Abstract
To increase insight into the structural basis of CXCR4 utilization in human immunodeficiency virus type 1 (HIV-1) infection, a new generation of three monoclonal antibodies (MAbs) was developed in WKA rats. The A80 MAb, which binds an epitope in the third extracellular loop (ECL3) of CXCR4, has unique biologic properties that provide novel insights into CXCR4 function. This agent enhanced syncytium formation in activated human peripheral blood mononuclear cells (PBMC) infected with X4 or R5 and CEM cells infected with X4 HIV-1 strains. Exposure to A80 increased the productive infection of activated CD4+ T cells and CEM cells with R5 and X4 viruses, respectively. This antibody uniquely induced agglutination of PBMC and CEM cells but did not activate calcium mobilization. Agglutination induced by A80 was inhibited by stromal cell-derived factor 1, T22, and phorbol 12-myristate 13-acetate but was not significantly altered by pretreatment of cells with pertussis toxin, wortmannin, or MAbs to LFA-1, ICAM-1, ICAM-2, and ICAM-3. The binding of the A145 and A120 MAbs was mapped to the N-terminal extracellular domain and a conformational epitope involving ECL1 and ECL2, respectively. Both of these MAbs inhibited HIV-1 infection and lacked the novel properties of A80. These results suggest a new role for CXCR4 in homologous lymphocyte adhesion that is ligand independent and in HIV-1 infection.
Human immunodeficiency virus type 1 (HIV-1) infects target cells through sequential binding of the gp120 subunit of envelope glycoprotein with cellular receptors. Binding to the primary receptor, CD4 (26, 47, 50, 51), induces a gp120 conformation that is permissive for interaction with a coreceptor, which is required for envelope-mediated fusion (3, 7, 21, 28, 30, 32, 35). CCR5 is the front line coreceptor for commonly transmitted forms of HIV-1 and CXCR4 serves this role for T-cell-tropic (T-tropic) strains that evolve late in the course of infection (22, 24, 28, 29, 60, 70). CCR5 and CXCR4 belong to the chemokine receptor family, which transmit signals through heterotrimeric G proteins (3, 8, 7, 35). T-tropic HIV-1, designated X4 strains based on the functional relationship with CXCR4, has been suggested to be more virulent than R5 or macrophage-tropic strains (7, 9, 23), possibly due to the wider spectrum of target cells that express CXCR4 (13).
The exclusive ligand of CXCR4 is stromal cell-derived factor 1 (SDF-1), a member of the family of chemo-attractant cytokines (54, 56). This chemokine has been demonstrated to play a critical role during embryologic development in the homing of hepatic hematopoietic precursors to bone marrow, the arborization of small blood vessels, the formation of the cerebellum, and B-cell lymphopoiesis (54, 71). SDF-1 regulates homing and directed the migration of lymphocytes and modulates the expression of cell surface adhesion molecules (18, 66). SDF-1 can interfere with infection by X4 strains of HIV-1 by receptor blockade and downmodulation from the cell surface (54, 56, 68). Activation of CXCR4 by SDF-1 or gp120 may induce cell activation and apoptosis of neurons and CD4+ cells (10, 12, 27, 39, 42, 55, 69).
The structural basis for the interaction of CXCR4 with SDF-1 and HIV-1 envelope glycoproteins has not yet been elucidated. Structure-function studies with chimeras, point mutants, or domain-specific monoclonal antibodies (MAbs) indicate that these functions involve multiple domains of the receptor and are not coincident (14, 16, 19, 20, 31, 33, 35, 41). Whereas the membrane-proximal region of the N-terminal (NT) extracellular domain and the third extracellular loop (ECL3) appear to be critical for SDF-1 binding and signaling, regions contiguous to the second ECL have been implicated in coreceptor activity (14, 15, 16, 31). Studies with CXCR4 mutants that are not coupled to G proteins have revealed that coreceptor activity is independent of signal transduction (31, 52). In contrast, it has been shown that signaling through CCR5 is required for fusion of R5 viruses with primary CD4+ T lymphocytes (2), although signal transduction is not necessary for infection of cell lines (4, 5, 34, 38).
Cell fusion with syncytium formation represents an important cytopathic effect of HIV-1 infection that may be a critical mechanism for depletion of CD4+ T lymphocytes (49, 50, 51, 62, 67). Syncytium formation results from the interaction of the gp120 subunit of envelope glycoprotein expressed on infected cells with CD4 and a coreceptor, typically CXCR4, on the surface of target cells (3, 11, 28, 32, 35, 50, 51, 62, 67). The involvement of cytoadhesion molecules in syncytium formation has been demonstrated by inhibition with MAbs to LFA-1 and ICAM-1 (17, 37, 40, 65) and the observation that LFA-1-deficient CD4+ T lymphocytes exhibit decreased syncytium formation (57). Moreover, this process can be enhanced by the modulation of LFA-1 conformation using the NKI-IL-16 MAb (6).
In the physiologic response to SDF-1 signaling through CXCR4, rolling of T lymphocytes and tight adhesion to endothelial cells is dependent upon LFA-1 activation (18, 25, 45). Similarly, SDF-1 activates integrins (VLA-4 and VLA-5) in CD34+ cells (57, 66). These findings link CXCR4 signaling to integrin activation in physiologic responses and implicate this mechanism in HIV-1 infection as well. Here we demonstrate that an MAb to the ECL3 of CXCR4, A80, has the unique properties of inducing cell agglutination and enhancing syncytium formation by HIV-1, providing additional evidence for the association between CXCR4 signaling and cell adhesion. This unique activity of the A80 MAb provides important insights into the mechanism for CXCR4 function in physiologic responses and HIV-1 envelope-mediated membrane fusion.
MATERIALS AND METHODS
Reagents.
RPMI 1640 medium (Sigma Chemical Company, St. Louis, Mo.) supplemented with 10% fetal calf serum (FCS) (Sigma), 100 U of penicillin per ml, and 100 μg of streptomycin per ml (hereinafter called RPMI medium) was used for cultivation of all of the cells used. Anti-human CD3 MAb (OKT-3) was purchased from the American Type Culture Collection (Rockville, Md.). Anti-human CD28 MAb was purchased from R&D (Minneapolis, Minn.). Magnetic beads conjugated with anti-CD8, and unconjugated tosylactivated beads (M-450) were purchased from Dynal; the latter were conjugated with anti-CD3 and anti-CD28 MAb (anti-CD3/CD28 beads) according to the manufacturer's recommendation. The fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled MAbs anti-human CD2, CD3, CD4, CD8, CD11a, CD14, CD18, CD20, CD28, CD29, CD50, CD54, CD69, and CD102 and FITC-labeled streptavidin were purchased from Beckman-Coulter. The mouse anti-human CXCR4 MAbs purchased were 12G5 (FAB170), FAB171, FAB172, and FAB173 (R&D). A rat immunoglobulin G1 (IgG1) MAb against hepatitis C virus (HCV), Mo-8 (44), was used as a rat isotype-matched negative control.
