Abstract
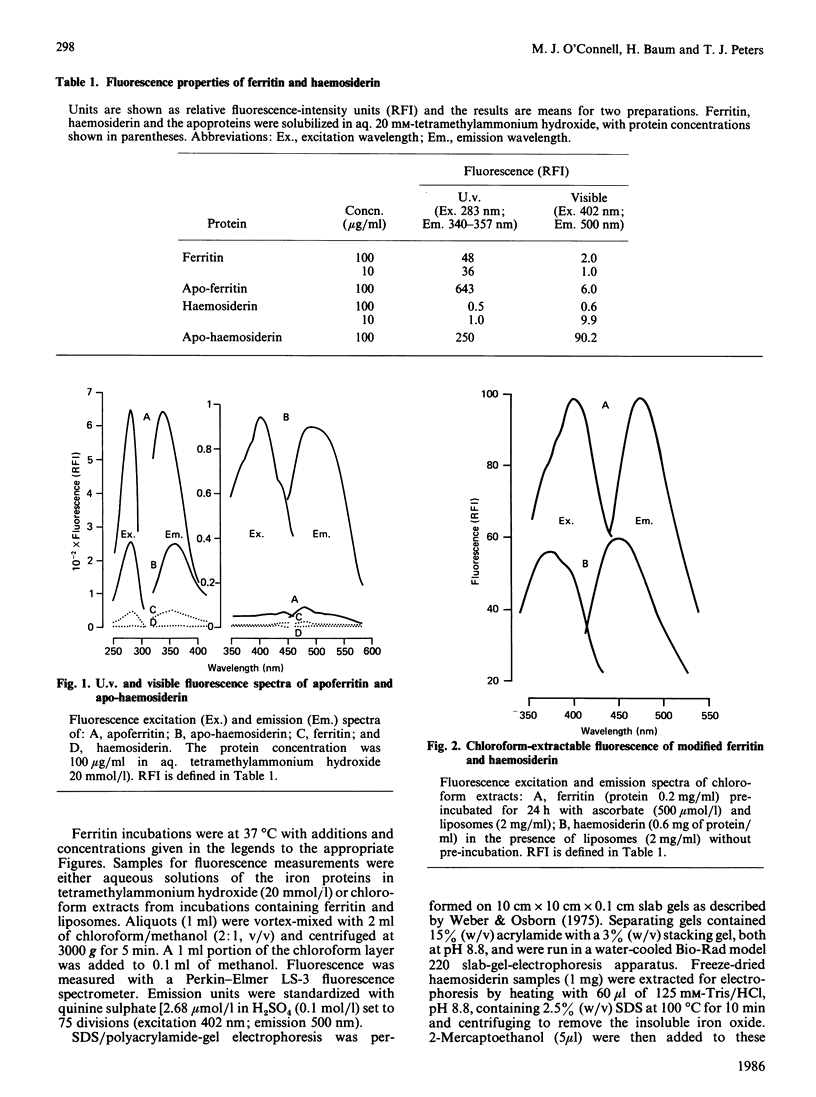
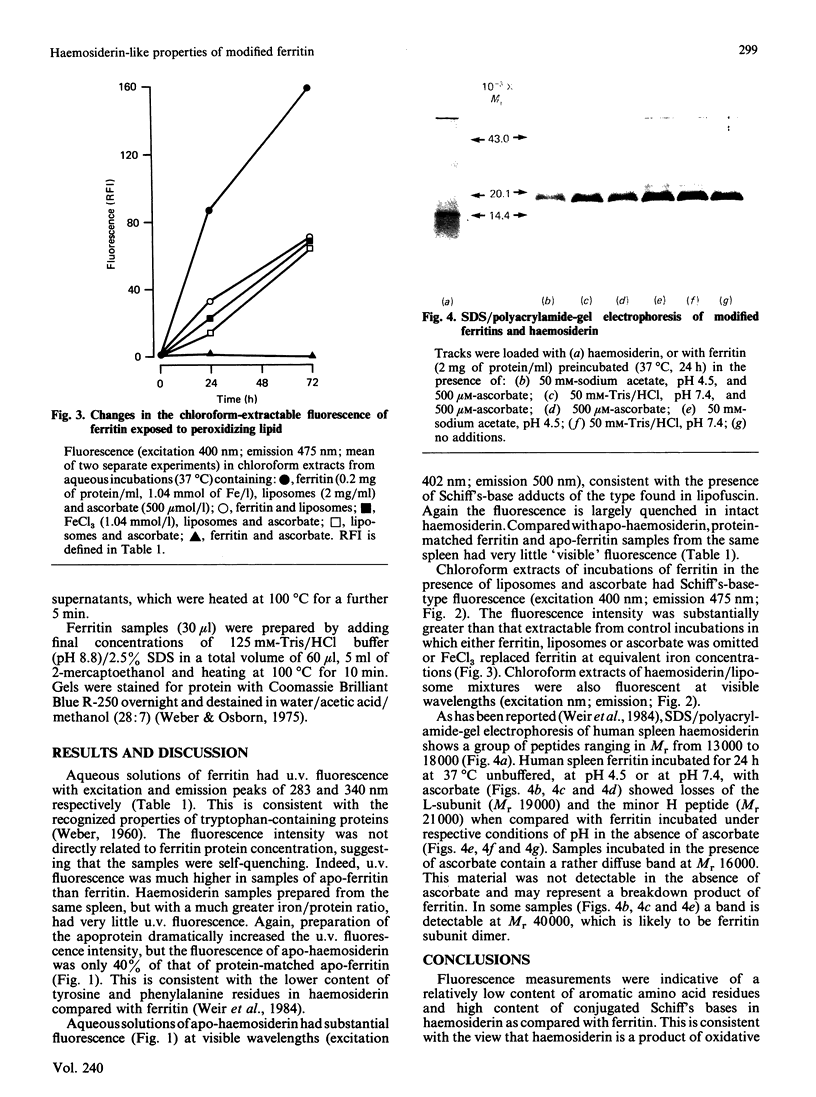
Conjugated-Schiff's-base-type fluorescence was measured in iron-depleted samples and chloroform extracts of human spleen haemosiderin. Incubation of ferritin with liposomes and ascorbate led to the formation of compounds with similar fluorescence properties. Analysis of protein subunits by SDS/polyacrylamide-gel electrophoresis confirmed that ferritin was damaged in incubations with ascorbate. Since previous studies have shown that intact ferritin is resistant to proteolytic degradation, it is suggested that haemosiderin may be a product of oxidative reactions involving ferritin and lipid.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cham B. E., Roeser H. P., Nikles A., Ridgway K. A procedure for the purification of ferritin from human liver by heating a methanol-treated homogenate. Anal Biochem. 1985 Dec;151(2):561–565. doi: 10.1016/0003-2697(85)90220-9. [DOI] [PubMed] [Google Scholar]
- Coffey J. W., De Duve C. Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J Biol Chem. 1968 Jun 25;243(12):3255–3263. [PubMed] [Google Scholar]
- Iancu T. C., Neustein H. B. Ferritin in human liver cells of homozygous beta-thalassaemia: ultrastructural observations. Br J Haematol. 1977 Dec;37(4):527–535. doi: 10.1111/j.1365-2141.1977.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Kim K., Rhee S. G., Stadtman E. R. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985 Dec 15;260(29):15394–15397. [PubMed] [Google Scholar]
