Abstract
Methamphetamine use and HIV disproportionately affect sexual and gender minority (SGM) people assigned male at birth. Identifying risk factors for methamphetamine use is crucial to inform preventive interventions. In this cohort study with 1,296 SGM people assigned male at birth, ages 16 to 29, and who resided in Chicago, Poisson regression analyses indicated the prevalence of methamphetamine use increased from 2015 to 2023 [Incidence Rate Ratio (IRR) = 1.07; 95% CI = 1.01 to 1.13; P = 0.02]. This increase was most pronounced among those ages 25 or older at baseline (IRR = 2.20; 95% CI = 1.33 to 3.63; P = 0.002), and 23.9 [Interquartile Range (IQR) = 22.1 to 26.9] was the median age of first-time methamphetamine use. In 826 participants with a prior HIV diagnosis or previous inflammatory measurements, Cox proportional-hazards models examined risk factors for incident, first-time methamphetamine use. Adjusting for other substance use, the rate of incident, first-time methamphetamine use was two-fold greater after HIV diagnosis [adjusted hazard ratio (aHR) = 2.02; 95% CI = 1.27 to 3.23; P = 0.003]. For each SD higher C-reactive protein, the rate of incident, first-time methamphetamine use was 18% greater (aHR = 1.18; 95% CI, 1.05 to 1.34; P = 0.008). HIV seroconversion and inflammation could increase the risk of initiating methamphetamine use in SGM people assigned male at birth.
Keywords: HIV, inflammation, men who have sex with men, methamphetamine
In the United States, a resurgent methamphetamine epidemic is disproportionately affecting racial, ethnic, sexual, and gender minority communities (1, 2). Methamphetamine use has several medical consequences including severe psychiatric symptoms, fatal overdose, and HIV (3–5). Among sexual and gender minority (SGM) people assigned male at birth, methamphetamine use is prevalent and associated with diminished effectiveness of biomedical HIV prevention (5–7).
Elucidating the bidirectional pathways linking methamphetamine use and inflammation could inform preventive interventions. Prior research focused extensively on how cooccurring substance use and HIV could modulate neuroimmune outcomes (8). Specifically, the use of stimulants such as methamphetamine has been associated with higher C-reactive protein (CRP) and immune activation markers irrespective of HIV status (9, 10). Few studies have examined the reverse direction, if HIV and inflammation potentiate methamphetamine use. The scientific premise that HIV and inflammation potentiate methamphetamine use is partially supported by findings where HIV transgenic (versus wild type) rats displayed faster reinstatement of methamphetamine use and greater neuroinflammation in the prefrontal cortex (11).
Inflammation-driven neurobehavioral changes could amplify risk for initiating methamphetamine use. Systemic inflammation decreases availability of striatal dopamine, altering reward processing and increasing anhedonia (12). The clinical relevance of inflammation is supported by time-lagged associations of higher CRP with greater risk of cannabis use and a tobacco use disorder in youth ages 9 to 12 (13). In another cohort of youth ages 12 to 13, higher interleukin-6 (IL-6) and tumor necrosis factor–alpha (TNF-α) were indirectly associated with substance use (i.e., alcohol, tobacco, cannabis, and other substances) via more functional difficulties (14). This study examined whether HIV and inflammation predated incident, first-time methamphetamine use in SGM people assigned male at birth.
Results
Mean baseline age was 21.5 (SD = 3.2) and most participants identified as Black/African American (32%) or Hispanic/Latine (30%), cisgender men (90%), and monosexual (67%). Most participants were HIV-negative (85%) and had some post-high school education (60%).
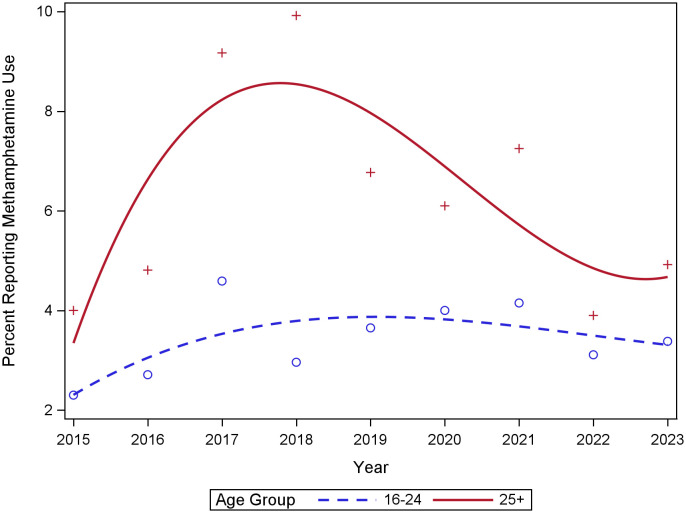
In Poisson regression analyses, the prevalence of self-reported methamphetamine use increased from 2015 to 2023 (Incidence Rate Ratio [IRR] = 1.07; 95% CI = 1.01 to 1.13; P = 0.02), particularly among individuals 25 years of age or older (IRR = 2.20; 95% CI = 1.33 to 3.63; P = 0.002). Prevalence peaked at 9.9% (in 2018) and 4.6% (in 2017) for individuals 25 years of age or older and 16 to 24 years of age, respectively (Fig. 1).
