfected had little effect although it produced a greater amount of the Rex protein. In contrast, in the rat cells Rex had almost no effect on CAT production in spite of an expression level of Rex protein that was sufficiently high. Increasing the amount of pSRαRex transfected to 0.5 μg did not induce CAT production in REF52 cells (data not shown). These results are consistent with an earlier observation (28) and indicate that Rex functions very poorly in rat cells.
FIG. 1.
Analysis of expression levels and activity of Rex in HeLa and REF52 cells. HeLa and REF52 cells were transfected with the indicated amounts of pSRαRex along with 0.5 μg of pDM128RxRE and 0.1 μg of pCDMβ-gal. A fraction of each sample was subjected to Western blotting to examine Rex protein synthesis, while the other fraction was used to measure Rex activity. The CAT/β-Gal ratios for all samples were calculated. The ratio for the control sample (HeLa cells transfected with pSRα296 instead of pSRαRex) was arbitrarily set at 1. Error bars, standard deviations.
We also tested whether the human immunodeficiency virus type 1 Rev protein, functional homologue to the Rex protein, works in REF52 cells by using pDM128, which allows Rev activity to be quantitated (18). Unlike the Rex protein, the Rev protein was able to enhance the production of CAT protein in a manner dependent on the levels of Rev expression in REF52 cells, although somewhat less efficiently than in HeLa cells (data not shown). These observations indicate that Rex activity is limited in REF52 cells to a greater degree than that of Rev. Therefore we did not investigate Rev function in rat cells anymore.
Cloning of rat CRM1 and expression.
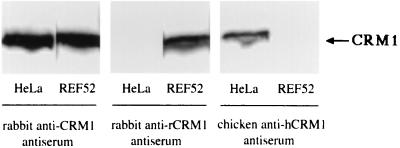
The major cofactor for Rex function is known to be CRM1, which plays crucial roles in both the export and multimerization of the Rex protein (11). To test whether the poor activity of Rex in rat cells may be due to the weaker ability of rCRM1 than of hCRM1 to support Rex functions, we cloned a cDNA encoding rCRM1. Sequence analysis revealed that the nucleotide sequence of the rcrm1 cDNA was 89% identical to that of hcrm1, while the predicted amino acid sequences were 97% identical, with only 24 aa being different (Fig. 2). In the next step, we generated antibodies that differentially recognize rCRM1 and hCRM1 on the basis of their carboxy-terminal amino acid sequences, where divergence between the two proteins is the highest. As shown in Fig. 3, Western blotting analyses demonstrated that our antibodies, termed rabbit anti-rCRM1 and chicken anti-hCRM1 antiserum, could distinguish between the two CRM1 proteins. In contrast, rabbit anti-CRM1 antiserum, elicited by a peptide whose sequence corresponds to aa 1024 to 1043 (30), recognized both rCRM1 and hCRM1. Similar blot patterns were observed for various human (Jurkat cells, U251 cells, astrocytes, CD4+T cells, CD8+ T cells, and macrophages) and rat (3Y1 cells) cell lines (data not shown). These blots also clearly showed that the rat and human cells contained similar amounts of CRM1.
FIG. 2.
Comparison of hCRM1 and rCRM1 amino acid sequences. The amino acids of rCRM1 different from those of hCRM1 are shown under the hCRM1 sequence in single-letter code.
FIG. 3.
Western blot analysis of endogenous hCRM1 and rCRM1 expression in HeLa and REF52 cells, respectively. Approximately 20 μg of total cellular protein was subjected to SDS-PAGE. Rabbit anti-CRM1, rabbit anti-rCRM1, or chicken anti-hCRM1 antiserum was used as the primary antibodies.
Effect of rCRM1 on Rex function.
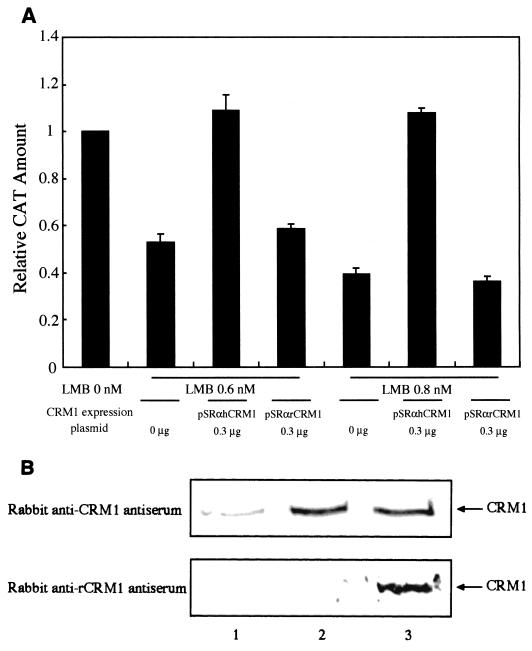
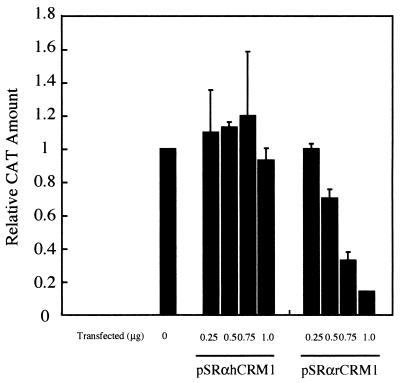
We investigated the capacity of rCRM1 to support Rex function relative to that of hCRM1. We measured Rex activity in REF52 cells cotransfected with pDM128RxRE, pSRαRex, and pCDMβ-gal along with various amounts of the hCRM1 or rCRM1 expression plasmid. Figure 4A shows that hCRM1 augmented Rex activity in a dose-dependent manner, whereas overexpression of rCRM1 had little effect. Western blotting of transfected REF52 cells using rabbit anti-CRM1 antiserum showed that nearly equivalent levels of CRM1 proteins are produced when the human and rat crm1 genes are transfected in rat cells (Fig. 4B). The fainter band seen in lane 1 indicates the endogenous rCRM1. Chicken anti-hCRM1 antiserum could also detect the expression of hCRM1 in