Goat anti-rat IgG (heavy and light chains) labeled with either FITC or peroxidase (POD) was purchased from American Corlex. Streptavidin-POD was purchased from Dako. PE-labeled MAb anti-HIV-1 p24 was purchased from Beckman Coulter. MAbs against adhesion molecules used for blocking of adhesion were anti-LFA-1 alpha chain (CD11a) (clone TP1/32; Upstate Biotechnology, Inc.), anti-LFA-1 beta chain (CD18, clone MHM23; Dako), anti-ICAM-1 (CD54, clone 84H10; Beckman-Coulter), anti-ICAM-2 (CD102, clone B-T1; Beckman-Coulter), anti-ICAM-3 (CD50, clone HP2/19; Beckman-Coulter), and anti-VLA beta chain (CD29, clone BB4; Beckman-Coulter). The CXCR4 ligands used were recombinant SDF-1 (R&D) and T22 (53). Pertussis toxin (PTX) (Seikagakukogyo, Tokyo, Japan) and wortmannin (WMN) (Wako Pure Chemicals, Kyoto, Japan) were used to block SDF-1 signal pathway. Phorbol 12-myrisate 13-acetate (PMA) was purchased from Sigma. WMN and PMA were dissolved at 100 μg/ml in dimethyl sulfoxide and diluted to a final concentration of 0.1 μg/ml in culture medium.
Cells.
Cell lines used included human T-cell lines (CEM, Molt-4, Jurkat, Hut78, and MT-2), human B-cell lines (BJAB and Raji), human myeloid cell line (THP-1), a human erythroblastoid cell line (K-562), a human glioblastoma cell line (U-87 MG), a monkey kidney cell line (Cos-1), a WKA rat kidney cell line (W7KSV) and a rat T-cell line (W7TM-1) (63), and a mouse myeloma cell line (SP2/0). Fresh peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood of healthy donors by Ficoll-Hypaque density gradient centrifugation. PBMC or PBMC depleted of CD8+ T cells by an immunomagnetic method with anti-CD8 antibody-conjugated magnetic beads were cultured at 2 × 106 cells/ml in RPMI medium containing 50 U of human recombinant interleukin-2 (rIL-2; Shionogi Pharmaceutical, Osaka, Japan)/ml in the presence of the anti-CD3 (OKT3 from the American Type Culture Collection)/CD28 beads at a cell/bead ratio of 1:1 in a 12-well plate (Falcon). On days 3 and 6, the cells were collected, diluted 1:4 with fresh RPMI medium with 50 U of rIL-2/ml, and restimulated with the anti-CD3/CD28 beads at a cell/bead ratio of 1:1.
Generation of rat MAbs.
The human CXCR4 expression plasmid was kindly provided by K. Matsushima (University of Tokyo). The CXCR4-expressing cells generated were A62 cells of Cos-1 origin, and the 0.5a cells were of W7TM-1 origin (63). The human CCR5 gene was isolated by PCR performed directly on a human genomic DNA, and its sequence was confirmed before transfer into XhoI and NotI sites in the expression vector, BCMGSNeo (46). The primers used for PCR amplification were as follows: sense, 5′-CTCGAGAACAAGATGGATTATCAA-3′; and antisense, 5′-GCGGCCGCGAGTCCGTGTCACAAGCCCACA-3′. The CCR5-expressing cells generated were A41 cells of Cos-1 origin, and the W516 cells were of of W7KSV origin. These transfectants were selected and maintained in the presence of 0.5 mg of G418 (Gibco)/ml. Expression of the transgenes was confirmed by a Northern blot assay and a flow cytometric analysis with commercial antibodies against human CXCR4 and human CCR5. Lines of MAb-producing hybridomas were generated as described previously (64). Briefly, WKA rat spleen cells immunized intraperitoneally with 0.5a or W516 cells at 107 cells/animal every 2 weeks for 2 to 4 months were fused with SP2/0 cells by using polyethylene glycol 4000 and were selected in hypoxanthine-aminopterin-thymidine medium. Specific antibody screenings were performed by an indirect immunofluorescence assay on A40 and A62 cells. Hybridomas were cloned by limiting dilution, and MAbs were produced in culture medium or in ascitic fluid of BALB/c nude mice. IgG fractions were obtained from ascitic fluid by a gel filtration chromatography with Superdex G200 (Amersham Pharmacia). The immunoglobulin isotype and subclass of a new MAb were determined by using an enzyme-linked immunosorbent assay (ELISA) kit (Zymed).
Immunofluorescence.
Sample cells (1 × 105 to 5 × 105) were incubated in a volume of 100 μl of phosphate-buffered saline (PBS) containing 2% FCS and 0.1% sodium azide (referred to as fluorescence-activated cell sorting [FACS] buffer) and 100 μg of normal human IgG/ml for 15 min on ice. The cells were reacted with 0.1 ml of hybridoma culture supernatants, 5 μg of purified antibody, or appropriately diluted FITC-labeled antibodies/ml for 30 min on ice, and then they were washed twice with FACS buffer. For the detection of unlabeled rat IgG, these cells were further incubated with 100 μl of 1:100 diluted goat anti-rat IgG labeled with FITC containing 100 μg of normal goat IgG/ml for 30 min on ice. After being washed, the cells were fixed with 1% paraformaldehyde in FACS buffer for 5 min at room temperature and then analyzed on a flow cytometer (FACSCalibur) by using the CellQuest software (Becton Dickinson). The area of positivity was determined by using an isotype-matched mouse MAb (Beckman-Coulter) or a rat IgG1 MAb (Mo-8).
Immunoprecipitation and immunoblot.
Cells (2 × 107cells) were lysed in 1 ml of lysis buffer containing either 1% Brij 97 (Sigma) or 0.5% NP-40 in 20 mM Tris-HCl (pH 8.2)–0.15 M NaCl–5 mM iodoacetamide–1 mM phenylmethylsulfonyl fluoride. For immunoprecipitation, a volume of 100 μl of cell lysates was incubated with 1 μg of MAb on ice for 30 min and precipitated with 10 μl of protein G-Sepharose (Amersham Pharmacia) at 4°C overnight. Washed precipitates were treated with the sample buffer at 37°C for 30 min in nonreducing conditions and separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS-10% PAGE). The separated proteins were transferred to polyvinylidene difluoride membrane (Pierce). The membranes were reacted with rat MAbs, followed by reaction with POD-labeled goat anti-rat IgG. The binding of MAb was visualized by using the ECL substrate (Pharmacia), followed by analysis with Fluor-S MAX MultiImager (Bio-Rad).
Cell agglutination assay.