Fig. 1.
Trends in prevalence of self-reported methamphetamine use from 2015 to 2023 among SGM youth ages 16 to 29 at baseline in the RADAR cohort (N = 1,296).
There were 130 incident, first-time methamphetamine use cases (i.e., any self-reported use or reactive urine toxicology) across 5,128 person-years (crude incidence rate = 2.53/100 person years; 95% CI = 2.13 to 3.00). The median age of incident, first-time methamphetamine use was 23.9 (IQR = 22.1 to 26.9). In a multivariate Cox proportional-hazards model, prior HIV diagnosis (adjusted hazard ratio [aHR] = 2.02, 95% CI, 1.27 to 3.23, P = 0.003), any cocaine use (aHR = 3.28, 95% CI, 2.07 to 5.19, P < 0.001), any cannabis use (aHR = 2.15, 95% CI, 1.09 to 4.25, P = 0.03), and each SD higher CRP (aHR = 1.18, 95% CI. 1.05 to 1.34, P = 0.008) were independently associated with greater rates of incident, first-time methamphetamine use (Table 1). There were no significant two-way interactions among these independent risk factors.
Table 1.
Bivariate and multivariate Cox proportional hazards models examining incident, first-time methamphetamine use (self-report or reactive urine toxicology) among SGM people asssigned male at birth [N = (826 participants, 6,129 observations)]
| N (%) | Bivariate models HR (95% CI) |
Multivariate model aHR (95% CI) |
|
|---|---|---|---|
| Race/ethnicity | |||
| Non-hispanic white | 190 (23.0) | – | – |
| Non-hispanic black | 311 (37.7) | 1.98 (1.18 to 3.35) | 1.84 (0.85 to 3.95) |
| Hispanic/latine | 258 (31.2) | 1.34 (0.76 to 2.36) | 1.57 (0.74 to 3.32) |
| Non-hispanic other | 67 (8.1) | 1.09 (0.50 to 2.42) | 1.98 (0.73 to 5.36) |
| High school graduate or less (ref = greater than high school) | 1,551 (25.3) | 1.48 (1.01 to 2.18) | 1.10 (0.69 to 1.75) |
| Prior HIV diagnosis (ref = negative) | 1,365 (22.3) | 3.05 (2.10 to 4.44) | 2.02 (1.27 to 3.23) |
| Any cannabis use (ref = no) | 4,472 (73.0) | 3.31 (1.82 to 6.01) | 2.15 (1.09 to 4.25) |
| Any cocaine use (ref = no) | 750 (12.2) | 4.31 (2.95 to 6.29) | 3.28 (2.07 to 5.19) |
| Any prescription drug use (ref = no) | 522 (8.5) | 1.99 (1.22 to 3.24) | 1.34 (0.70 to 2.55) |
| CRP categories | |||
| 0 to 0.99 | 2,814 (45.9) | – | – |
| 1.00 to 1.99 | 1,113 (18.2) | 1.08 (0.62 to 1.89) | – |
| 2.00 to 2.99 | 568 (9.3) | 0.71 (0.30 to 1.67) | – |
| 3.00 or greater | 1,634 (26.7) | 1.16 (0.72 to 1.89) | – |
| Median (IQR) | HR (95% CI) | aHR (95% CI) | |
| Age | 24.0 (4.8) | 1.07 (1.01 to 1.14) | 1.03 (0.96 to 1.10) |
| Continuous TNF-α* | 1.4 (1.0) | 1.06 (0.87 to 1.28)† | – |
| Continuous IL-6* | 0.5 (0.5) | 0.90 (0.65 to 1.26)† | – |
| Continuous CRP* | 1.2 (2.8) | 1.19 (1.06 to 1.32)† | 1.18 (1.05 to 1.34) |
*Median and IQR are reported in raw units (pg/mL for IL-6 and TNF-alpha; mg/L for CRP).
