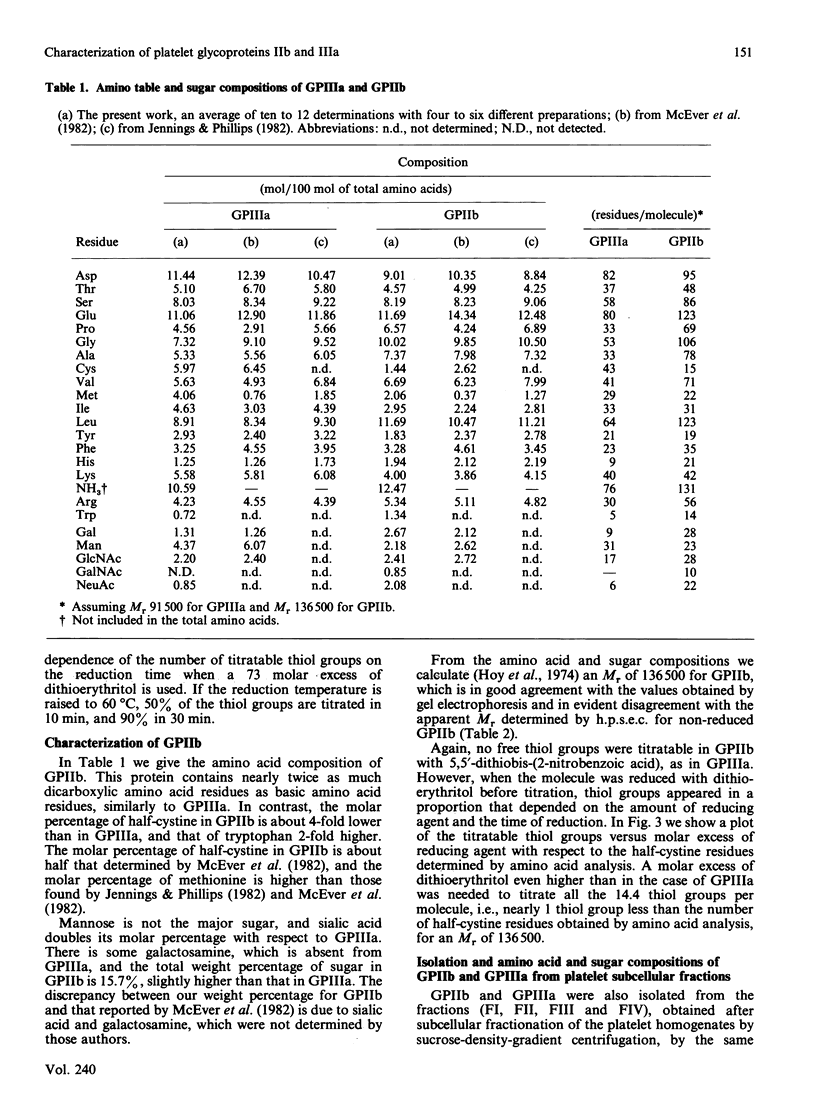
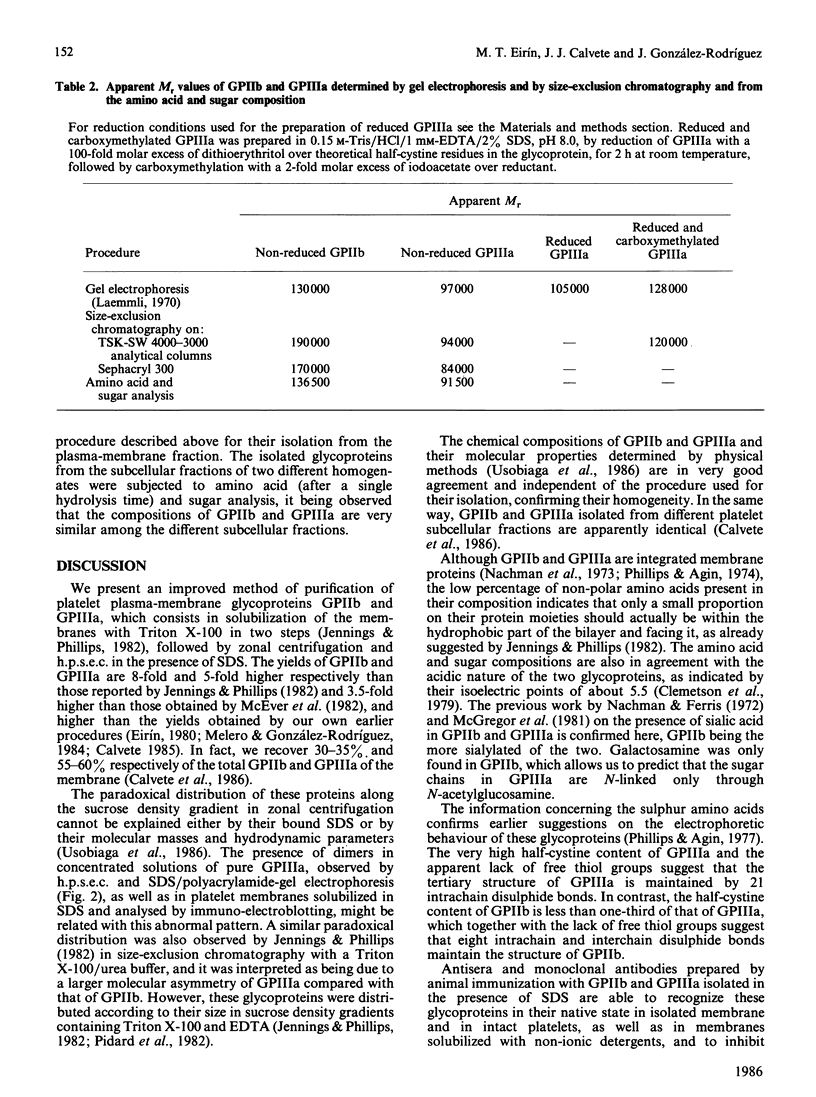
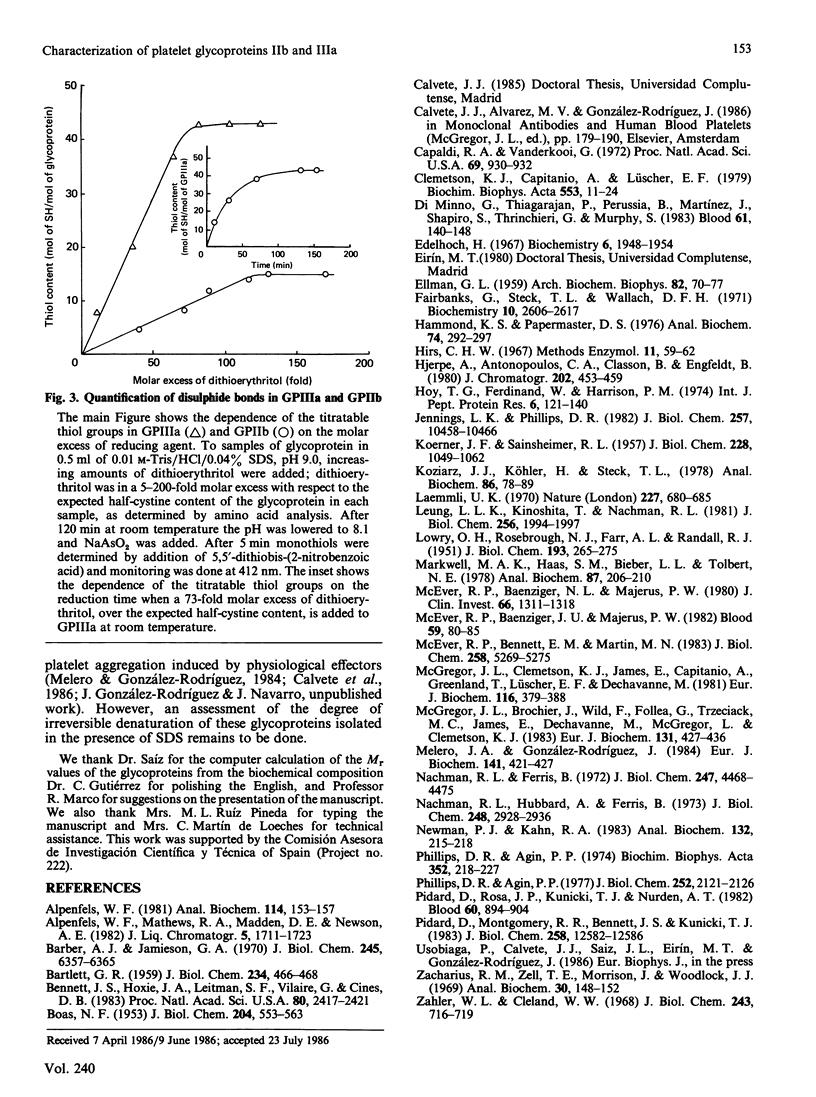
Abstract
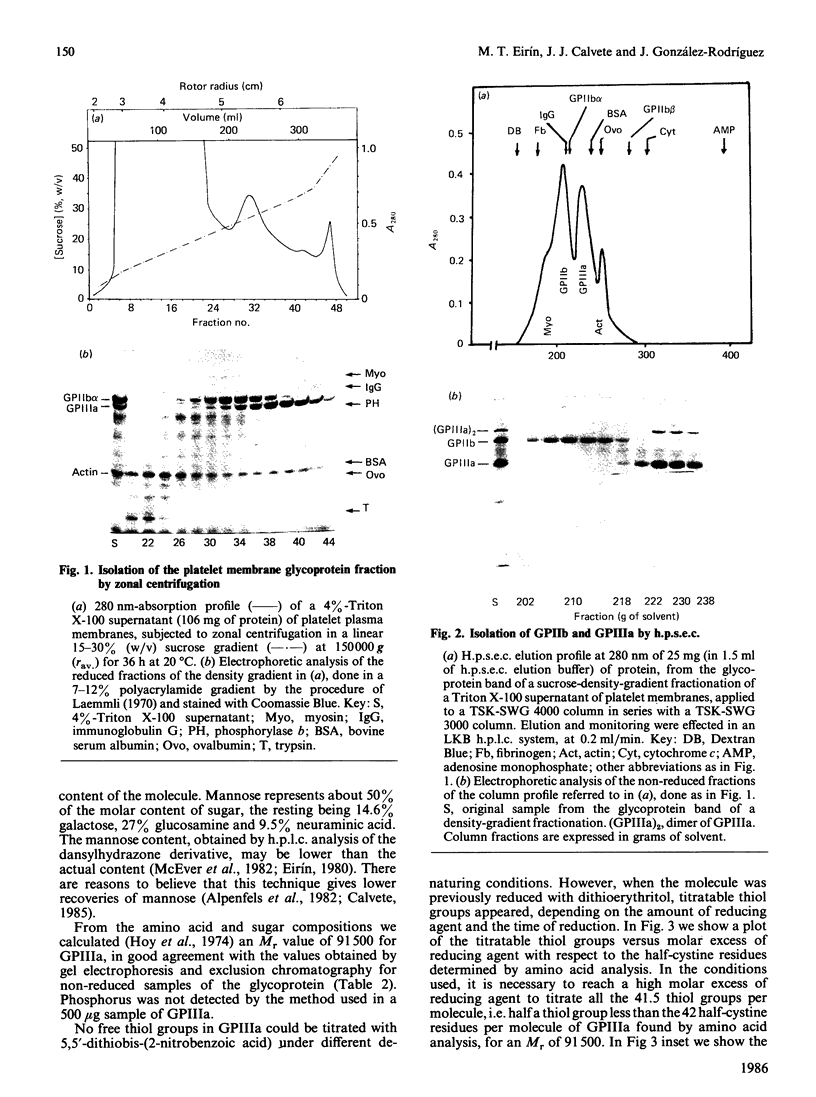
We describe a new procedure for isolation of glycoproteins IIb (GPIIb) and IIIa (GPIIIa) from human platelet plasma membrane with high yields (2.7 mg of GPIIb and 3.3 mg of GPIIIa per 100 mg of starting platelet membrane proteins), equivalent to a recovery of 35% and 55% respectively of the total GPIIb and GPIIIa of the membrane. The procedure involves Triton X-100 differential extraction of platelet membranes, SDS solubilization of the 4%-Triton X-100 supernatant, zonal centrifugation in a sucrose density gradient, and preparative high-performance size-exclusion chromatography. The weight percentage of sugar is 15.7% for GPIIb and 12.5% for GPIIIa. Neuraminic acid is present in both glycoproteins, representing 30% and 15% respectively of the total sugar weight of GPIIb and GPIIIa. Mannose, galactose and glucosamine account for 45%, 13% and 28% respectively of the sugars of GPIIIa, whereas galactosamine was not detected. Mannose, galactose, glucosamine and galactosamine represent 17%, 21%, 24% and 10% respectively of the sugar content of GPIIb. The molar percentages of half-cystine and methionine are 4-fold and 2-fold higher respectively in GPIIIa than in GPIIb. From the amino acid and sugar compositions we confirmed the acidic nature of both glycoproteins. The Mr values obtained, 136,500 for GPIIb and 91,500 for GPIIIa, are in very good agreement with those obtained by physical methods. The apparent lack of free thiol groups in both glycoproteins indicates that the tertiary structure of GPIIIa is maintained by 21 intrachain disulphide bonds, and that there are eight intrachain and interchain disulphide groups in GPIIb.