Anti-CD3/CD28 bead-activated PBMC, CEM cells, and BJAB cells were washed once and resuspended in RPMI medium at 2 × 106 cells/ml, separated into aliquots in 48-well flat-bottom plates (0.5 ml/well), and then preincubated with 1 μg of PTX/ml, 100 μM WMN, 1 μg of SDF-1α/ml, 5 μM T22, or 20 μg of MAbs (including anti-CD11a, CD18, CD50, CD54, CD102, and CD29)/ml for 30 or 60 min at 37°C, followed by incubation with 2 μg of A80 IgG/ml. After overnight incubation, cells were observed for agglutination under an inverted microscope at magnification of ×40 or ×100. PMA was used to activate LFA-1 on BJAB cells and to downmodulate CXCR4 on CEM cells at 0.1 μg/ml.
HIV-1 infection.
The HIV-1 strains used were molecular clones of NL4-3 (1) and JR-CSF (48). These HIV-1 stocks produced in Cos-1 cells were obtained from Y. Koyanagi (Tohoku University). Infectious titers of each virus stock were determined by endpoint dilution assay with activated PBMC and are expressed as 50% tissue culture infective doses (TCID50). Either ten million PBMC activated for 6 days with anti-CD3/CD28 beads and depleted of CD8+ T cells or CEM cells were washed once with RPMI medium and incubated with 1,000 TCID50 of HIV-1 in a 0.2-ml volume for 3 h at 37°C. The infected PBMC and CEM cells were subsequently washed twice with fresh RPMI medium and cultured at 2 × 105 cells/ml for 5 and 8 days, respectively, in 48-well culture plates (0.5 ml/well) in the presence or absence of 10 μg of MAbs/ml. CEM cell cultures were split 1:2 with fresh medium on days 4 and 6. Culture supernatants were examined for production of cell-free HIV-1 p24 by using an ELISA kit (Zepto Metrix Corporation). The number of syncytia per culture well was microscopically determined by using a hemocytometer at a magnification of ×100. Data were analyzed by using the Student's t test.
Epitope mapping.
Peptides with amino acid sequences of four of the extra cellular regions of the human CXCR4 (35)—i.e., amino acids 1 to 39 (MEGISIYTSDNYTEEMGSGDYDSMKEPCFREENANFNKI), amino acids 97 to 110 (DAVANWYFGNFLCK), amino acids 176 to 201 (NVSEADDRYICDRFYPNDLWVVVFQFQ), and amino acids 262 to 282 (DSFILLEIIKQGCEFENTVHK)—and those of the human CCR5 (59)—i.e., amino acids 1 to 31 (MDYQVSSPIYDINYYTSEPCQKINVKQIAAR), amino acids 89 to 102 (YAAAQWDFGNTMCQ), amino acids 168 to 197 (RSQKEGLHYTCSSHFPYSQYQFWKNFQTLK), and amino acids 258 to 279 (NTFQEFFGLNNCSSSNRLDQAM)—were synthesized by the step-wise solid-phase procedure of Na9-fluorenylmethoxycarbonyl chemistry on an automated peptide synthesizer (PSSM-8; Shimadzu, Kyoto, Japan). All peptides used were >90% pure as judged by a high-pressure liquid chromatographic analysis (data not shown). These peptides were immobilized onto 96-well flat-bottom ELISA plates (Nunc) by incubation at 100 μl/well of a 10-μg/ml concentration of peptide in PBS at 4°C overnight. The immobilized peptides were then tested for reactivity with an antibody by an ELISA as described previously (64). Epitopes recognized by anti-CXCR4 MAbs were also determined by using chimeric receptors. The chimeric receptors composed of CXCR4 and CXCR2 were constructed by the PCR-ligation-PCR approach as described previously (52). Cos-1 cells transfected with each receptor plasmid by means of electroporation by using the Gene Pulser II (Bio-Rad). After culture for 48 h, the cells were harvested and examined for reactivity with MAbs by an indirect immunofluorescence assay.
Ca2+ mobilization assay.
CEM cells at 107 cells/ml were loaded with 4 μM fluo-3-acetylozymethyl ester (Fluo-3; Molecular Probes, Eugene, Oreg.), a Ca2+ indicator, for 30 min at 37°C. After either stimulation with 0.5 μg of SDF-1α/ml or treatment with each MAb at 10, 50, and 100 μg/ml, the intracellular Ca2+ concentration was then measured by use of FACSCalibur with analysis software (Flow Jo; Tree Star, Inc.).
RESULTS
Generation of novel MAbs to CXCR4: A145 is active in immunoblotting.
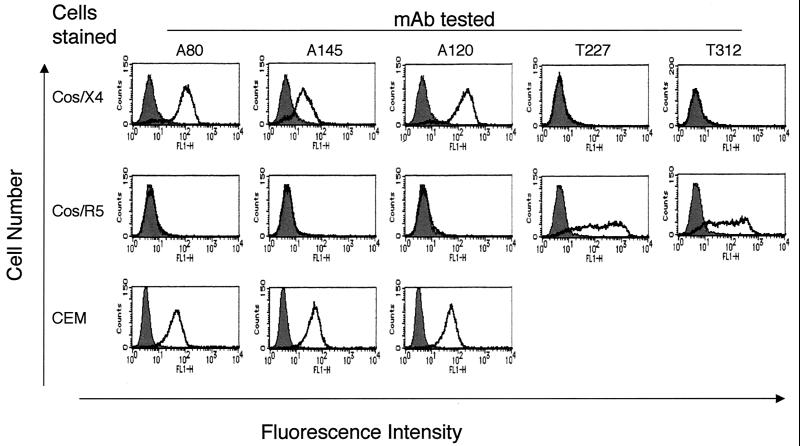
Three MAbs to human CXCR4 were generated by immunization of WKA rats with a syngeneic transfectant cell line programmed to express human CXCR4. The specificity of these IgG1 MAbs, designated A80, A120, and A145, was established by indirect immunofluorescence analysis of CXCR4 transfectants by using anti-CCR5 MAbs as control myeloma proteins. These MAbs lacked reactivity with control cells and CCR5 transfectants. Analysis of a panel of cell lines demonstrated that all known to express CXCR4, including CEM (Fig. 1), Molt4, Jurkat, Hut78, K562, THP-1, BJAB, and Raji cells (data not shown), stained positively with A80, A120, and A145. The three MAbs did not react with U-87 MG cells, which lack CXCR4 expression, or with CCR1, CCR2B, CCR3, or CCR5 HOS-CD4 transfectants (data not shown).
FIG. 1.
Reactivity of MAbs generated from WKA rats. Cos-1 cells expressing human CXCR4 (Cos/X4) and CCR5 (Cos/R5) and CEM cells were reacted with 5 μg of MAbs/ml, followed by incubation with goat anti-rat IgG-FITC. The binding of MAb was determined by flow cytometry. The shaded area indicates cells stained with negative control rat IgG1 MAb (Mo-8) specific for HCV. T227 and T312 were control rat IgG MAbs to anti-human CCR5. Representative results from four independent experiments are shown.
