Abstract
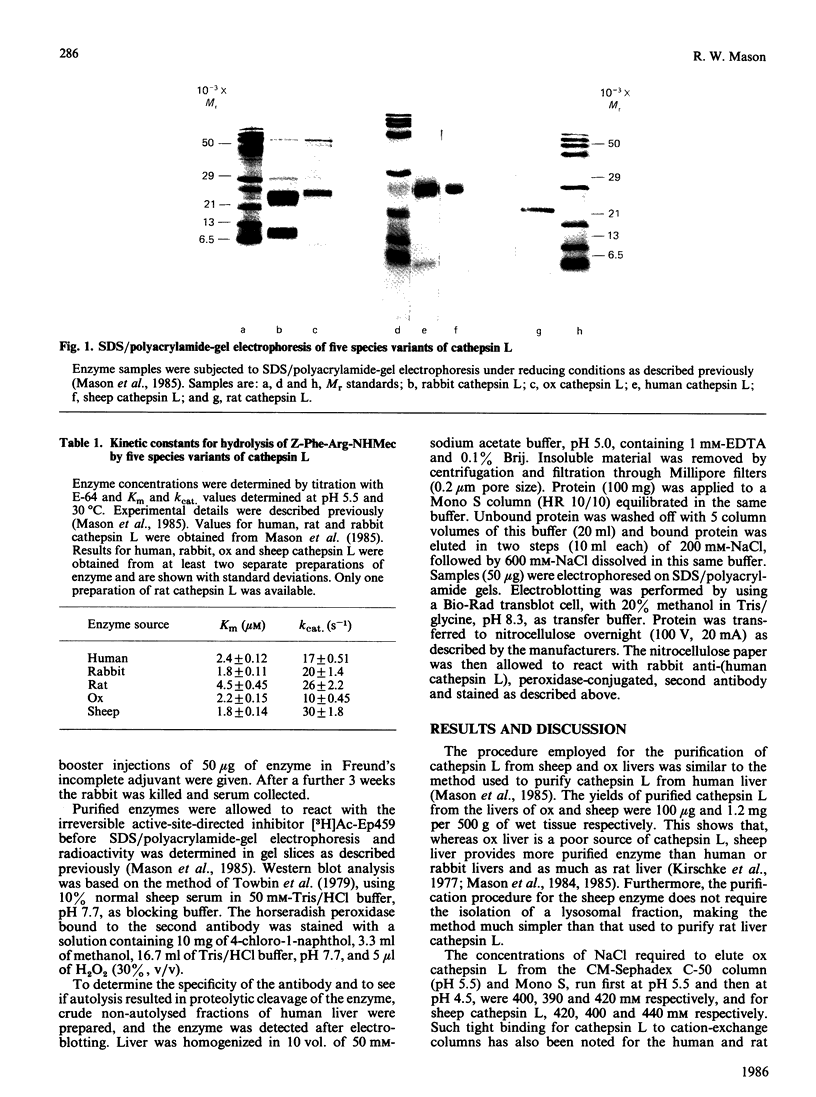
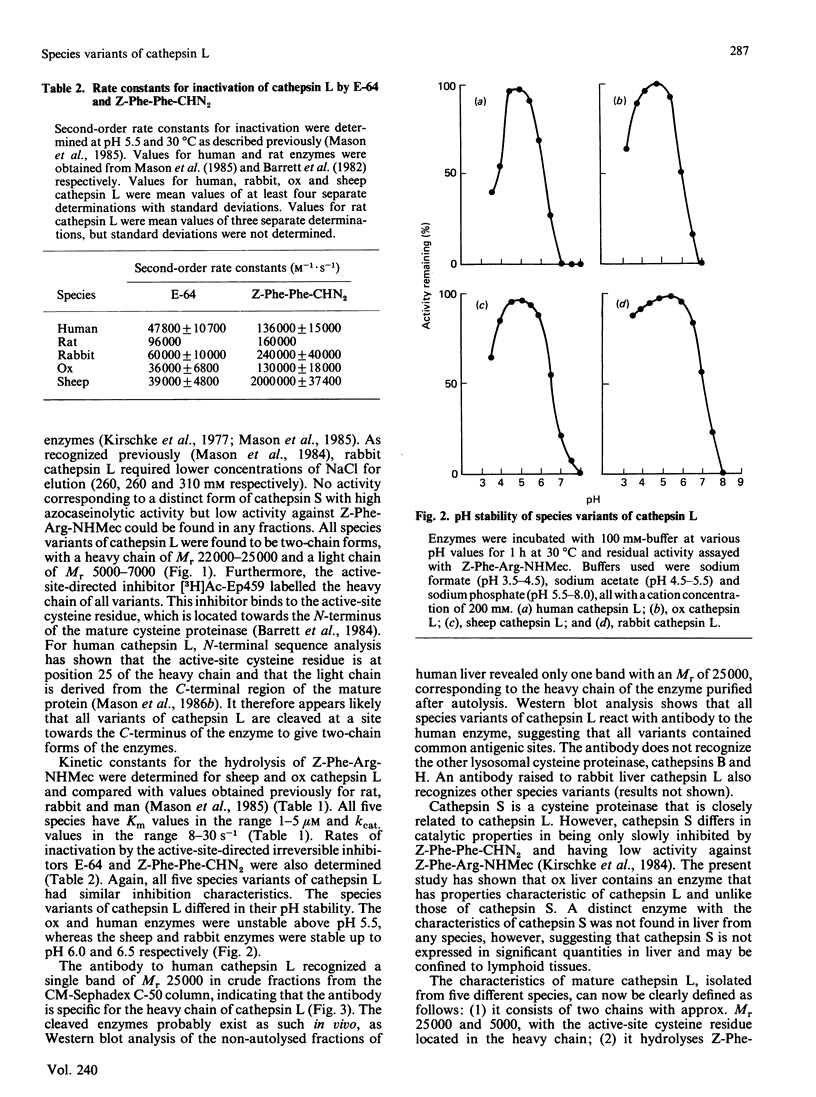
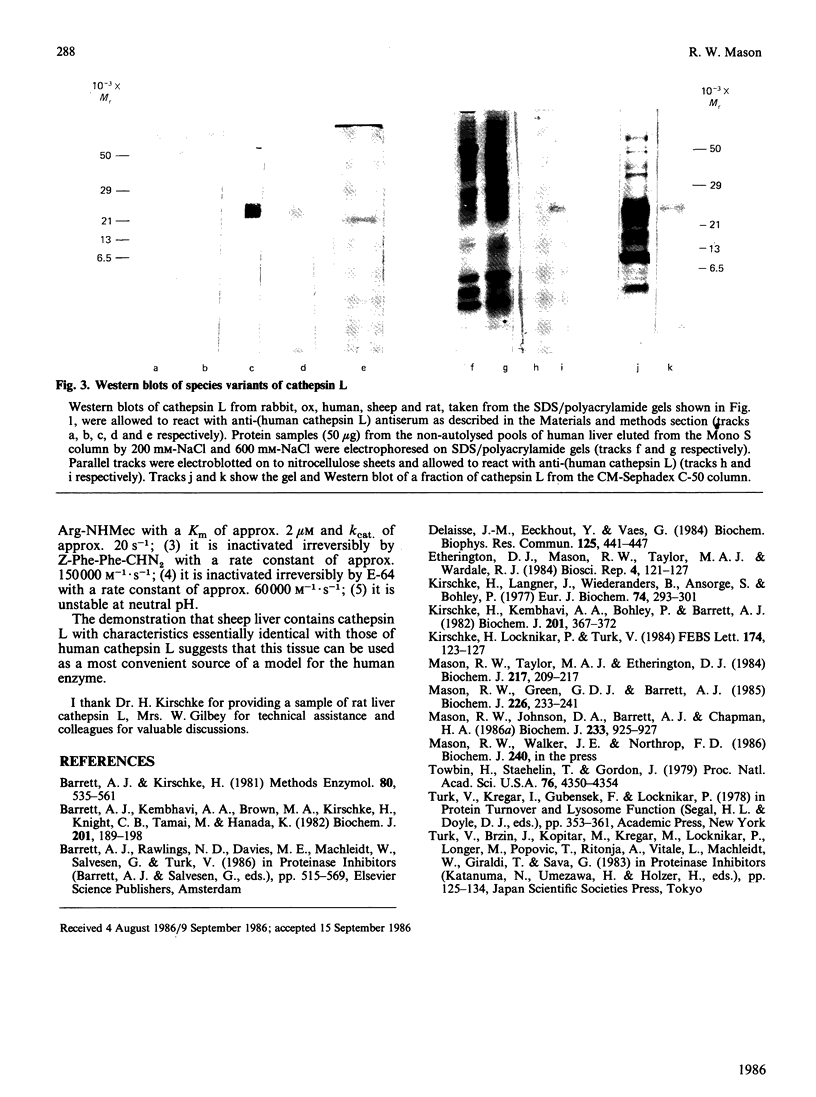
Cathepsin L variants purified from sheep and ox liver are shown to have similar catalytic properties to those from rat, rabbit and man with regard to activity against the substrate benzyloxycarbonyl-Phe-Arg-7-(4-methyl)coumarylamide and inhibition by benzyloxycarbonyl-Phe-Phe-diazomethane, thus identifying cathepsin L in these species for the first time. All five variants of cathepsin L are shown to be immunologically related by their interaction with antibodies raised to the human enzyme. Sheep liver was found to yield more enzyme than any other species, suggesting that this tissue is a good source of cathepsin L. Cathepsin S, a closely related enzyme, could not be detected in livers of any of these species.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Delaissé J. M., Eeckhout Y., Vaes G. In vivo and in vitro evidence for the involvement of cysteine proteinases in bone resorption. Biochem Biophys Res Commun. 1984 Dec 14;125(2):441–447. doi: 10.1016/0006-291x(84)90560-6. [DOI] [PubMed] [Google Scholar]
- Etherington D. J., Mason R. W., Taylor M. A., Wardale R. J. Production of a monospecific antiserum to cathepsin L: the histochemical location of enzyme in rabbit fibroblasts. Biosci Rep. 1984 Feb;4(2):121–127. doi: 10.1007/BF01120308. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Kembhavi A. A., Bohley P., Barrett A. J. Action of rat liver cathepsin L on collagen and other substrates. Biochem J. 1982 Feb 1;201(2):367–372. doi: 10.1042/bj2010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P. Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977 Apr 1;74(2):293–301. doi: 10.1111/j.1432-1033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Locnikar P., Turk V. Species variations amongst lysosomal cysteine proteinases. FEBS Lett. 1984 Aug 20;174(1):123–127. doi: 10.1016/0014-5793(84)81089-3. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Johnson D. A., Barrett A. J., Chapman H. A. Elastinolytic activity of human cathepsin L. Biochem J. 1986 Feb 1;233(3):925–927. doi: 10.1042/bj2330925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Taylor M. A., Etherington D. J. The purification and properties of cathepsin L from rabbit liver. Biochem J. 1984 Jan 1;217(1):209–217. doi: 10.1042/bj2170209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]




