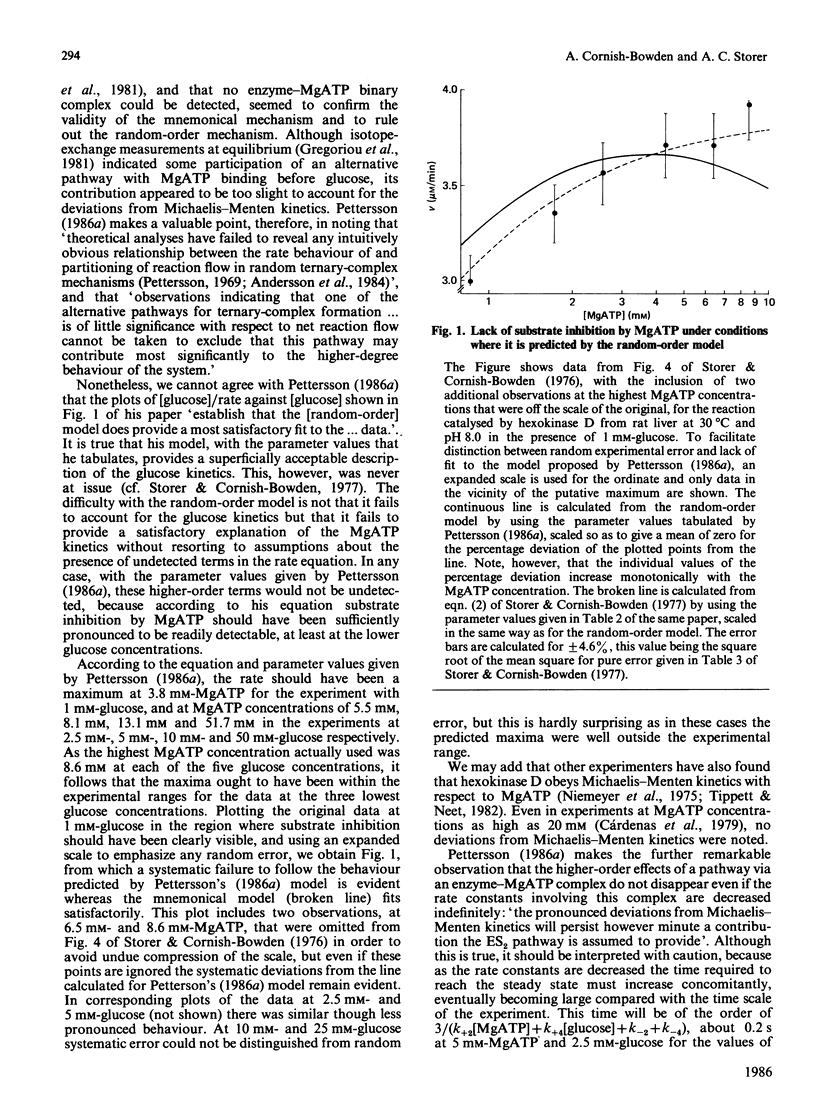
Abstract
Two recent proposals to account for the kinetic co-operativity of hexokinase D ('glucokinase') from rat liver are examined. A model in which the deviations from Michaelis-Menten kinetics result from a random order of binding of the substrates [Pettersson (1986) Biochem. J. 233, 347-350] accounts satisfactorily for the behaviour as a function of glucose concentrations, but it also predicts observable substrate inhibition by MgATP, which is in fact not observed. An alternative proposal in which the deviations arise from recycling of an enzyme-MgADP complex [Pettersson (1986) Eur. J. Biochem. 154, 167-170] also accounts satisfactorily for some of the data, but the required enzyme-MgADP complex could not be detected in isotope-exchange measurements. Thus the mnemonical mechanism proposed originally [Storer & Cornish-Bowden (1977) Biochem. J. 165, 61-69], which explains the deviations in terms of a relatively slow interconversion between two forms of free enzyme, remains the most parsimonious explanation of the behavior of hexokinase D.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson P., Kvassman J., Oldén B., Pettersson G. Catalytic significance of binary enzyme-aldehyde complexes in the liver alcohol dehydrogenase reaction. Eur J Biochem. 1984 Mar 15;139(3):519–527. doi: 10.1111/j.1432-1033.1984.tb08036.x. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Trayer I. P. Reaction of rat hepatic glucokinase with substrate-related and other alkylating agents. Eur J Biochem. 1979 Sep;99(2):299–308. doi: 10.1111/j.1432-1033.1979.tb13257.x. [DOI] [PubMed] [Google Scholar]
- Cárdenas M. L., Rabajille E., Niemeyer H. Kinetic cooperativity of glucokinase with glucose. Arch Biol Med Exp (Santiago) 1979 Dec;12(5):571–580. [PubMed] [Google Scholar]
- Cárdenas M. L., Rabajille E., Niemeyer H. Suppression of kinetic cooperativity of hexokinase D (glucokinase) by competitive inhibitors. A slow transition model. Eur J Biochem. 1984 Nov 15;145(1):163–171. doi: 10.1111/j.1432-1033.1984.tb08536.x. [DOI] [PubMed] [Google Scholar]
- Gregoriou M., Trayer I. P., Cornish-Bowden A. Isotope-exchange evidence for an ordered mechanism for rat-liver glucokinase, a monomeric cooperative enzyme. Biochemistry. 1981 Feb 3;20(3):499–506. doi: 10.1021/bi00506a009. [DOI] [PubMed] [Google Scholar]
