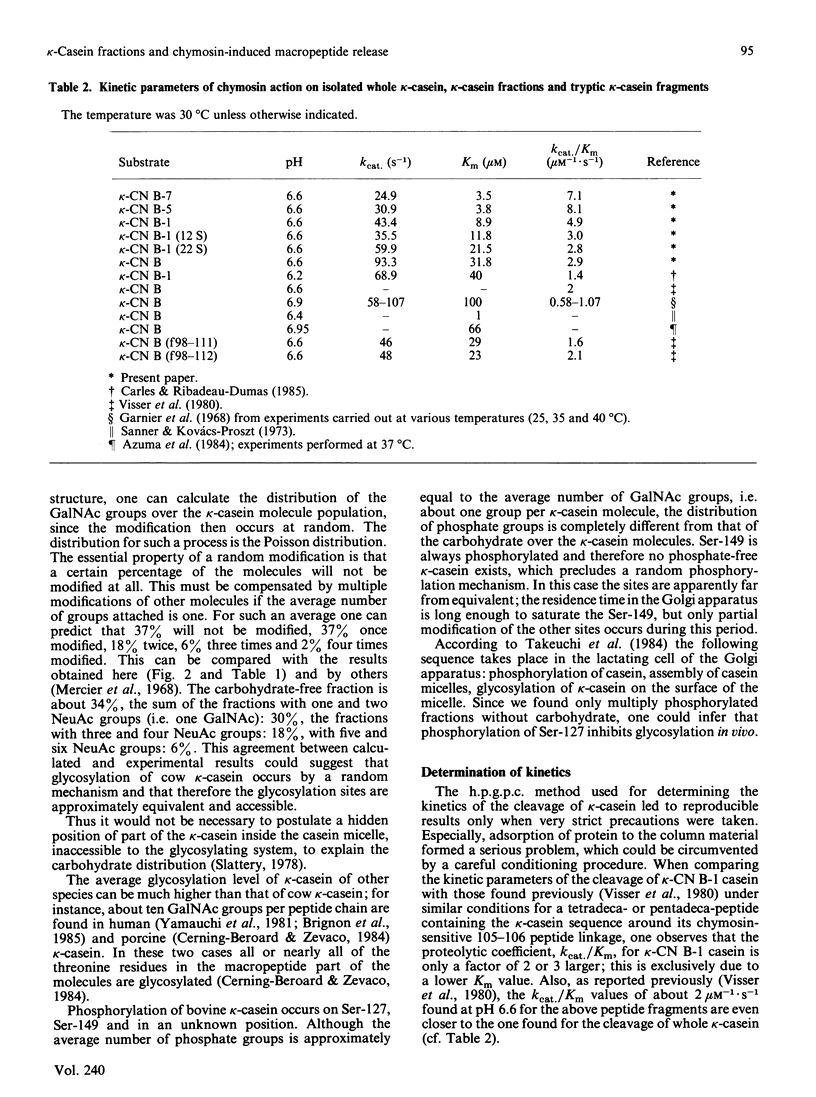
Abstract
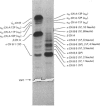
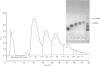
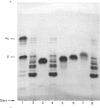
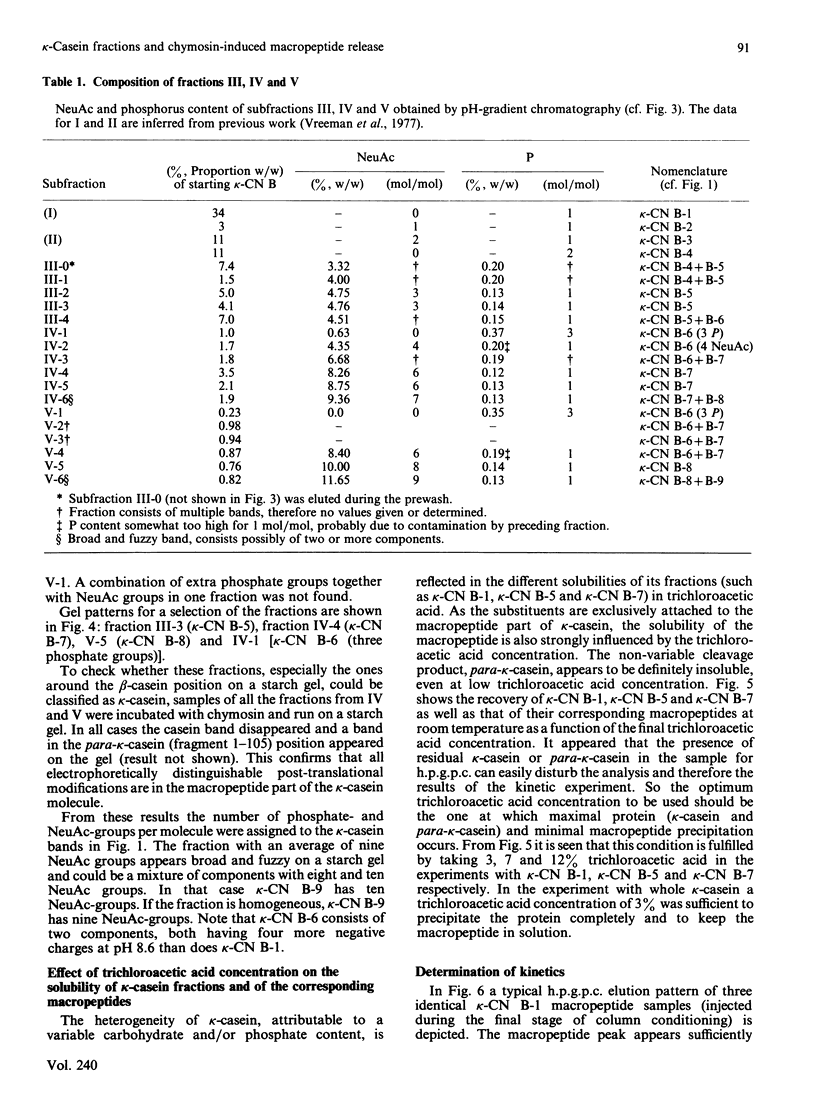
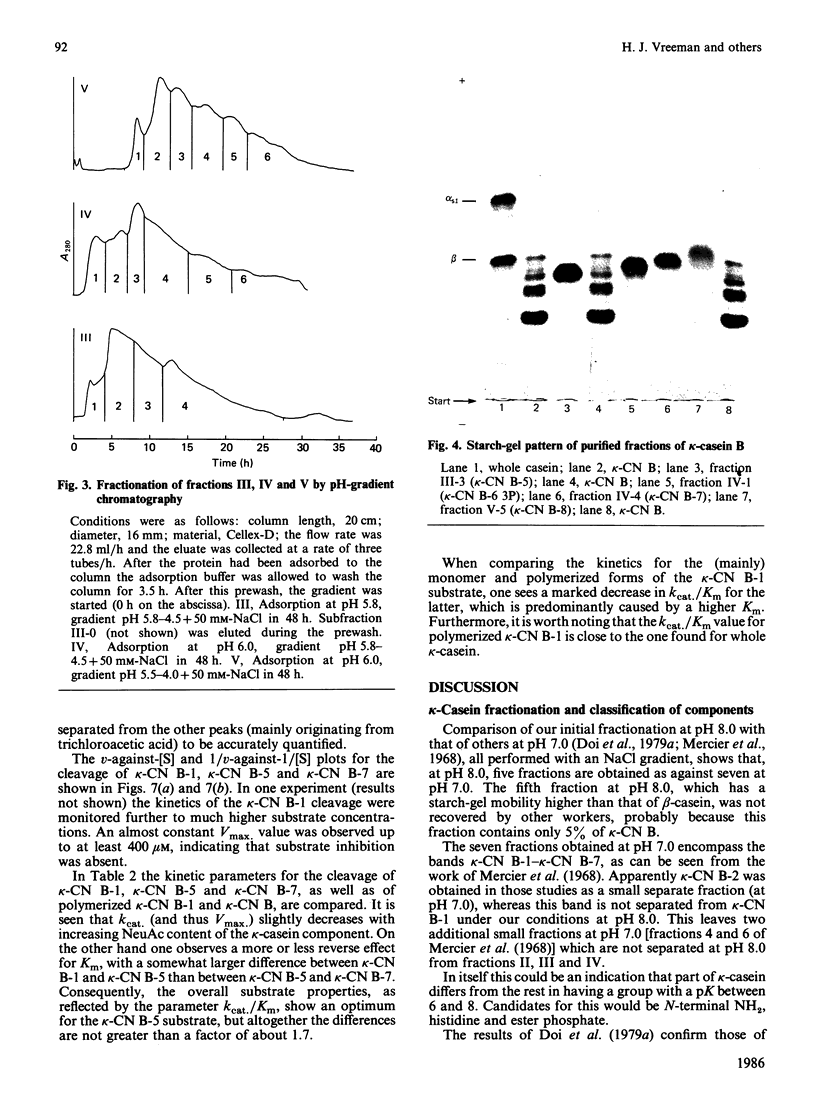
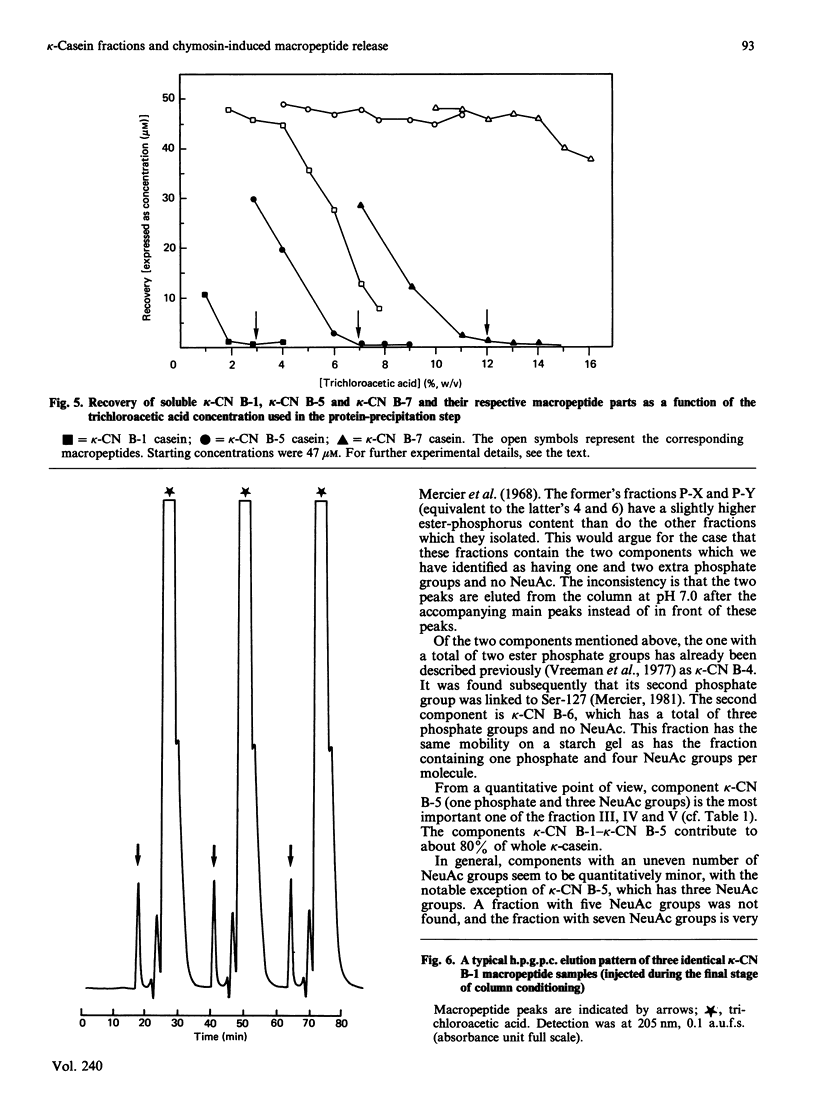
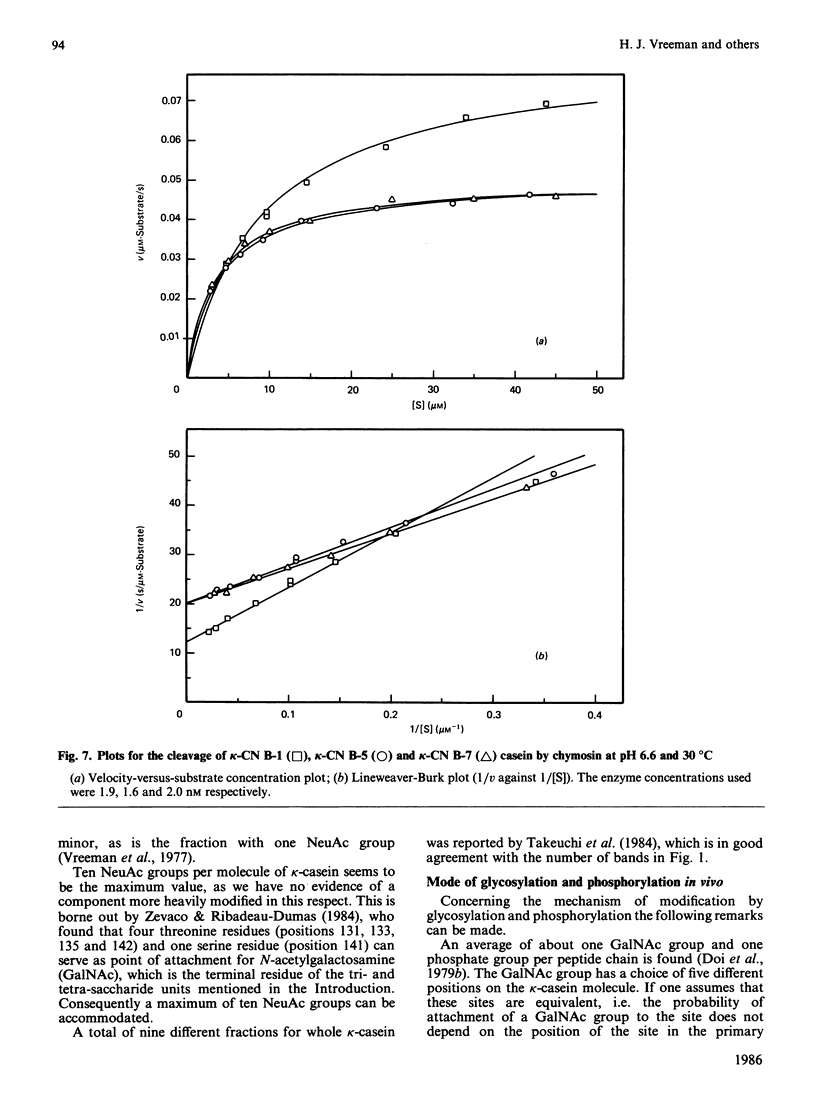
Bovine kappa-casein was fractionated at pH 8.0 on DEAE-Sepharose with an NaCl gradient, followed by DEAE-cellulose chromatography using a decreasing pH gradient from pH 6.0 to 4.5. At least ten components could be identified, each differing in N-acetylneuraminic acid (NeuAc) and/or phosphorus content. Two components appeared to be multiply-phosphorylated, but did not contain NeuAc. The possible significance of this finding in relation to the mode of phosphorylation and glycosylation in vivo is discussed. A carbohydrate-free fraction as well as two NeuAc-containing fractions were compared in their substrate behaviour towards the action of the milk-clotting enzyme chymosin at pH 6.6 and 30 degrees C. To this end the trichloroacetic acid-soluble reaction products were analysed by high-performance gel-permeation chromatography. In order of increasing carbohydrate content the kcat. values found ranged from 40 to 25 s-1 and the Km values from 9 to 3 microM; the overall substrate properties