- O'Connell M. J., Ward R. J., Baum H., Peters T. J. The role of iron in ferritin- and haemosiderin-mediated lipid peroxidation in liposomes. Biochem J. 1985 Jul 1;229(1):135–139. doi: 10.1042/bj2290135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M., Halliwell B., Moorhouse C. P., Aruoma O. I., Baum H., Peters T. J. Formation of hydroxyl radicals in the presence of ferritin and haemosiderin. Is haemosiderin formation a biological protective mechanism? Biochem J. 1986 Mar 15;234(3):727–731. doi: 10.1042/bj2340727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel W. Lipofuscin pigment accumulation and distribution in five rat organs as a function of age. J Gerontol. 1968 Apr;23(2):145–153. doi: 10.1093/geronj/23.2.145. [DOI] [PubMed] [Google Scholar]
- Schuessler H., Schilling K. Oxygen effect in the radiolysis of proteins. Part 2. Bovine serum albumin. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Mar;45(3):267–281. doi: 10.1080/09553008414550381. [DOI] [PubMed] [Google Scholar]
- Selden C., Owen M., Hopkins J. M., Peters T. J. Studies on the concentration and intracellular localization of iron proteins in liver biopsy specimens from patients with iron overload with special reference to their role in lysosomal disruption. Br J Haematol. 1980 Apr;44(4):593–603. doi: 10.1111/j.1365-2141.1980.tb08714.x. [DOI] [PubMed] [Google Scholar]
- WEBER G. Fluorescence-polarization spectrum and electronic-energy transfer in proteins. Biochem J. 1960 May;75:345–352. doi: 10.1042/bj0750345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir M. P., Gibson J. F., Peters T. J. Biochemical studies on the isolation and characterization of human spleen haemosiderin. Biochem J. 1984 Oct 1;223(1):31–38. doi: 10.1042/bj2230031. [DOI] [PMC free article] [PubMed] [Google Scholar]