- Holroyde M. J., Allen M. B., Storer A. C., Warsy A. S., Chesher J. M., Trayer I. P., Cornish-Bowden A., Walker D. G. The purification in high yield and characterization of rat hepatic glucokinase. Biochem J. 1976 Feb 1;153(2):363–373. doi: 10.1042/bj1530363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J. C., Buc J., Navarro A., Ricard J. Regulatory behavior of monomeric enzymes. 2. A wheat-germ hexokinase as a mnemonical enzyme. Eur J Biochem. 1974 Nov 1;49(1):209–223. doi: 10.1111/j.1432-1033.1974.tb03826.x. [DOI] [PubMed] [Google Scholar]
- Niemeyer H., de la Luz Cárdenas M., Rabajille E., Ureta T., Clark-Turri L., Peñaranda J. Sigmoidal kinetics of glucokinase. Enzyme. 1975;20(6):321–333. doi: 10.1159/000458957. [DOI] [PubMed] [Google Scholar]
- Pettersson G. Mechanistic origin of the kinetic cooperativity of hexokinase type L1 from wheat germ. Eur J Biochem. 1986 Jan 2;154(1):167–170. doi: 10.1111/j.1432-1033.1986.tb09374.x. [DOI] [PubMed] [Google Scholar]
- Pettersson G. Mechanistic origin of the sigmoidal rate behaviour of glucokinase. Biochem J. 1986 Jan 15;233(2):347–350. doi: 10.1042/bj2330347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard-Knight D., Cornish-Bowden A. Solvent isotope effects on the glucokinase reaction. Negative co-operativity and a large inverse isotope effect in 2H2O. Eur J Biochem. 1984 May 15;141(1):157–163. doi: 10.1111/j.1432-1033.1984.tb08170.x. [DOI] [PubMed] [Google Scholar]
- Ricard J., Meunier J. C., Buc J. Regulatory behavior of monomeric enzymes. 1. The mnemonical enzyme concept. Eur J Biochem. 1974 Nov 1;49(1):195–208. doi: 10.1111/j.1432-1033.1974.tb03825.x. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Kinetic evidence for a 'mnemonical' mechanism for rat liver glucokinase. Biochem J. 1977 Jul 1;165(1):61–69. doi: 10.1042/bj1650061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Kinetics of rat liver glucokinase. Co-operative interactions with glucose at physiologically significant concentrations. Biochem J. 1976 Oct 1;159(1):7–14. doi: 10.1042/bj1590007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippett P. S., Neet K. E. An allosteric model for the inhibition of glucokinase by long chain acyl coenzyme A. J Biol Chem. 1982 Nov 10;257(21):12846–12852. [PubMed] [Google Scholar]