of these components as reflected by the kinetic parameter kcat./Km ranged from 5 to 8 microM-1 X S-1. Irreversible polymerization of the carbohydrate-free fraction brought about a more-than-2-fold increase in Km, the kcat. value remaining virtually constant. The kcat./Km found for the cleavage of whole kappa-casein at pH 6.6 was of the same magnitude as the kcat./Km found for the polymerized carbohydrate-free fraction (i.e. about 3 microM-1 X S-1). No indication of substrate inhibition was found for the carbohydrate-free fraction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brignon G., Chtourou A., Ribadeau-Dumas B. Preparation and amino acid sequence of human kappa-casein. FEBS Lett. 1985 Aug 19;188(1):48–54. doi: 10.1016/0014-5793(85)80872-3. [DOI] [PubMed] [Google Scholar]
- Carles C., Ribadeau Dumas B. Kinetics of the action of chymosin (rennin) on a peptide bond of bovine alpha s1-casein. Comparison of the behaviour of this substrate with that of beta- and kappa o-caseins. FEBS Lett. 1985 Jun 17;185(2):282–286. doi: 10.1016/0014-5793(85)80923-6. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Doi H., Kawaguchi N., Ibuki F., Kanamori M. Susceptibility of kappa-casein components to various proteases. J Nutr Sci Vitaminol (Tokyo) 1979;25(1):33–41. doi: 10.3177/jnsv.25.33. [DOI] [PubMed] [Google Scholar]