REF52 cells. These results indicate that rCRM1 can only weakly support Rex function, suggesting that this may be the primary reason why Rex has limited activity in rat cells. It is also possible, however, that there may be another limiting factor that hampers the cofactor activity of rCRM1 but not hCRM1 in rat cells. To exclude this possibility, we tested the ability of rat and human CRM1 to support Rex function in human cells. HeLa cells cotransfected with pSRαRex, pDM128RxRE, pCDMβ-gal, and either pSRαhCRM1 or pSRαrCRM1 were placed in the presence of LMB 8 h posttransfection. LMB specifically inactivates CRM1 in HeLa cells (31), and thus it inhibits Rex activity. We found, however, that when hCRM1 is overexpressed, Rex activity is restored (Fig. 5A). In contrast, rCRM1 did not restore Rex activity even though nearly equivalent amounts of the two CRM1 proteins were produced exogenously, as shown by Western blotting (Fig. 5B). Rabbit anti-rCRM1 antiserum also demonstrated that rCRM1 is abundantly expressed in HeLa cells. It is unlikely that rCRM1 is much more sensitive to LMB than hCRM1 (see the description below; Table 1). Taken together, these results indicate that rCRM1, unlike hCRM1, has only very weak activity as a cofactor of Rex.
FIG. 4.
Effect of overexpressing rat and human CRM1 on Rex activity. (A) REF52 cells were transfected with 0.075 μg of pSRαRex along with 0.5 μg of pDM128RxRE and 0.1 μg of pCDMβ-gal in combination with various amounts of pSRαhCRM1 (⧫) or pSRαrCRM1 (▪). The CAT/β-Gal ratios of all samples were calculated. The ratio of the sample transfected with pSRαRex in the absence of either the hCRM1 or rCRM1 expression plasmid was arbitrarily set to 1. (B) CRM1 proteins produced in REF52 cells that were transfected with 0.3 μg of either rCRM1 or hCRM1 expression plasmids (lane 1, pSRα296; lane 2, pSRαhCRM1; lane 3, pSRαrCRM1) were analyzed by Western blotting as described in Materials and Methods.
FIG. 5.
Ability of hCRM1 and rCRM1 to support Rex function in HeLa cells. (A) HeLa cells were transfected with 0.05 μg of pSRαRex, 0.5 μg of pDM128RxRE, and 0.1 μg of pCDMβ-gal together with various amounts of pSRαhCRM1 or pSRαrCRM1. At 8 h posttransfection, the medium was replaced with medium containing LMB at 0.6 or 0.8 nM. At 24 h posttransfection, the cells were subjected to CAT and β-Gal measurement. The CAT/β-Gal ratios of all samples were calculated. The ratio of the control sample transfected with pSRαRex in the absence of either hCRM1 or rCRM1 expression plasmids and in the absence of LMB was arbitrarily set to 1. The amounts of CAT and the β-Gal activity in the control sample were over 300 pg and 2.5 × 10−3 U, respectively. (B) CRM1 proteins produced in HeLa cells transfected with 0.3 μg of each CRM1 expression plasmid (lane 1, pSRα296; lane 2, pSRαhCRM1; lane 3, pSRαrCRM1) were analyzed by Western blotting.
TABLE 1.
Effect of overexpression of hCRM1 and rCRM1 with or without LMB on subcellular localization of Rex in HeLa and REF52 cellsa
| Protein(s) | LMB concn (nM) | % Main localization for:
|
|||
|---|---|---|---|---|---|
| HeLa cells in:
|
REF52 cells in:
|
||||
| Nucleus | Cytoplasm | Nucleus | Cytoplasm | ||
| Rex | 0 | >99 | <1 | >99 | <1 |
| Rex + hCRM1 | 0 | 32 | 68 | 26 | 74 |
| Rex + rCRM1 | 0 | 31 | 69 | 16 | 84 |
| Rex + hCRM1 | 0.3 | 50 | 50 | 46 | 54 |
| Rex + rCRM1 | 0.3 | 67 | 43 | 44 | 56 |
| Rex + hCRM1 | 1.0 | 74 | 26 | 95 | 5 |
| Rex + rCRM1 | 1.0 | 67 | 33 | 84 | 16 |
| Rex + hCRM1 | 2.0 | 87 | 13 | 92 | 8 |
| Rex + rCRM1 | 2.0 | 81 | 19 | 95 | 5 |
| Rex + hCRM1 | 10 | 98 | 2 | >99 | <1 |
| Rex + rCRM1 | 10 | >99 | <1 | >99 | <1 |
HeLa and REF52 cells were transfected with Rex expression plasmid in combination with CRM1 expression plasmid as shown in Fig. 6. The transfected cells were treated with LMB at the indicated concentrations for 2 h before fixation with paraformaldehyde and were subjected to immunofluorescence microscopy. The stained cells whose nuclei or cytoplasms were mainly stained were counted, and their percentages were calculated. The typical examples of the cells in which Rex is mainly localized in the nucleus are shown in Fig. 6A, C, E, F, H, and J. The cells exhibiting main localization of Rex in the cytoplasm are depicted in Fig. 6B, D, G, and I. More than 200 cells were counted. Two independent experiments were done, and similar results were obtained.