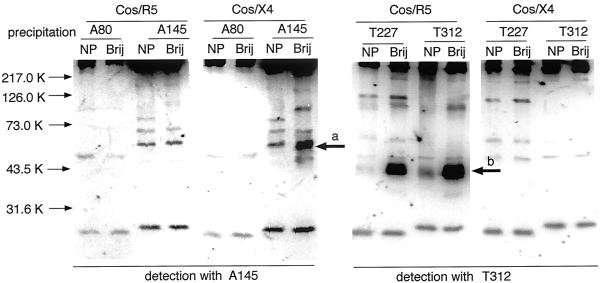
The reactivity of these MAbs with CXCR4 was further analyzed by immunoprecipitation, followed by immunoblotting. Analysis of CEM cell lysates by immunoblotting revealed that A145, but not A80 or A120, detected a 50-kDa protein corresponding to CXCR4 (data not shown). Figure 2 shows that A145 had the unique ability among the three MAbs to specifically immunoprecipitate CXCR4 from detergent lysates (Brij 97 or NP-40) of Cos-1 cells transfected with CXCR4 but not CCR5.
FIG. 2.
Immunoprecipitation and immunoblot of CXCR4 by MAb. Cos-1 cells expressing human CXCR4 (Cos/X4) and CCR5 (Cos/R5) were lysed with either NP-40 (NP) or Brij 97 (Brij), and the lysates were reacted with anti-CXCR4 or anti-CCR5 MAbs. Immunocomplexes collected by protein G-Sepharose were separated by SDS-PAGE, blotted on polyvinylidene difluoride sheets, and reacted with either anti-CXCR4 A145 or anti-CCR5 T312, followed by reaction with POD-labeled goat anti-rat IgG. Arrows a and b indicate 50-kDa CXCR4 and 40-kDa CCR5 molecules, respectively. Representative results from three independent experiments are shown.
Effects of anti-CXCR4 MAbs on HIV-1 infection: enhancement by A80.
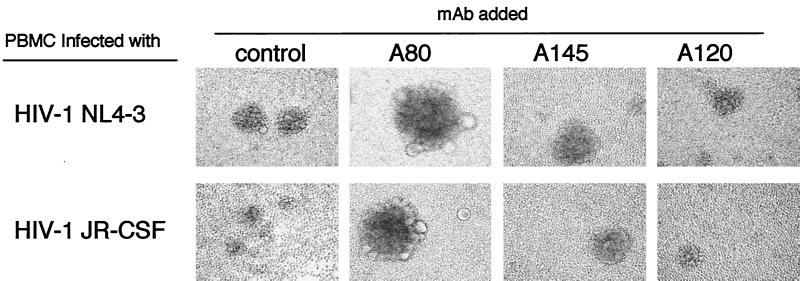
The effects of the three anti-CXCR4 MAbs on HIV-1 infection were determined in experiments with R5 and X4 strains and activated PBMC and CEM cells as targets. Figure 3 shows that, unlike A120 and A145, A80 induced syncytium formation in PBMC cultures infected with either X4 or R5 strains. A80 also significantly enhanced productive infection of CD8-depleted PBMC with R5 HIV-1 and of CEM cells with X4 HIV-1 (P < 0.05), as demonstrated in Table 1. A80 also induced syncytium formation by R5 and X4 strains in the PBMC and X4 strains in CEM cells (Table 1). The novel properties of inducing syncytium formation and enhancing infection are unique among MAbs to CXCR4, including the commercial MAbs, 12G5, F171, F172, and F173.
FIG. 3.
Induction of large syncytia in activated PBMC infected by HIV-1 in the presence of A80. Anti-CD3/CD28 bead-activated PBMC were infected with X4 HIV-1NL4-3 or R5 HIV-1JR-CSF at a multiplicity of infection of 0.005 for 3 h. After being washed, the cells were cultured in the presence of various MAbs at 10 μg/ml for 5 days. The control culture contained Mo-8. Syncytia were observed under an inverted microscope at an original magnification of ×100. Representative results from five independent experiments are shown.
TABLE 1.
Effects of anti-CXCR4 MAbs on HIV-1 productive infection and syncytium formation
| Target cells | Virus | Mean HIV-1 production and syncytium formation ± SD in the presence ofa:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Control
|
A80
|
A120
|
A145
|
||||||
| p24 (ng/ml) | n | p24 (ng/ml) | n | p24 (ng/ml) | n | p24 (ng/ml) | n | ||
| Activated PBMC | R5 HIV-1JR-CSF | 8.6 ± 0.4 | 703 ± 20 | 19.9 ± 1.3 | 5,386 ± 670 | 4.3 ± 0.4 | 206 ± 41 | 8.1 ± 0.8 | 620 ± 87 |
| Activated PBMC | X4 HIV-1NL4-3 | 9.8 ± 0.3 | 106 ± 46 | 12.4 ± 0.7 | 3,140 ± 225 | 1.0 ± 0.1 | 0 | 5.5 ± 0.2 | 0 |
| CEM cells | X4 HIV-1NL4-3 | 3.8 ± 0.3 | 0 | 10.8 ± 0.5 | 1,386 ± 122 | 0.2 ± 0.1 | 0 | 1.4 ± 0.4 | 0 |
A total of 2 × 105 CD8-depleted activated PBMC or CEM cells were infected with either HIV-1NL4-3 or HIV-1JR-CSF at a multiplicity of infection of 0.005 for 3 h. After being washed, the PBMC and CEM cells were cultured for 5 and 8 days, respectively, in the presence of 10 μg of MAb/ml. The negative control MAb used was anti-HCV Mo-8. CEM cells were split 1:2 in fresh medium with each MAb on days 3 and 6. The HIV-1 gag p24 (p24) produced in the culture supernatants was determined by ELISA. The number of syncytia (n) consisting of at least 10 nuclei was determined microscopically by using a hemocytometer. The numbers represent the means ± standard deviations of triplicate cultures. Representative results from four independent experiments are shown.
Induction of cell agglutination by A80.