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpenfels W. F. A rapid and sensitive method for the determination of monosaccharides as their dansyl hydrazones by high-performance liquid chromatography. Anal Biochem. 1981 Jun;114(1):153–157. doi: 10.1016/0003-2697(81)90466-8. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Barber A. J., Jamieson G. A. Isolation and characterization of plasma membranes from human blood platelets. J Biol Chem. 1970 Dec 10;245(23):6357–6365. [PubMed] [Google Scholar]
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemetson K. J., Capitanio A., Lüscher E. F. High resolution two-dimensional gel electrophoresis of the proteins and glycoproteins of human blood platelets and platelet membranes. Biochim Biophys Acta. 1979 May 3;553(1):11–24. doi: 10.1016/0005-2736(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Di Minno G., Thiagarajan P., Perussia B., Martinez J., Shapiro S., Trinchieri G., Murphy S. Exposure of platelet fibrinogen-binding sites by collagen, arachidonic acid, and ADP: inhibition by a monoclonal antibody to the glycoprotein IIb-IIIa complex. Blood. 1983 Jan;61(1):140–148. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hammond K. S., Papermaster D. S. Fluorometric assay of sialic acid in the picomole range: a modification of the thiobarbituric acid assay. Anal Biochem. 1976 Aug;74(2):292–297. doi: 10.1016/0003-2697(76)90210-4. [DOI] [PubMed] [Google Scholar]
- Hoy T. G., Ferdinand W., Harrison P. M. A computer-assisted method for determining the nearest integer ratios of amino acid residues in purified proteins. Int J Pept Protein Res. 1974;6(3):121–140. doi: 10.1111/j.1399-3011.1974.tb02369.x. [DOI] [PubMed] [Google Scholar]