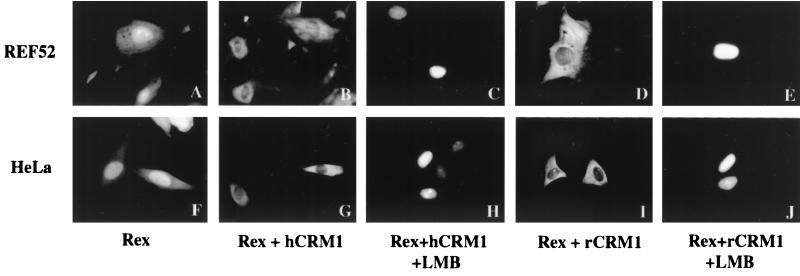
Both rat and human CRM1 proteins can export Rex to the cytoplasm.
As the data described above indicate that rCRM1 does not help Rex export RxRE-bearing mRNA, we wondered whether rCRM1 was even able to export the Rex protein itself. Subcellular localization studies of Rex in REF52 and HeLa cells transfected with pSRαRex, either alone or with plasmids expressing hCRM1 or rCRM1 (Fig. 6), showed that, in the absence of the exogenously expressed CRM1 proteins, the Rex protein was predominantly localized in the nucleus and nucleolus in HeLa cells (Fig. 6F). This was true also for REF52 cells (Fig. 6A). However, when either hCRM1 or rCRM1 was overexpressed by transfection of the relevant plasmids, the Rex protein was mainly detected in the cytoplasm; that is probably due to the enhanced transport to the cytoplasm by overexpression of its export receptor, CRM1 (Fig. 6B, D, G, and I). This notion was supported by the strong accumulation of the Rex protein in the nuclei in the presence of LMB even under the condition of the overexpression of hCRM1 or rCRM1 (Fig. 6C, E, H, and J). Furthermore it is consistent with the previous report showing cytoplasmic localization of Rex to be dependent on the presence of an intact NES domain in Rex (11). Thus, our data indicate that both rCRM1 and hCRM1 are able to support Rex protein export. When the immunofluorescence microscopy was done quantitatively, it appeared that the rat and human CRM1 proteins were equivalent in their abilities to help the Rex protein move into the cytoplasm (Table 1). When the cells overexpressing either hCRM1 or rCRM1 were treated with LMB at various concentrations, the enhanced transport of Rex to the cytoplasm, which was otherwise induced by overexpressing CRM1s, was similarly inhibited in a manner dependent on LMB concentration (Table 1). These results indicated that hCRM1 and rCRM1 have similar sensitivities to LMB.
FIG. 6.
Subcellular localization of Rex in REF52 and HeLa cells by immunofluorescence microscopy. REF52 and HeLa cells were transfected with 0.1 μg of pSRαRex (A and F) or with 0.25 μg of either pSRαhCRM1 (B and G) or pSRαrCRM1 (D and I). Some transfected samples were treated with LMB at 2 nM for 2 h before fixation with paraformaldehyde (C, E, H, and J). The procedure for immunofluorescence analysis was done as indicated in Materials and Methods.
Rex protein-binding affinities of rCRM1 and hCRM1.
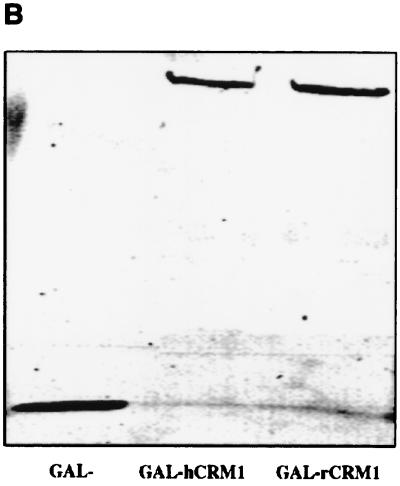
Next, we examined the Rex protein-binding affinities of the rat and human CRM1s in rat cells by the two-hybrid assay. Here rat and human CRM1 proteins were expressed as GAL fusion proteins in the presence of the Rex-VP fusion protein. The Luc/β-Gal ratios indicated that rCRM1 interacts with Rex as efficiently as hCRM1 (Fig. 7A). Western blotting using a mouse anti-GAL4 antibody indicated that the rat and human GAL-CRM1 fusion proteins were expressed at equivalent levels (Fig. 7B). rCRM1 bound much less efficiently to NES mutant RexM90 than to wild-type Rex, indicating that, as for hCRM1, the binding of rCRM1 to Rex is specifically mediated by the Rex NES (data not shown).
FIG. 7.
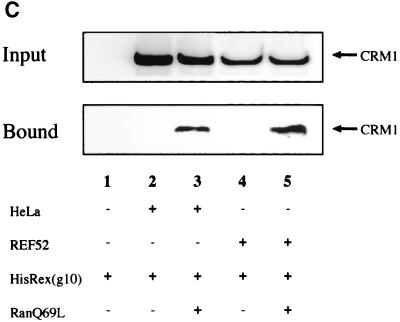
Interaction of Rex with either hCRM1 or rCRM1. (A) REF52 cells were transfected with the plasmid expressing either GAL-hCRM1 or GAL-rCRM1 in combination with pRexVP, pG5BLuc, and pCDMβ-gal. The Luc/β-Gal ratios of all samples were calculated. GAL-, plasmid expressing only the GAL4 region, which was used as a negative control. (B) Western blot of GAL-hCRM1 and GAL-rCRM1 proteins synthesized in transfected REF52 cells. (C) Pull-down assay using recombinant Rex protein. Purified His-Rex(g10) proteins immobilized on chelating Sepharose were incubated with HeLa or REF52 cell extract in the absence (lanes 2 and 4) or presence (lanes 3 and 5) of GTP-charged recombinant RanQ69L protein. The sample of lane 1 contained neither cell lysate nor recombinant RanQ69L proteins during incubation. The volume of the cell extract subjected to the binding reaction was nine times of that of the input fraction.