- Foltmann B., Pedersen V. B., Jacobsen H., Kauffman D., Wybrandt G. The complete amino acid sequence of prochymosin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2321–2324. doi: 10.1073/pnas.74.6.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. A., CHESSEMAN G. C. Action of rennin on casein: the function of the neuraminic acid residues. Biochim Biophys Acta. 1962 Jan 29;56:354–356. doi: 10.1016/0006-3002(62)90575-9. [DOI] [PubMed] [Google Scholar]
- Garnier J., Mocquot G., Ribadeau-Dumas B., Maubois J. L. Coagulation du lait par la présure: aspects scientifiques et technologiques. Ann Nutr Aliment. 1968;22(2):B495–B552. [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier J. C., Maubois J. L., Poznanski S., Ribadeau-Dumas B. Fractionnement préparatif des caséines de vache et de brebis par chromatographie sur D.E.A.E. cellulose, en milieu urée et 2-mercaptoéthanol. Bull Soc Chim Biol (Paris) 1968;50(3):521–530. [PubMed] [Google Scholar]
- Mercier J. C. Phosphorylation of caseins, present evidence for an amino acid triplet code posttranslationally recognized by specific kinases. Biochimie. 1981 Jan;63(1):1–17. doi: 10.1016/s0300-9084(81)80141-1. [DOI] [PubMed] [Google Scholar]
- SCHMIDT D. G. STARCH-GEL ELECTROPHORESIS OF K-CASEIN. Biochim Biophys Acta. 1964 Aug 19;90:411–414. doi: 10.1016/0304-4165(64)90210-7. [DOI] [PubMed] [Google Scholar]