†Exposure was standardized (M = 0; SD = 1) prior to inclusion in Cox proportional-hazards models.
Exposure was standardized (M = 0; SD = 1) prior to inclusion in Cox proportional-hazards models.
Significant associations (p < 0.05) are bolded.
Discussion
Methamphetamine use is an established risk factor for HIV and inflammation, and the converse may also be true. SGM people assigned male at birth had two-fold greater rates of first-time methamphetamine use following HIV diagnosis and each SD greater CRP was associated with 18% greater rates of first-time methamphetamine use. Future studies should examine the neurobehavioral mechanisms (e.g., reward processing) whereby HIV, inflammation, cocaine use, and cannabis use are associated with greater rates of incident, first-time methamphetamine use (12, 14). Mechanistic research should also determine whether HIV-associated alterations in the gut–brain axis amplify the risk for incident, first-time methamphetamine use (15).
Findings should be interpreted in the context of some limitations. Methamphetamine use prevalence was lower in this cohort, reflecting regional variation. Although urine toxicology screening partially addressed misclassification due to underreporting, hair toxicology testing provides more durable estimates. More comprehensive efforts are needed to characterize distinct trajectories of polysubstance use or specific patterns of couse (e.g., cannabis and cocaine) that could amplify the risk of initiating methamphetamine use. Future studies should also include more comprehensive panels of inflammatory markers and measure neuroinflammation in cerebrospinal fluid. Finally, this study was underpowered to test the interactions of the risk factors identified. Despite these limitations, this study will inform selective preventive interventions for SGM people with HIV, higher CRP, and current cocaine or cannabis use to decrease the risk of initiating methamphetamine use.
Materials and Methods
RADAR enrolled 1,302 SGM people living in the Chicago metropolitan area. Participants were assigned male at birth, between 16 and 29 years of age, and spoke English. All participants reported a sexual encounter with a man in the previous year or identified as a SGM person. RADAR visits were conducted twice per year for a median of 5.0 y (IQR = 2.0 to 7.1).
Measures.
Demographics. Age, sexual orientation, gender identity, race, ethnicity, and education were assessed.
HIV status. People with HIV were enrolled at baseline (n = 195) or seroconverted during follow-up (n = 89). HIV status was determined by rapid point-of-care testing for in-person visits.
Systemic inflammation. The MESO QuickPlex SQ 120 electrochemiluminescence immunoassay platform (Meso Scale Discovery, MSD) quantified plasma levels of CRP (MSD V-PLEX Plus Human CRP kit [dynamic range: 0.00000133 to 49.6 mg/L]) and cytokines that can induce CRP, namely IL-6 and TNF-α (MSD V-PLEX Custom Proinflammatory Panel 1 [human] kit [IL-6 dynamic range: 0.06 to 488 pg/mL; TNF-α dynamic range: 0.04 to 248 pg/mL]). Inflammatory markers were standardized (M = 0; SD = 1) to facilitate interpretation.
Substance use. Cannabis, cocaine, or methamphetamine use was classified as any self-reported use of each substance in the past six months or reactive urine toxicology results. Prescription drug use was classified as self-reported nonprescribed use of stimulants, depressants, or opioids in the past six months. We were unable to examine prescription opioid use separately due to the low prevalence in this cohort.
Statistical Analyses.
Poisson regression using robust SE assessed annual trends in self-reported methamphetamine use between 2015 and 2023, stratified by baseline age. An offset equal to the log of the total time at risk per year accounted for varying exposure time per participant. Participants with available data on self-reported methamphetamine use at baseline were included in analyses examining changes in methamphetamine use prevalence over calendar time (n = 1,296).
Cox proportional-hazards analyses were conducted using both time-dependent [i.e., age, education, HIV status, substance use, and systemic inflammation (where applicable)] and time-independent [i.e., race, ethnicity, and systemic inflammation (where applicable)] exposures. Time-independent exposures were measured at the baseline visit while time-dependent exposures were taken from the visit preceding the event. The timescale was measured in days and time-at-risk for those who initiated methamphetamine use was computed as the midpoint between the date of first use and the preceding visit. For noninitiators, time-at-risk ceased at their last visit. Events with the same survival time were modeled using an exact method. Bivariate Cox proportional-hazards models guided the selection of exposures (i.e., P < 0.10) for the multivariate model. Fit indices for continuous (AIC = 1,089 and BIC = 1,114) versus categorical (AIC = 1,096 and BIC = 1,126) CRP indicated that continuous CRP had better model fit. In total, 826 participants where HIV diagnosis or inflammatory measurements preceded incident, first-time methamphetamine use were included in Cox proportional-hazards analyses.