To ascertain the binding of Rex to each CRM1, a pull-down assay using a recombinant Rex protein was done. As shown in Fig. 7C, Rex bound to hCRM1 and rCRM1 with similar efficiencies in the presence of GTP-charged RanQ69L protein, a GTPase-deficient Ran mutant. The requirement for Ran-GTP guarantees specific binding of both hCRM1 and rCRM1 to Rex since the trimeric complex of CRM1, leucine-rich NES, and Ran-GTP has been well documented (7, 39, 45). These results coincide with the results of the two-hybrid assay (Fig. 7A). Together with the indirect immunofluorescence data described above (Fig. 6 and Table 1), our data suggest that rCRM1 can bind to and export Rex protein as efficiently as hCRM1 in rat cells.
The dominant-negative effect of rCRM1.
The facts that rCRM1 is able to interact with the Rex protein but did not support its function might suggest a dominant-negative effect of rCRM1 over hCRM1 in Rex functioning. To assess this possibility, HeLa cells were transfected with pSRαRex along with various amounts of pSRαhCRM1 or pSRαrCRM1. As expected, the activity of Rex was not affected by overexpression of hCRM1. On the other hand, Rex activity was significantly reduced by overexpression of rCRM1 in a dose-dependent manner (Fig. 8). This result suggests that rCRM1 has dominant-negative phenotype and is one more piece of evidence supporting the idea that rCRM1 hardly works as a cofactor of Rex although rCRM1 can interact with the Rex protein similarly to hCRM1.
FIG. 8.
The dominant-negative effect of rCRM1 on Rex activity in HeLa cells. HeLa cells were transfected with 0.05 μg of pSRαRex, 0.5 μg of pDM128RxRE, and 0.1 μg of pCDMβ-gal together with various amounts of pSRαhCRM1 or pSRαrCRM1. At 24 h posttransfection, the cells were subjected to CAT and β-Gal measurement. The CAT/β-Gal ratios of all samples were calculated. The ratio of the control sample transfected with pSRαRex in the absence of either hCRM1 or rCRM1 expression plasmid was arbitrarily set to 1. The amounts of CAT and the β-Gal activity in the control sample were over 350 pg and 3.0 × 10−3 U, respectively.
rCRM1 does not support a Rex-Rex interaction.
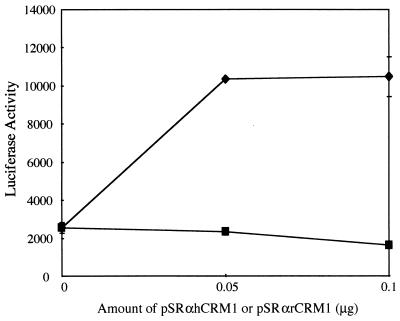
It has been reported that Rex has to multimerize on the cognate RNA before the complex can be transported to the cytoplasm. This multimerization depends on the ability of Rex proteins to form homodimers via protein-protein interaction. We had previously reported that hCRM1 contributes to the Rex-Rex interaction (11). To evaluate whether rCRM1 can similarly support the Rex-Rex interaction, a two-hybrid assay wherein both Gal-Rex and Rex-VP fusion proteins were coexpressed in REF52 cells was employed (Fig. 9). In the absence of the exogenously expressed CRM1 protein, the extent of the Rex-Rex interaction was very small because it was similar to that of the negative control in which GAL- and Rex-VP were coexpressed (data not shown). But overexpression of hCRM1 efficiently enhanced the Rex-Rex interaction. However, when rCRM1 was overexpressed, the enhancement of Rex-Rex interactions could hardly be detected. The overexpressed CRM1 proteins in each sample were confirmed by Western blotting (data not shown).
FIG. 9.
Induction of Rex-Rex dimerization by hCRM1 but not rCRM1 in REF52 cells. REF52 cells were transfected with pGALRex, pRexVP, pG5BLuc, and pCDMβ-gal in combination with various amounts of pSRαhCRM1 (⧫) or pSRαrCRM1 (▪). At 24 h posttransfection, the cells were harvested to measure the luciferase and β-Gal activities. The Luc/β-Gal ratios of all samples were calculated.
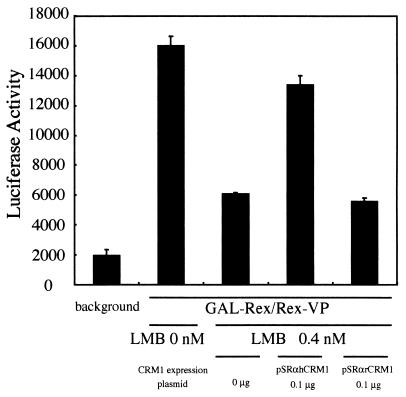
As LMB has been reported to specifically inhibit CRM1 function (31), we tested the effect of LMB on the Rex-Rex interaction in HeLa cells. The Rex-Rex interaction in the presence of 0.4 nM LMB was reduced by 40% relative to the value in the absence of LMB (Fig. 10), which confirms that Rex dimerization requires hCRM1. We next examined whether Rex-Rex interactions that had been inhibited by LMB could be restored by overexpression of rCRM1 or hCRM1. We found that hCRM1 could almost completely restore Rex-Rex interactions but that rCRM1 could not (Fig. 10). Similar results were obtained when 0.6 nM LMB was used (data not shown). Thus, it appears that rCRM1 is unable to support the Rex-Rex interaction.