- Sanner T., Kovács-Proszt G. Kinetic studies on the aggregation of kappa-casein by Mucor pusillus protease. Biochim Biophys Acta. 1973 Mar 23;303(1):68–76. doi: 10.1016/0005-2795(73)90149-9. [DOI] [PubMed] [Google Scholar]
- Sinkinson G., Wheelock J. V. Carbohydrates of the glycopeptides released by the action of rennin on whole milk. Biochim Biophys Acta. 1970 Sep 22;215(3):517–521. doi: 10.1016/0304-4165(70)90102-9. [DOI] [PubMed] [Google Scholar]
- Slattery C. W. Variation in the glycosylation pattern of bovine kappa-casein with micelle size and its relationship to a micelle model. Biochemistry. 1978 Mar 21;17(6):1100–1104. doi: 10.1021/bi00599a025. [DOI] [PubMed] [Google Scholar]
- Visser S., Rollema H. S. Quantification of chymosin action on nonlabeled kappa-casein-related peptide substrates by ultraviolet spectrophotometry: description of kinetics by the analysis of progress curves. Anal Biochem. 1986 Mar;153(2):235–241. doi: 10.1016/0003-2697(86)90087-4. [DOI] [PubMed] [Google Scholar]
- Visser S., Van Rooijen P. J., Schattenkerk C., Kerling K. E. Peptide substrates for chymosin (rennin). Kinetic studies with bovine kappa-casein-(103-108)-hexapeptide analogues. Biochim Biophys Acta. 1977 Mar 15;481(1):171–176. doi: 10.1016/0005-2744(77)90148-6. [DOI] [PubMed] [Google Scholar]