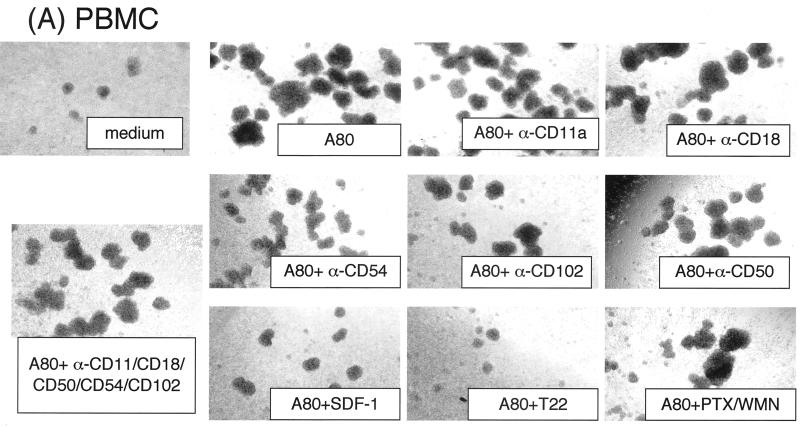
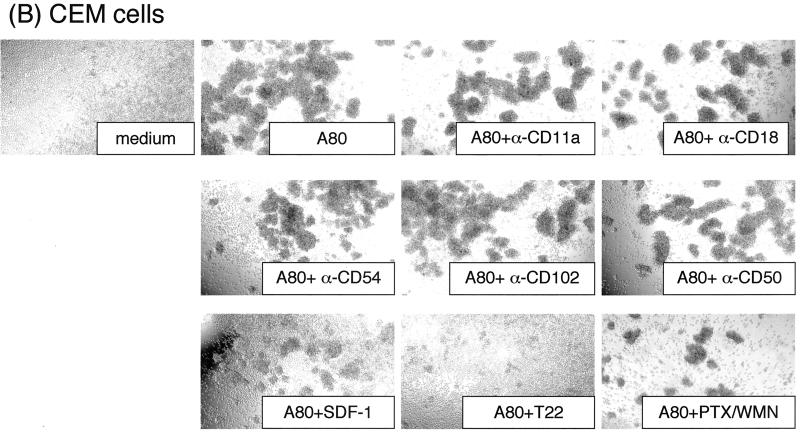
It was reasoned that the induction of syncytium formation and enhancement of HIV-1 infection by A80 may be the result of increased intercellular adhesion. The role of cytoadhesion molecules and CXCR4 signaling in the mechanism for the development of agglutination of cells by A80 in the absence of HIV-1 infection was determined. As shown in Fig. 4A and B, MAbs to LFA-1 (CD11a and CD18), ICAM-1 (CD54), ICAM-2 (CD102), and ICAM-3 (CD50) had minimal effects on agglutination mediated by A80. In addition, a MAb to the beta chain of VLA (CD29) lacked inhibitory activity (data not shown), and it is doubtful that LFA-2 (CD2) plays a role because CEM cells did not express this protein.
FIG. 4.
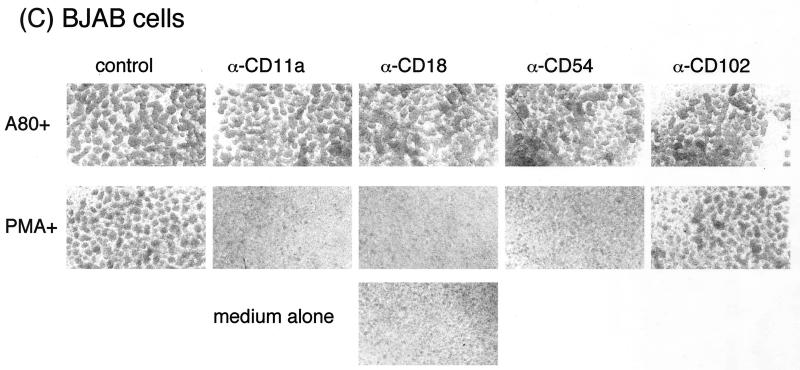
Effects of MAbs against various adhesion molecules, CXCR4 ligand, CXCR4 antagonist, and SDF-1 signal-blocking reagents on A80-induced cell agglutination. Activated PBMC (A), CEM cells (B), and BJAB cells (C) were preincubated with 20 μg of anti-LFA-1 CD11a, anti-LFA-1 CD18, anti-ICAM-1 (CD54), or anti-ICAM-2 (CD102) and anti-ICAM-3 (CD50)/ml; 1 μg of SDF-1α/ml; 5 μM T22; 0.1 μg of PTX/ml; 0.1 μg of WMN/ml; or various mixtures thereof at 37°C. After 1 h, either A80 (final concentration, 2 μg/ml) (A and B) or PMA (0.1 μg/ml) (C) was added, and the mixtures were incubated at 37°C overnight. Cell agglutination was observed under an inverted microscope at an original magnification of ×40. The “medium” panel was cultured in the absence of A80. Representative results from three independent experiments are shown.
Blocking studies were performed to ensure that the MAbs to cytoadhesion molecules inhibit adhesion of BJAB cells induced by PMA. As shown in Fig. 4C, agglutination after exposure to PMA was completely blocked by the MAbs to LFA-1 (CD11a and CD18) and ICAM-1 (CD54) but not by the MAb to ICAM-2. In contrast, A80 induced agglutination of BJAB cells that was not inhibited by the MAbs to LFA-1, ICAM-1, or ICAM-2. These results confirm that binding of A80 to CXCR4 on the cell surface induces agglutination via a novel mechanism.
Engagement of CXCR4 by SDF-1 or T22, a polypeptide CXCR4 antagonist, did not result in intercellular agglutination, but exposure to both inhibited the ability of A80 to induce this effect (Fig. 4A and B). Downmodulation of CXCR4 with PMA (61) completely abolished the agglutination of CEM cells after A80 binding (data not shown). Figure 4A and B also show that inhibitors of Gαi, PTX, and G-protein-coupled receptor signaling, WMN, did not influence the induction of agglutination by A80. These findings indicate that CXCR4 signaling is not required for the A80-induced agglutination but that the role of CXCR4 is critical.
A80 binds to ECL3 of CXCR4.
The epitopes recognized by A80, A120, and A145 MAbs were mapped by enzyme-linked immunosorbent assay (ELISA) with a panel of synthetic peptides spanning the four extracellular domains of CXCR4. An analogous panel of peptides from CCR5 served as controls. As shown in Table 2, A145 bound specifically to the peptide corresponding to the NT domain of CXCR4 and A80 and A120 did not bind to any of the peptides. To determine whether the latter two MAbs bound to epitopes that required expression of the native receptor on the cell surface, they were tested for binding to an array of CXCR4/CXCR2 chimeras in transient-expression experiments. The binding of A80 to chimeras corresponded to the presence of CXCR4-ECL3 (Table 3). In contrast, A120 showed optimal binding to chimeras that contained ECL1 and ECL2 of CXCR4, a result similar to that for MAb 12G5.
TABLE 2.
Reactivity of MAb to CXCR4 and CCR5 peptidesa
| Receptor | Peptide (aa) | Reactivity of MAb with peptide (OD450)
|
|||||
|---|---|---|---|---|---|---|---|
| Mo-8 | A80 | A120 | A145 | T227 | T312 | ||
| CXCR4 | NT (1–39) | <0.05 | <0.05 | <0.05 | 1.89 | <0.05 | <0.05 |
| ECL1 (97–110) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
| ECL2 (176–201) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
| ECL3 (262–282) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
| CCR5 | NT (1–31) | <0.05 | <0.05 | <0.05 | <0.05 | 1.78 | 1.65 |
| ECL1 (89–102) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
| ECL2 (168–197) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
| ECL3 (258–279) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
Microtiter plate wells coated with peptides were incubated with rat MAbs at 5 μg/ml for 30 min at room temperature, followed by incubation with anti-rat IgG conjugated with POD for 30 min. Then, the level of POD activity bound to wells was measured. Representative results from four independent experiments are shown. aa, amino acids; OD450, optical density at 450 nm.