FIG. 10.
Restoration of Rex-Rex dimerization in HeLa cells by overexpression of hCRM1 but not rCRM1. HeLa cells were transfected with pGALRex, pRexVP, pG5BLuc, and pCDMβ-gal in combination with 0.1 μg of pSRαhCRM1 or pSRαrCRM1. At 8 h posttransfection, treatment with 0.4 nM LMB commenced. At 24 h posttransfection, the cells were harvested and the Luc/β-Gal ratios of all samples were calculated.
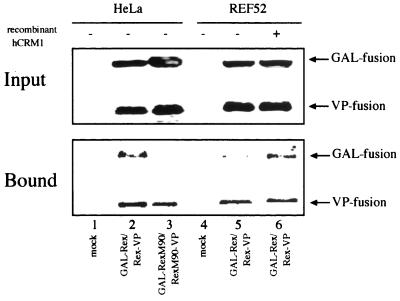
To confirm the above results, the interaction between differently tagged Rex proteins was examined by the coimmunoprecipitation method. HeLa and REF52 cells were transfected with GAL-Rex and Rex-VP expression plasmids. As a control, RexM90 fusion derivatives were used since RexM90 could not form dimers, as judged by a two-hybrid assay (11). The cell extracts were subjected to immunoprecipitation with an anti-VP16 antibody in the presence of GTP-charged RanQ69L, and the coprecipitated GAL-fused proteins were evaluated by Western blotting. The recombinant RanQ69L protein was used since it has been suggested that Rex-Rex dimerization requires the interaction of Rex with CRM1 and, consequently, Ran-GTP. GAL-Rex was coimmunoprecipitated with Rex-VP in HeLa cell lysate but not in REF52 cell lysate (Fig. 11, lanes 2 and 5). In contrast, GAL-RexM90 was not coimmunoprecipitated with RexM90-VP (Fig. 11, lane 3), consistent with our previous two-hybrid data (11). Another multimerization-deficient mutant, RexM64, was not coprecipitated as expected (data not shown). Addition of the recombinant hCRM1 protein to REF52 cell lysate during incubation enhanced coimmunoprecipitation of GAL-Rex compared to that for the sample without the recombinant hCRM1 protein (Fig. 11, lane 5 and 6). These results indicate that the ability of rCRM1 to support the Rex-Rex interaction is considerably less than that of hCRM1 although both CRM1s can bind efficiently to the Rex protein. This property of rCRM1 may be responsible for the inability of the Rex protein in rat cells to transport gag/env mRNAs into the cytoplasm.
FIG. 11.
Rex-Rex dimerization analyzed by the coimmunoprecipitation method. HeLa and REF52 cells were transfected with the plasmids which express GAL-Rex and Rex-VP or their derivatives as indicated. At 48 h posttransfection, the cells were harvested and the supernatants were incubated with anti-VP antibodies immobilized on protein-G Sepharose in the presence of GTP-charged recombinant RanQ69L. The recombinant hCRM1 protein was added to the sample, which was derived from the REF52 cells transfected with GAL-Rex and Rex-VP (lane 6) during incubation. The volume of the cell extract subjected to the coimmunoprecipitation reaction was nine times of that of input fraction. Rabbit anti-Rex C terminus antibodies were used for Western blotting.
DISCUSSION
In this study, we aimed to determine why HTLV-1 replicates so poorly in rats. We particularly focused on Rex protein function in rat cells because Koya et al. have reported that, although Tax/Rex-encoding mRNA is abundant in rat cells, the viral mRNAs encoding Gag and Env proteins are produced at low levels (28). We show here that Rex functions very inefficiently in rat cells because rCRM1, unlike hCRM1, works very poorly as a cofactor for Rex activity. We found that rCRM1 binds to and exports the Rex protein into the cytoplasm as efficiently as hCRM1 (Fig. 6 and 7 and Table 1), indicating that rCRM1 may be able to act as an export receptor for the Rex protein and other NES-bearing proteins. LMB sensitivities of hCRM1 and rCRM1 seem to be quite similar (Fig. 6 and Table 1), coincident with conservation of the amino acid sequence around the cysteine residue at aa 528, which may be a binding site of LMB (31) (Fig. 2). On the other hand, rCRM1 is unable to induce Rex-Rex homodimer formation (Fig. 9 to 11), which is required for Rex multimerization on target RNA (2) destined for export to the cytoplasm. Thus, the limited ability of rCRM1 to act as a cofactor for Rex may be responsible for the poor replication of HTLV-1 in rats. Our results suggest that better rat models of HTLV-1 infection could be developed by constructing transgenic rats expressing hCRM1. This notion is partly supported by our observation that, as shown in Fig. 4A, overexpression of hCRM1 could restore Rex activity in rat cells. Formal proof of this concept would be the observation that HTLV-1 actually does replicate better in rat cells that express hCRM1. However, a possible problem with such transgenic rat models may be that the presence of rCRM1 in rat cells could have a dominant-negative effect on hCRM1 because rCRM1 binds to Rex as efficiently as hCRM1 does. This dominant-negative effect on Rex activity was detected when rCRM1 was overexpressed in HeLa cells, although a relatively large amount of rCRM1 was required (Fig. 8). Thus, more investigation is necessary to optimize the conditions under which Rex will fully function in rat cells.