- Visser S., Van Rooijen P. J., Schattenkerk C., Kerling K. E. Peptide substrates for chymosin (rennin). Kinetic studies with peptides of different chain length including parts of the sequence 101-112 of bovine k-casein. Biochim Biophys Acta. 1976 Jun 7;438(1):265–272. doi: 10.1016/0005-2744(76)90242-4. [DOI] [PubMed] [Google Scholar]
- Vreeman H. J., Both P., Brinkhuis J. A., van der Spek C. Purification and some physicochemical properties of bovine kappa-casein. Biochim Biophys Acta. 1977 Mar 28;491(1):93–103. doi: 10.1016/0005-2795(77)90044-7. [DOI] [PubMed] [Google Scholar]
- Vreeman H. J., Brinkhuis J. A., van der Spek C. A. Some association properties of bovine SH-kappa-casein. Biophys Chem. 1981 Oct;14(2):185–193. doi: 10.1016/0301-4622(81)85018-1. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Yamauchi K., Azuma N., Kobayashi H., Kaminogawa S. Isolation and properties of human kappa-casein. J Biochem. 1981 Oct;90(4):1005–1012. doi: 10.1093/oxfordjournals.jbchem.a133552. [DOI] [PubMed] [Google Scholar]
- van Halbeek H., Dorland L., Vliegenthart J. F., Fiat A. M., Jolles P. A 360-MHz 1H-NMR study of three oligosaccharides isolated from cow kappa-casein. Biochim Biophys Acta. 1980 Jun 26;623(2):295–300. doi: 10.1016/0005-2795(80)90257-3. [DOI] [PubMed] [Google Scholar]