TABLE 3.
Reactivity of anti-CXCR4 MAbs to chimeric receptors composed of CXCR4 and CXCR2a
| Chimera | % Positive cells of transfectants stained with:
|
||||
|---|---|---|---|---|---|
| Mo-8 | 12G5 | A80 | A120 | A145 | |
| Vector alone | 5.7 | 4.0 | 4.4 | 4.8 | 4.9 |
| 2222 | 4.1 | 4.3 | 2.3 | 2.6 | 4.6 |
| 4444 | 3.1 | 18.8 | 22.0 | 19.1 | 20.3 |
| 2444 | 4.9 | 20.4 | 27.8 | 20.9 | 4.9 |
| 2442 | 2.7 | 17.8 | 2.4 | 19.7 | 4.1 |
| 2242 | 2.4 | 2.6 | 2.2 | 4.6 | 4.3 |
| 4442 | 2.9 | 20.3 | 2.5 | 21.8 | 18.2 |
| 2224 | 3.1 | 4.1 | 22.7 | 5.1 | 5.5 |
Cos-1 cells transfected with CXCR4 and CXCR2 chimeric genes by means of electroporation and cultured for 2 days were stained with MAbs, followed by treatment with goat anti-mouse or rat IgG-FITC, and analyzed on a flow cytometer. Mo-8, negative control. Representative results from three independent experiments are shown.
A80, A120, and A145 do not induce CXCR4 signaling.
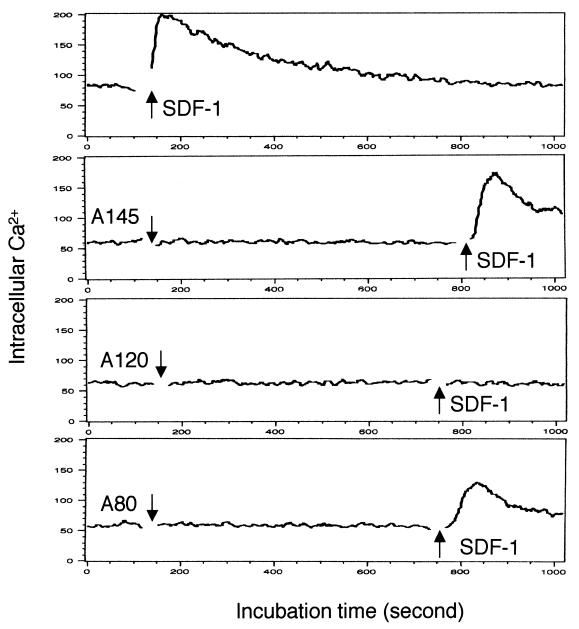
Since the binding of A80 to CXCR4 induced agglutination of CEM cells and PBMC, experiments were performed to determine whether binding of any of the three MAbs to CXCR4 induced a prototypic Gαi signaling response, i.e., mobilization of cytosolic calcium ions (Fig. 5). Whereas SDF-1 stimulated an influx of free calcium ions in CEM cells labeled with Fluo-3, exposure to A80, A120, or A145 did not induce calcium mobilization. Pretreatment of CEM cells with A120, but not A80 or A145, blocked the calcium response induced by SDF-1.
FIG. 5.
Ca2+ influx assay. CEM cells were loaded with Fluo-3 and then treated with 0.5 μg of SDF-1α/ml either before or after treatment with 20 μg of MAb/ml. Ca2+ influx was monitored by flow cytometry. The arrows indicate the time when SDF-1α or MAbs were added. Representative results from four independent experiments are shown.
DISCUSSION
Here we report the characterization of a new set of three rat MAbs to human CXCR4, one of which, A80, has unique biologic properties. Binding of A80 to ECL3 of CXCR4 on the surface of target cells enhanced productive infection by X4 and R5 strains of HIV-1 and augmented syncytium formation. A80 also induced agglutination of PBMC and CEM cells. The agglutination induced by A80 was blocked by SDF-1 and T22, a specific ligand and an antagonist of CXCR4, respectively, but not by inhibitors of CXCR4 signal transduction or MAbs to cytoadhesion molecules. The A120 MAb recognized an epitope that involved ECL1 and ECL2, and A145 bound to a peptide corresponding to the NT extracellular domain. A145 MAb bound CXCR4 in immunoprecipitation and Western blotting experiments, indicating that A145 bound an epitope that was not dependent upon native conformation of this domain. A120 blocked infection via CXCR4.
The mechanism for the enhancement of HIV-1 infection by A80 is novel. This effect was not altered by inhibition of signaling by the Gαi protein pathway or the phosphatidylinositol 3-kinase cascade and was accompanied by the induction of syncytium formation. A80 alone did not affect G-protein-mediated signaling, and SDF-1 did not induce agglutination; thus, the mechanism for stimulating this intercellular adhesion must involve an alternative pathway. The engagement of CXCR4 by SDF-1 or T22 blocks the effect of A80 on the agglutination of target cells. Analysis of structural requirements for the activation of signaling by SDF-1 has implicated ECL3 and adjacent transmembrane domains of CXCR4 (31). Thus, it is possible that binding of this CXCR4 domain by A80 may induce conformational changes in the receptor that activate a novel signaling pathway. The agglutination mediated by A80 binding to CXCR4 involves the development of intercellular adhesion. The stimulation of T lymphocyte and hematopoietic progenitor interactions with endothelial cells by SDF-1 has been shown to involve LFA-1 and VLA4/5, respectively (18, 45, 58). However, although a single MAb against a single epitope on an adhesion molecule may be not sufficient to interfere with adhesion function, cell adhesion blocking experiments with a set of MAbs against LFA-1, ICAMs, and CD29 (a common chain of the β1 integrin family) suggested that A80-mediated cell agglutination is independent of LFA-1, VLA4, and VLA5. Recent studies have demonstrated that the CX3C chemokine (fractalkine or neurotactin [fractalkine/neurotactin]) is capable of mediating firm intercellular adhesion through CX3CR1 that is resistant to pertussis toxin, exposure to EDTA or EGTA, and MAbs to β1 or β2 integrins (36, 43). Both CXCR4 and CX3CR1 effects are independent of receptor coupling to Gαi signaling and the function of the β1 or β2 integrins, but the latter mechanism requires that the chemokine module of fractalkine/neurotactin be attached to the mucin stalk that tethers it to the cell surface (43). Our preliminary studies showed that A80-mediated cell agglutination did not occur in the presence of 1 mM EDTA or EGTA and at 4°C (data not shown), suggesting that the cell agglutination is dependent on both calcium and temperature. Future experiments will determine whether monovalent fragments of A80 can induce syncytium formation and agglutination.