With respect to the formation of the transport complex composed of viral RNA, Rex, and possibly other cellular factors, we believe that either a Rex–hCRM1–Ran-GTP complex initiates binding to the high-affinity site contained in RxRE or that Rex binds to the high-affinity site at the same time as it associates with hCRM1 with the aid of Ran-GTP. Following this step, additional Rex molecules then bind to the viral RNA through its nonspecific RNA-binding activity (13), eventually provoking Rex polymerization along the viral RNA transcript via its multimerization domain with the aid of hCRM1. This process would allow a number of Rex proteins to cover the RNA, thus increasing the number of hCRM1 molecules that associate with the complex. The association of multiple CRM1 molecules may be important to overcome the factors that retain RNAs in the nucleus (34) and would allow the RNAs to pass efficiently through nuclear pores.
Two possible mechanisms by which CRM1 induces Rex multimerization can be envisaged. The association of CRM1 with the NES region of the Rex protein may trigger a conformational change in Rex that leads directly to its multimerization. Alternatively, the binding of CRM1 to the NES may be necessary but not sufficient, and additional interactions between Rex and CRM1 with or without the aid of another putative factor may be required for multimerization. The second possibility is supported by our observation that rCRM1 binds Rex as efficiently as hCRM1 but does not support Rex multimerization, which indicates that Rex multimerization induced by CRM1 is separable from the simple binding of Rex NES to CRM1. We infer that hCRM1 may possess a domain required for its additional interaction with Rex to induce the Rex-Rex interaction. As rCRM1 differs from hCRM1 by only 24 aa, these two proteins are particularly suitable for future research into the structure-function relationships involved in Rex-Rex interaction induced by CRM1.
Our observations underscore the importance of Rex multimerization in RNA export. Rex-dependent RNA export is different from Rex protein export in that Rex multimerization is essential for successful RNA export. Multimerization may be a general feature of RNA-binding proteins that are involved in RNA metabolism, since hnRNP A1, C1, E2, I, K, and L proteins have all been reported to multimerize (27). Although the role and mechanism of multimerization of these proteins have not been studied in detail, studies investigating the multimerization of Rex may provide insights into these phenomena.
ACKNOWLEDGMENTS
We thank M. Yoshida for LMB and anti-CRM1 antiserum, T. Kanno for REF52 cells, M. Komoda for pG5BLuc, Y. Yoneda for pET3dRanQ69L, D. Görlich for pET3ahCRM1, J. Katahira for HisRex(g10), K. Nishiike and F. Kokusen for excellent technical assistance, and Y. Okuda and A. Okuhara for manuscript preparation.
This investigation was supported by grants from the Ministry of Sports and Culture (Japan) and the Ministry of Health and Welfare (Japan). Y. Hakata is a JSPS Research Fellow.
REFERENCES
- 1.Ballaun C, Farrington G K, Dobrovnik M, Rusche J, Hauber J, Bohnlein E. Functional analysis of human T-cell leukemia virus type I rex-response element: direct RNA binding of Rex protein correlates with in vivo activity. J Virol. 1991;65:4408–4413. doi: 10.1128/jvi.65.8.4408-4413.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogerd H, Greene W C. Dominant negative mutants of human T-cell leukemia virus type I Rex and human immunodeficiency virus type 1 Rev fail to multimerize in vivo. J Virol. 1993;67:2496–2502. doi: 10.1128/jvi.67.5.2496-2502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogerd H P, Fridell R A, Benson R E, Hua J, Cullen B R. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogerd H P, Huckaby G L, Ahmed Y F, Hanly S M, Greene W C. The type I human T-cell leukemia virus (HTLV-I) Rex trans-activator binds directly to the HTLV-I Rex and the type 1 human immunodeficiency virus Rev RNA response elements. Proc Natl Acad Sci USA. 1991;88:5704–5708. doi: 10.1073/pnas.88.13.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnlein E, Berger J, Hauber J. Functional mapping of the human immunodeficiency virus type 1 Rev RNA binding domain: new insights into the domain structure of Rev and Rex. J Virol. 1991;65:7051–7055. doi: 10.1128/jvi.65.12.7051-7055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang J, Kushida S, Feng R, Tanaka M, Kawamura T, Abe H, Maeda N, Onobori M, Hori M, Uchida K, Miwa M. Transmission of human T-cell leukemia virus type 1 to mice. J Virol. 1998;72:3952–3957. doi: 10.1128/jvi.72.5.3952-3957.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 8.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 10.Grassmann R, Berchtold S, Aepinus C, Ballaun C, Boehnlein E, Fleckenstein B. In vitro binding of human T-cell leukemia virus rex proteins to the rex-response element of viral transcripts. J Virol. 1991;65:3721–3727. doi: 10.1128/jvi.65.7.3721-3727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hakata Y, Umemoto T, Matsushita S, Shida H. Involvement of human CRM1 (exportin 1) in the export and multimerization of the Rex protein of human T-cell leukemia virus type 1. J Virol. 1998;72:6602–6607. doi: 10.1128/jvi.72.8.6602-6607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanly S M, Rimsky L T, Malim M H, Kim J H, Hauber J, Duc Dodon M, Le S Y, Maizel J V, Cullen B R, Greene W C. Comparative analysis of the HTLV-I Rex and HIV-1 Rev trans-regulatory proteins and their RNA response elements. Genes Dev. 1989;3:1534–1544. doi: 10.1101/gad.3.10.1534. [DOI] [PubMed] [Google Scholar]