- Jennings L. K., Phillips D. R. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982 Sep 10;257(17):10458–10466. [PubMed] [Google Scholar]
- KOERNER J. F., SINSHEIMER R. L. A deoxyribonuclease from calf spleen. II. Mode of action. J Biol Chem. 1957 Oct;228(2):1049–1062. [PubMed] [Google Scholar]
- Koziarz J. J., Köhler H., Steck T. L. A system for preparative polyacrylamide gel electrophoresis in sodium dodecyl sulfate. Anal Biochem. 1978 May;86(1):78–89. doi: 10.1016/0003-2697(78)90320-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung L. L., Kinoshita T., Nachman R. L. Isolation, purification, and partial characterization of platelet membrane glycoproteins IIb and IIIa. J Biol Chem. 1981 Feb 25;256(4):1994–1997. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Baenziger J. U., Majerus P. W. Isolation and structural characterization of the polypeptide subunits of membrane glycoprotein IIb-IIIa from human platelets. Blood. 1982 Jan;59(1):80–85. [PubMed] [Google Scholar]
- McEver R. P., Baenziger N. L., Majerus P. W. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980 Dec;66(6):1311–1318. doi: 10.1172/JCI109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- McGregor J. L., Brochier J., Wild F., Follea G., Trzeciak M. C., James E., Dechavanne M., McGregor L., Clemetson K. J. Monoclonal antibodies against platelet membrane glycoproteins. Characterization and effect on platelet function. Eur J Biochem. 1983 Mar 15;131(2):427–436. doi: 10.1111/j.1432-1033.1983.tb07281.x. [DOI] [PubMed] [Google Scholar]