The set of new anti-CXCR4 MAbs will also serve as critical domain-specific reagents to study the function of CXCR4. Whereas A120, which recognizes an epitope involving ECL1 and ECL2 of CXCR4, blocked infection with X4 HIV-1, A80 enhanced productive infection. Binding of A145 to the NT extracellular domain resulted in low-level inhibition of HIV-1 infection. ECL2 and adjacent segments of CXCR4 have been implicated in its coreceptor activity with T-tropic envelope glycoproteins, but chimeras containing the NT extracellular domain did not support envelope-mediated fusion with dual- and T-tropic envelope glycoproteins (14–16, 19, 20, 31). Similarly, A120, but not A80 or A145, inhibited SDF-1 signaling through CXCR4. Binding and signaling studies with CXCR4/CXCR2 chimeras have implicated the segment between Cys28 and the interface with the first transmembrane helix of CXCR4 in the interaction with SDF-1 (31). It is therefore likely that A145 binds with the portion of this domain that is N terminal to Cys28, which contains more residues that are divergent between human and rodent CXCR4 homologs. The distinct biologic properties of these three MAbs establish the specificity of these reagents and illustrate the complexity of the structural basis of CXCR4 functions, both physiologic and pathological. The activities of the MAbs to epitopes in the NT and ECL1/2 domains show excellent correlation with our understanding of CXCR4 function derived from molecular genetic approaches. This set of domain-specific immunologic reagents should be a critical tool for dissecting the structural basis for CXCR4 functions, including novel activities in cytoadhesion.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Health Sciences of Organization for Drug ADR Relief, R&D Promotion, and Product Review of Japan; the Ministry of Health, Labor, and Welfare of Japan; the Japan Human Heath Sciences Foundation; and CREST of the Japan Science and Technology Corporation (JST).
We are grateful to Y. Koyanagi for providing HIV-1 and to H. Sato and Y. Takebe for unpublished data on HOS-CD4 cells.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–289. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfano M, Schmidtmayerova H, Amella C A, Pushkarsky T, Bukrinsky M. The B-oligomer of pertussis toxin deactivates CC chemokine receptor 5 and blocks entry of M-tropic HIV-1 strains. J Exp Med. 1999;190:597–606. doi: 10.1084/jem.190.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 4.Alkhatib G, Locati M, Kennedy P E, Murphy P M, Berger E A. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 5.Aramori I, Ferguson S S, Bieniasz P D, Zhang J, Cullen B, Cullen M G. Molecular mechanism of desensitization of the chemokine receptor CCR-5: receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO J. 1997;16:4606–4616. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbeau B, Fortin J F, Genois N, Tremblay M J. Modulation of human immunodeficiency virus type 1-induced syncytium formation by the conformational state of LFA-1 determined by a new luciferase-based syncytium quantitative assay. J Virol. 1998;72:7125–7136. doi: 10.1128/jvi.72.9.7125-7136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 8.Berger E A, Doms R W, Fenyo E M, Korber B T, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 9.Berkowitz R D, Alexander S, Bare C, Linquist-Stepps V, Bogan M, Moreno M E, Gibson L, Wieder E D, Kosek J, Stoddart C A, McCune J M. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J Virol. 1998;72:10108–10117. doi: 10.1128/jvi.72.12.10108-10117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berndt C, Mopps B, Angermuller S, Gierschik P, Krammer P H. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4+ T cells. Proc Natl Acad Sci USA. 1998;95:12556–12561. doi: 10.1073/pnas.95.21.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bieniasz P D, Fridell R A, Aramori I, Ferguson S S, Caron M G, Cullen B R. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco J, Jacotot E, Cabrera C, Cardona A, Clotet B, De Clercq E, Este J A. The implication of the chemokine receptor CXCR4 in HIV-1 envelope protein-induced apoptosis is independent of the G protein-mediated signalling. AIDS. 1999;13:909–917. doi: 10.1097/00002030-199905280-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brelot A, Heveker N, Adema K, Hosie M J, Willett B, Alizon M. Effect of mutations in the second extracellular loop of CXCR4 on its utilization by human and feline immunodeficiency viruses. J Virol. 1999;73:2576–2586. doi: 10.1128/jvi.73.4.2576-2586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brelot A, Heveker N, Montes M, Alizon M. Identification of residues of CXCR4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities. J Biol Chem. 2000;31:23736–23744. doi: 10.1074/jbc.M000776200. [DOI] [PubMed] [Google Scholar]
- 16.Brelot A, Heveker N, Pleskoff O, Sol N, Alizon M. Role of the first and third extracellular domains of CXCR-4 in human immunodeficiency virus coreceptor activity. J Virol. 1997;71:4744–4751. doi: 10.1128/jvi.71.6.4744-4751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butini L, De Fougerolles A R, Vaccarezza M, Graziosi C, Cohen D I, Montroni M, Springer T A, Pantaleo G, Fauci A S. Intercellular adhesion molecules (ICAM)-1, ICAM-2, and ICAM-3 function as counter-receptors for lymphocyte function-associated molecule 1 in human immunodeficiency virus-mediated syncytium formation. Eur J Immunol. 1994;24:2191–2195. doi: 10.1002/eji.1830240939. [DOI] [PubMed] [Google Scholar]
- 18.Campbell J J, Hedrick J, Zlotnik A, Siani M A, Thompson D A, Butcher E C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 19.Chabot D J, Chen H, Dimitrov D S, Broder C C. N-linked glycosylation of CXCR4 masks coreceptor function for CCR5-dependent human immunodeficiency virus type 1 isolates. J Virol. 2000;74:4404–4413. doi: 10.1128/jvi.74.9.4404-4413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chabot D J, Zhang P F, Quinnan G V, Broder C C. Mutagenesis of CXCR4 identifies important domains for human immunodeficiency virus type 1 X4 isolate envelope-mediated membrane fusion and virus entry and reveals cryptic coreceptor activity for R5 isolates. J Virol. 1999;73:6598–6609. doi: 10.1128/jvi.73.8.6598-6609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 22.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connor R I, Ho D D. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J Virol. 1994;68:4400–4408. doi: 10.1128/jvi.68.7.4400-4408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connor R I, Ho D D. Transmission and pathogenesis of human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 1994;10:321–323. doi: 10.1089/aid.1994.10.321. [DOI] [PubMed] [Google Scholar]
- 25.Constantin G, Majeed M, Giagulli C, Piccio L, Kim J V, Butcher E C, Laudanna C. Chemokines trigger immediate β2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 26.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 27.Davis C B, Dikic I, Unutmaz D, Hill C M, Arthos J, Siani M A, Thompson D A, Schlessinger J, Littman D R. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;38:1661–1666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 29.Dimitrov D S, Xiao X, Chabot D J, Broder C C. HIV coreceptors. J Membr Biol. 1998;166:75–90. doi: 10.1007/s002329900450. [DOI] [PubMed] [Google Scholar]