- 13.Heaphy S, Dingwall C, Ernberg I, Gait M J, Green S M, Karn J, Lowe A D, Singh M, Skinner M A. HIV-1 regulator of virion expression (Rev) protein binds to an RNA stem-loop structure located within the Rev response element region. Cell. 1990;60:685–693. doi: 10.1016/0092-8674(90)90671-z. [DOI] [PubMed] [Google Scholar]
- 14.Heger P, Rosorius O, Koch C, Casari G, Grassmann R, Hauber J. Multimer formation is not essential for nuclear export of human T-cell leukemia virus type 1 Rex trans-activator protein. J Virol. 1998;72:8659–8668. doi: 10.1128/jvi.72.11.8659-8668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidaka M, Inoue J, Yoshida M, Seiki M. Post-transcriptional regulator (rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 1988;7:519–523. doi: 10.1002/j.1460-2075.1988.tb02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hieda M, Tachibana T, Yokoya F, Kose S, Imamoto N, Yoneda Y. A monoclonal antibody to the COOH-terminal acidic portion of ran inhibits both the recycling of ran and nuclear protein import in living cells. J Cell Biol. 1999;144:645–655. doi: 10.1083/jcb.144.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K I, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hope T J, Huang X J, McDonald D, Parslow T G. Steroid-receptor fusion of the human immunodeficiency virus type 1 Rev transactivator: mapping cryptic functions of the arginine-rich motif. Proc Natl Acad Sci USA. 1990;87:7787–7791. doi: 10.1073/pnas.87.19.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibuki K, Funahashi S I, Yamamoto H, Nakamura M, Igarashi T, Miura T, Ido E, Hayami M, Shida H. Long-term persistence of protective immunity in cynomolgus monkeys immunized with a recombinant vaccinia virus expressing the human T cell leukemia virus type I envelope gene. J Gen Virol. 1997;78:147–152. doi: 10.1099/0022-1317-78-1-147. [DOI] [PubMed] [Google Scholar]
- 20.Inoue J, Yoshida M, Seiki M. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc Natl Acad Sci USA. 1987;84:3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishiguro N, Abe M, Seto K, Sakurai H, Ikeda H, Wakisaka A, Togashi T, Tateno M, Yoshiki T. A rat model of human T lymphocyte virus type I (HTLV-I) infection. 1. Humoral antibody response, provirus integration, and HTLV-I-associated myelopathy/tropical spastic paraparesis-like myelopathy in seronegative HTLV-I carrier rats. J Exp Med. 1992;176:981–989. doi: 10.1084/jem.176.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson S, Raine C S, Mingioli E S, McFarlin D E. Isolation of an HTLV-1-like retrovirus from patients with tropical spastic paraparesis. Nature. 1988;331:540–543. doi: 10.1038/331540a0. [DOI] [PubMed] [Google Scholar]
- 23.Kalyanaraman V S, Sarngadharan M G, Nakao Y, Ito Y, Aoki T, Gallo R C. Natural antibodies to the structural core protein (p24) of the human T-cell leukemia (lymphoma) retrovirus found in sera of leukemia patients in Japan. Proc Natl Acad Sci USA. 1982;79:1653–1657. doi: 10.1073/pnas.79.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katahira J, Ishizaki T, Sakai H, Adachi A, Yamamoto K, Shida H. Effects of translation initiation factor eIF-5A on the functioning of human T-cell leukemia virus type I Rex and human immunodeficiency virus Rev inhibited trans-dominantly by a Rex mutant deficient in RNA binding. J Virol. 1995;69:3125–3133. doi: 10.1128/jvi.69.5.3125-3133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato H, Koya Y, Ohashi T, Hanabuchi S, Takemura F, Fujii M, Tsujimoto H, Hasegawa A, Kannagi M. Oral administration of human T-cell leukemia virus type 1 induces immune unresponsiveness with persistent infection in adult rats. J Virol. 1998;72:7289–7293. doi: 10.1128/jvi.72.9.7289-7293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim F J, Beeche A A, Hunter J J, Chin D J, Hope T J. Characterization of the nuclear export signal of human T-cell lymphotropic virus type 1 Rex reveals that nuclear export is mediated by position-variable hydrophobic interactions. Mol Cell Biol. 1996;16:5147–5155. doi: 10.1128/mcb.16.9.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J H, Hahm B, Kim Y K, Choi M, Jang S K. Protein-protein interaction among hnRNPs shuttling between nucleus and cytoplasm. J Mol Biol. 2000;298:395–405. doi: 10.1006/jmbi.2000.3687. [DOI] [PubMed] [Google Scholar]
- 28.Koya Y, Ohashi T, Kato H, Hanabuchi S, Tsukahara T, Takemura F, Etoh K, Matsuoka M, Fujii M, Kannagi M. Establishment of a seronegative human T-cell leukemia virus type 1 (HTLV-1) carrier state in rats inoculated with a syngeneic HTLV-1-immortalized T-cell line preferentially expressing Tax. J Virol. 1999;73:6436–6443. doi: 10.1128/jvi.73.8.6436-6443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota S, Nosaka T, Cullen B R, Maki M, Hatanaka M. Effects of chimeric mutants of human immunodeficiency virus type 1 Rev and human T-cell leukemia virus type I Rex on nucleolar targeting signals. J Virol. 1991;65:2452–2456. doi: 10.1128/jvi.65.5.2452-2456.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo N, Khochbin S, Nishi K, Kitano K, Yanagida M, Yoshida M, Horinouchi S. Molecular cloning and cell cycle-dependent expression of mammalian CRM1, a protein involved in nuclear export of proteins. J Biol Chem. 1997;272:29742–29751. doi: 10.1074/jbc.272.47.29742. [DOI] [PubMed] [Google Scholar]