Acknowledgments
The RADAR cohort is supported by the National Institute on Drug Abuse (U01-DA036939; B.M., Principal Investigator (PI)) and additional support was provided by the Third Coast Center for AIDS Research (P30-AI117943; B.M. and R.T.D., PIs).
Author contributions
A.W.C., M.N., R.T.D., and B.M. designed research; J.M.S., T.W.M., M.N., R.T.D., and B.M. performed research; A.W.C., D.T.R., and J.B. analyzed data; J.M.S., T.W.M., M.N., and R.T.D. provided feedback on analyses and manuscript drafts; and A.W.C., B.S.D., and B.M. wrote the paper.
Competing interests
The authors declare no competing interest.
Data, Materials, and Software Availability
Metadata and code for variables in this manuscript data have been deposited in Nortwestern University Research and Data Repository: https://arch.library.northwestern.edu (https://doi.org/10.21985/n2-jze7-xw88) (16). There are no data underlying this work.
References
- 1.Han B., Compton W. M., Jones C. M., Einstein E. B., Volkow N. D., Methamphetamine use, methamphetamine use disorder, and associated overdose deaths among US adults. JAMA Psychiatry 78, 1329–1342 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera A. V., Harriman G., Carrillo S. A., Braunstein S. L., Trends in methamphetamine use among men who have sex with men in New York City, 2004–2017. AIDS Behav. 25, 1210–1218 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKetin R., et al. , Mental health outcomes associated with the use of amphetamines: A systematic review and meta-analysis. eClinicalMedicine 16, 81–97 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han B., et al. , Methamphetamine overdose deaths in the US by sex and race and ethnicity. JAMA Psychiatry 78, 564–567 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grov C., Westmoreland D., Morrison C., Carrico A. W., Nash D., The crisis we are not talking about: One-in-three annual HIV seroconversions among sexual and gender minorities were persistent methamphetamine users. J. Acquir Immune. Defic. Syndr. 85, 272–279 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross E. J., et al. , Overamped: Stimulant Use and HIV Pathogenesis. Curr. HIV/AIDS Rep. 20, 321–332 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Viamonte M., Ghanooni D., Reynolds J. M., Grov C., Carrico A. W., Running with scissors: A systematic review of substance use and the pre-exposure prophylaxis care continuum among sexual minority men. Curr. HIV/AIDS Rep. 19, 235–250 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saloner R., et al. , Methamphetamine and cannabis: A tale of two drugs and their effects on HIV, brain, and behavior. J. Neuroimmune Pharmacol. 15, 743–764 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan E., Taylor H. E., Ryan D. T., D’Aquila R., Mustanski B., Systemic inflammation is elevated among both HIV-uninfected and HIV-infected young MSM. AIDS 33, 757–759 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherenack E. M., et al. , Stimulant use, HIV, and immune dysregulation among sexual minority men. Drug Alcohol. Depend. 251, 110942 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Guglielmo G., et al. , Increases in compulsivity, inflammation, and neural injury in HIV transgenic rats with escalated methamphetamine self-administration under extended-access conditions. Brain Res. 1726, 146502 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucido M. J., et al. , Aiding and abetting anhedonia: Impact of inflammation on the brain and pharmacological implications. Pharmacol. Rev. 73, 1084–1117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costello E. J., Copeland W. E., Shanahan L., Worthman C. M., Angold A., C-reactive protein and substance use disorders in adolescence and early adulthood: A prospective analysis. Drug Alcohol. Depend. 133, 712–717 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chat I. K.-Y., et al. , Concurrent and prospective associations of inflammatory signaling, specific depressive symptoms, and substance use in adolescence. Brain Behav. Immunity 110, 85–94 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrico A. W., et al. , Through the looking-glass: Psychoneuroimmunology and the microbiome-gut-brain axis in the modern antiretroviral therapy era. Psychosomatic Med. 84, 984 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustanski B., Data from “HIV, inflammation, and initiation of methamphetamine use in sexual and gender minorities assigned male at birth.” Figshare. 10.21985/n2-jze7-xw88. Deposited 9 July 2024. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Metadata and code for variables in this manuscript data have been deposited in Nortwestern University Research and Data Repository: https://arch.library.northwestern.edu (https://doi.org/10.21985/n2-jze7-xw88) (16). There are no data underlying this work.