- 30.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 31.Doranz B J, Orsini M J, Turner J D, Hoffman T L, Berson J F, Hoxie J A, Peiper S C, Brass L F, Doms R W. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J Virol. 1999;73:2752–2761. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 33.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 34.Farzan M, Choe H, Martin K A, Sun Y, Sidelko M, Mackay C R, Gerard N P, Sodroski J, Gerard C. HIV-1 entry and macrophage inflammatory protein-1-mediated signaling are independent functions of the chemokine receptor CCR5. J Biol Chem. 1997;272:6854–6857. doi: 10.1074/jbc.272.11.6854. [DOI] [PubMed] [Google Scholar]
- 35.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 36.Fong A M, Robinson L A, Steeber D A, Tedder T F, Yoshie O, Imai T, Patel D D. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortin J F, Barbeau B, Hedman H, Lundgren E, Tremblay M J. Role of the leukocyte function antigen-1 conformational state in the process of human immunodeficiency virus type 1-mediated syncytium formation and virus infection. Virology. 1999;257:228–238. doi: 10.1006/viro.1999.9687. [DOI] [PubMed] [Google Scholar]
- 38.Gosling J, Monteclaro F S, Atchison R E, Arai H, Tsou C L, Goldsmith M A, Charo I F. Molecular uncoupling of C-C chemokine receptor 5-induced chemotaxis and signal transduction from HIV-1 coreceptor activity. Proc Natl Acad Sci USA. 1997;94:5061–5066. doi: 10.1073/pnas.94.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson D L, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1α is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 40.Hildreth J E, Orentas R J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989;244:1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- 41.Hori T, Sakaida H, Sato A, Nakajima T, Shida H, Yoshie O, Uchiyama T. Detection and delineation of CXCR-4 (fusin) as an entry and fusion cofactor for T-tropic [correction of T cell-tropic] HIV-1 by three different monoclonal antibodies. J Immunol. 1998;160:180–188. [PubMed] [Google Scholar]
- 42.Horuk R. Chemokine receptors and HIV-1: the fusion of two major research fields. Immunol Today. 1999;20:89–94. doi: 10.1016/s0167-5699(98)01396-6. [DOI] [PubMed] [Google Scholar]
- 43.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall T J, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1977;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 44.Inudo M, Kato N, Tanaka Y. New monoclonal antibodies against a recombinant second envelope protein of hepatitis C virus. Microbiol Immunol. 1998;42:875–877. doi: 10.1111/j.1348-0421.1998.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 45.Kantele J M, Kurk S, Jutila M A. Effects of continuous exposure to stromal cell-derived factor-1α on T cell rolling and tight adhesion to monolayers of activated endothelial cells. J Immunol. 2000;164:5035–5040. doi: 10.4049/jimmunol.164.10.5035. [DOI] [PubMed] [Google Scholar]
- 46.Karasuyama H, Tohyama N, Tada T. Autocrine growth and tumorigenicity of interleukin 2-dependent helper T cells transfected with IL-2 gene. J Exp Med. 1989;169:13–25. doi: 10.1084/jem.169.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 48.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S Y. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 49.LaBonte J A, Patel T, Hofmann W, Sodroski J. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J Virol. 2000;74:10690–10698. doi: 10.1128/jvi.74.22.10690-10698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lifson J D, Reyes G R, McGrath M S, Stein B S, Engleman E G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986;232:1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- 51.Lifson J D, Feinberg M B, Reyes G R, Rabin L, Banapour B, Chakrabarti S, Moss B, Wong-Staal F, Steimer K S, Engleman E G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986;323:725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- 52.Lu Z, Berson J F, Chen Y, Turner J D, Zhang T, Sharron M, Jenks M H, Wang Z, Kim J, Rucker J, Hoxie J A, Peiper S C, Doms R W. Evolution of HIV-1 coreceptor usage through interactions with distinct CCR5 and CXCR4 domains. Proc Natl Acad Sci USA. 1997;94:6426–6431. doi: 10.1073/pnas.94.12.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, Kishimoto T, Yamamoto N, Nagasawa T. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389–1393. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul C C, Yoshie O, Matsushima K, Yoshida N, Springer T A, Kishimoto T. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci USA. 1996;93:14726–14729. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nanki T, Lipsky P E. Stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. J Immunol. 2000;164:5010–5014. doi: 10.4049/jimmunol.164.10.5010. [DOI] [PubMed] [Google Scholar]
- 56.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 57.Pantaleo G, Butini L, Graziosi C, Poli G, Schnittman S M, Greenhouse J J, Gallin J I, Fauci A S. Human immunodeficiency virus (HIV) infection in CD4+ T lymphocytes genetically deficient in LFA-1: LFA-1 is required for HIV-mediated cell fusion but not for viral transmission. J Exp Med. 1991;173:511–514. doi: 10.1084/jem.173.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav M M, Nagler A, Lider O, Alon R, Zipori, Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34+ cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 59.Samson M, Labbe O, Mollereau C, Parmentir G. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 60.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Signoret N, Oldridge J, Pelchen-Matthews A, Klasse P J, Tran T, Brass L F, Rosenkilde M M, Schwartz T W, Holmes W, Dallas W, Luther M A, Wells T N, Hoxie J A, Marsh M. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sodroski J, Goh W C, Rosen C, Campbell C, Haseltine W A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature. 1986;322:470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka Y, Tozawa H, Koyanagi Y, Shida H. Recognition of human T cell leukemia virus type I (HTLV-I) gag and pX gene products by MHC-restricted cytotoxic T lymphocytes induced in rats against syngeneic HTLV-I-infected cells. J Immunol. 1990;144:4202–4211. [PubMed] [Google Scholar]
- 64.Tanaka Y, Zeng L, Shiraki H, Shida H, Tozawa H. Identification of a neutralization epitope on the envelope gp46 antigen of human T cell leukemia virus type 1 and induction of neutralizing antibody by peptide immunization. J Immunol. 1991;147:354–360. [PubMed] [Google Scholar]
- 65.Valentin A, Lundin K, Patarroyo M, Asjo B. The leukocyte adhesion glycoprotein CD18 participates in HIV-1-induced syncytia formation in monocytoid and T cells. J Immunol. 1990;144:934–937. [PubMed] [Google Scholar]
- 66.Wang J F, Park I W, Groopman J E. Stromal cell-derived factor-1α stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C. Blood. 2000;95:2505–2513. [PubMed] [Google Scholar]
- 67.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 68.Yang O O, Swanberg S L, Lu Z, Dziejman M, McCoy J, Luster A D, Walker B D, Herrmann S H. Enhanced inhibition of human immunodeficiency virus type 1 by Met-stromal-derived factor 1β correlates with down-modulation of CXCR4. J Virol. 1999;73:4582–4589. doi: 10.1128/jvi.73.6.4582-4589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng J, Ghorpade A, Niemann D, Cotter R L, Thylin M R, Epstein L, Swartz J M, Shepard R B, Liu X, Nukuna A, Gendelman H E. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: implications for human immunodeficiency virus type 1-associated dementia. J Virol. 1999;73:8256–8267. doi: 10.1128/jvi.73.10.8256-8267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 71.Zou Y R, Kottmann A H, Kuroda M, Taniuchi I, Littman D R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]