- McGregor J. L., Clemetson K. J., James E., Capitanio A., Greenland T., Lüscher E. F., Dechavanne M. Glycoproteins of platelet membranes from Glanzmann's thrombasthenia. A comparison with normal using carbohydrate-specific or protein-specific labelling techniques and high-resolution two-dimensional gel electrophoresis. Eur J Biochem. 1981 May 15;116(2):379–388. doi: 10.1111/j.1432-1033.1981.tb05346.x. [DOI] [PubMed] [Google Scholar]
- Melero J. A., Gonzalez-Rodriguez J. Preparation of monoclonal antibodies against glycoprotein IIIa of human platelets. Their effect on platelet aggregation. Eur J Biochem. 1984 Jun 1;141(2):421–427. doi: 10.1111/j.1432-1033.1984.tb08208.x. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Ferris B. Studies on the proteins of human platelet membranes. J Biol Chem. 1972 Jul 25;247(14):4468–4475. [PubMed] [Google Scholar]
- Nachman R. L., Hubbard A., Ferris B. Iodination of the human platelet membrane. Studies of the major surface glycoprotein. J Biol Chem. 1973 Apr 25;248(8):2928–2936. [PubMed] [Google Scholar]
- Newman P. J., Kahn R. A. Purification of human platelet membrane glycoproteins IIb and IIIa using high-performance liquid chromatography gel filtration. Anal Biochem. 1983 Jul 1;132(1):215–218. doi: 10.1016/0003-2697(83)90450-5. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Thrombin substrates and the proteolytic site of thrombin action on human-platelet plasma membranes. Biochim Biophys Acta. 1974 Jun 13;352(2):218–227. doi: 10.1016/0005-2736(74)90213-2. [DOI] [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Pidard D., Rosa J. P., Kunicki T. J., Nurden A. T. Further studies on the interaction between human platelet membrane glycoproteins IIb and IIIa in triton X-100. Blood. 1982 Oct;60(4):894–904. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]
- Zahler W. L., Cleland W. W. A specific and sensitive assay for disulfides. J Biol Chem. 1968 Feb 25;243(4):716–719. [PubMed] [Google Scholar]