- 31.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner E P, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushida S, Mizusawa H, Matsumura M, Tanaka H, Ami Y, Hori M, Yagami K, Kameyama T, Tanaka Y, Yoshida A, Nyunoya H, Shimotono K, Iwasaki Y, Uchida K, Miwa M. High incidence of HAM/TSP-like symptoms in WKA rats after administration of human T-cell leukemia virus type 1-producing cells. J Virol. 1994;68:7221–7226. doi: 10.1128/jvi.68.11.7221-7226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q X, Camerini D, Xie Y, Greenwald M, Kuritzkes D R, Chen I S. Syncytium formation by recombinant HTLV-II envelope glycoprotein. Virology. 1996;218:279–284. doi: 10.1006/viro.1996.0192. [DOI] [PubMed] [Google Scholar]
- 34.Nakielny S, Dreyfuss G. The hnRNP C proteins contain a nuclear retention sequence that can override nuclear export signals. J Cell Biol. 1996;134:1365–1373. doi: 10.1083/jcb.134.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neville M, Stutz F, Lee L, Davis L I, Rosbash M. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr Biol. 1997;7:767–775. doi: 10.1016/s0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 36.Nosaka T, Siomi H, Adachi Y, Ishibashi M, Kubota S, Maki M, Hatanaka M. Nucleolar targeting signal of human T-cell leukemia virus type I rex-encoded protein is essential for cytoplasmic accumulation of unspliced viral mRNA. Proc Natl Acad Sci USA. 1989;86:9798–9802. doi: 10.1073/pnas.86.24.9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi T, Hanabuchi S, Kato H, Koya Y, Takemura F, Hirokawa K, Yoshiki T, Tanaka Y, Fujii M, Kannagi M. Induction of adult T-cell leukemia-like lymphoproliferative disease and its inhibition by adoptive immunotherapy in T-cell-deficient nude rats inoculated with syngeneic human T-cell leukemia virus type 1-immortalized cells. J Virol. 1999;73:6031–6040. doi: 10.1128/jvi.73.7.6031-6040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 39.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 40.Paraskeva E, Izaurralde E, Bischoff F R, Huber J, Kutay U, Hartmann E, Lührmann R, Görlich D. CRM1-mediated recycling of snurportin 1 to the cytoplasm. J Cell Biol. 1999;145:255–264. doi: 10.1083/jcb.145.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rimsky L, Dodon M D, Dixon E P, Greene W C. Trans-dominant inactivation of HTLV-I and HIV-1 gene expression by mutation of the HTLV-I Rex transactivator. Nature. 1989;341:453–456. doi: 10.1038/341453a0. [DOI] [PubMed] [Google Scholar]
- 42.Seiki M, Inoue J, Hidaka M, Yoshida M. Two cis-acting elements responsible for posttranscriptional trans-regulation of gene expression of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:7124–7128. doi: 10.1073/pnas.85.19.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shida H, Tochikura T, Sato T, Konno T, Hirayoshi K, Seki M, Ito Y, Hatanaka M, Hinuma Y, Sugimoto M, Nishmaki F-T, Maruyama T, Miki K, Suzuki K, Morita M, Sashiyama H, Yoshimura N, Hayami M. Effect of the recombinant vaccinia viruses that express HTLV-I envelope gene on HTLV-I infection. EMBO J. 1987;6:3379–3384. doi: 10.1002/j.1460-2075.1987.tb02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siomi H, Shida H, Nam S H, Nosaka T, Maki M, Hatanaka M. Sequence requirements for nucleolar localization of human T cell leukemia virus type I pX protein, which regulates viral RNA processing. Cell. 1988;55:197–209. doi: 10.1016/0092-8674(88)90043-8. [DOI] [PubMed] [Google Scholar]
- 45.Stade K, Ford C S, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 46.Sutton R E, Littman D R. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J Virol. 1996;70:7322–7326. doi: 10.1128/jvi.70.10.7322-7326.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka Y, Tanaka R, Terada E, Koyanagi Y, Miyano-Kurosaki N, Yamamoto N, Baba E, Nakamura M, Shida H. Induction of antibody responses that neutralize human T-cell leukemia virus type I infection in vitro and in vivo by peptide immunization. J Virol. 1994;68:6323–6331. doi: 10.1128/jvi.68.10.6323-6331.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uemura Y, Kotani S, Yoshimoto S, Fujishita M, Yano S, Ohtsuki Y, Miyoshi I. Oral transmission of human T-cell leukemia virus type I in the rabbit. Jpn J Cancer Res. 1986;77:970–973. [PubMed] [Google Scholar]
- 50.Weichselbraun I, Berger J, Dobrovnik M, Bogerd H, Grassmann R, Greene W C, Hauber J, Bohnlein E. Dominant-negative mutants are clustered in a domain of the human T-cell leukemia virus type I Rex protein: implications for trans dominance. J Virol. 1992;66:4540–4545. doi: 10.1128/jvi.66.7.4540-4545.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida M, Suzuki T, Fujisawa J, Hirai H. HTLV-1 oncoprotein tax and cellular transcription factors. Curr Top Microbiol Immunol. 1995;193:79–89. doi: 10.1007/978-3-642-78929-8_4. [DOI] [PubMed] [Google Scholar]