Highlights
-
•
The histological relationship between suprasellar craniopharyngiomas and the third ventricle in the two subtypes (PCPs/ACPs) was studied, potentially explaining the less aggressive behavior of PCPs compared to ACPs.
-
•
•The topographical differences between PCPs and ACPs were systematically summarized, providing valuable guidelines for diagnosis and intra-operative strategies.
-
•
•Post-operative hypothalamic outcomes were detailed analyzed in craniopharyngioma. Patients with PCPs suffered less weight gain, daytime sleepiness and memory difficulty than ACPs.
-
•
•Differences between PCPs and ACPs in clinical features, operative techniques, and surgical outcomes were extensively discussed based on our single-institute experience.
Keywords: Papillary craniopharyngioma, Clinical characteristics, Tumor topography, Surgical technique, Third ventricle floor, Hypothalamic outcomes
Abstract
Objective
Understanding the differences of suprasellar papillary and adamantinomatous craniopharyngiomas (PCPs/ACPs) is pivotal for target therapy, surgical strategy or postoperative management. Here, the clinical features, surgical nuances and postoperative hypothalamic outcomes of PCPs were systematically recapitulated.
Methods
24 PCPs and 52 ACPs underwent initial surgery were retrospectively reviewed. Clinical data, quantified third ventricle (3rd V) occupation and optic chiasm distortion were compared, as well as intra-operative findings, operating notes and prognosis. Moreover, analysis of tumor/3rd V relationship and hypothalamic outcomes were also performed.
Results
Tumors were more likely to occupies the 3rd V cavity in PCPs. Chiasm distortion of “compressed forward” was the most common pattern (45.8 %) in PCPs, whereas “stretched forward” pattern accounted the highest (42.5 %) in ACPs. Besides, round-shaped with less calcification, duct-like recess, solid consistency, rare subdiaphragmatic invasion, visible lower stalk and improved postoperative visual outcome were more frequently observed in PCPs. The basal membranes of the tumor epithelium and the reactive gliosis were separated by a layer of collagen fibers in most PCPs, which differs from ACPs in the morphological examination of tumor/3rd V floor interface. In daytime sleepiness and memory difficulty, the PCPs showed significantly better outcomes than the ACPs groups, and PCPs suffered less postoperative weight gain (p < 0.05) than ACPs among adult-onset cases.
Conclusion
PCPs are different from ACPs regards the clinical features, operative techniques and outcomes. If necessary, PCPs are suggested more amenable to total removal since its less invasiveness to the 3rd V floor and better hypothalamic outcomes.
Introduction
Craniopharyngiomas (CPs) are benign tumors that embryologically derive from ectopic embryonic remnants of the hypophysiopharyngeal duct (Rathke's pouch), which divided into papillary CPs (PCPs) and adamantinomatous CPs (ACPs) [1]. Although considered histologically benign, suprasellar CPs have complex topographical features of ventricular and suprasellar occupation that associating a high surgical risk and worse outcomes, particularly when tumor derives from the pars tuberalis in infundibulo-tuberal region. PCPs commonly develop in this region but account for only 10–20 % of all CPs [2]. Since PCPs has been reported with promising inhibition to the BRAF–MEK targeted therapy [3], outlining the nuances of clinical features, operating notes, and prognosis will be pivotal for therapy selection or surgery if necessary. While extensive literature exists on the clinical features, surgical notes, and outcomes of ACPs, comprehensive descriptions of PCPs and comparisons between the two types are rare.
Sequencing studies [4] have demonstrated that CPs have distinct genetic origins which may lead to the heterogeneity of drug susceptibility, topographical features and hypothalamic involvement patterns. Therefore, the systematic understanding of characteristics between PCPs and ACPs are imperative before tailoring appropriate treatment. It's been reported [5,6] that PCPs are characterized by lack of calcifications, hypointense cysts and tendency to involve the third ventricle (3rd V). Besides, Pascual et.al categorize the different distortion patterns of the optic chiasm [7] in CPs and proposed duct-like recess [8,9] at the infundibular portion as a simple MRI sign that represents a distinctive hallmark of PCPs. However, many of above were based on the indirect analysis of radiology may associate with the possibility of bias in slice selection. It's been proposed that the use of endoscopic endonasal approach (EEA) allows visualization of retro-chiasmatic compartment especially for the ventral hypothalamus, which provides a direct and accurate spatial understanding of tumor topography when surgery seem inevitable [10]. Meanwhile, the knowledge based on surgical observation, further understanding of tumor/3rd V floor interface and hypothalamic outcomes in PCPs are still lacking. Thus, it's of great importance to summarize the clinical features, technique nuances and hypothalamic outcomes of PCPs for the needs of precision medical care.
Based on our single-institution experience, we retrospectively analyzed the quantified third ventricle occupation, optic chiasm distortion, and CP-3rd V/hypothalamus relationship in PCPs and ACPs using radiological and direct surgical evidence via EEA. Together with the systematic comparison of clinical features, technique nuances and hypothalamic outcomes, we aim for our work to serve as a reference for more appropriate therapy selection, surgical strategy, and postoperative management in CP treatment.
Methods
Patient population
All patients with CPs treated by EEA that histologically identified at our institution between September 2017 to June 2022 were retrospectively reviewed. Patients subjected to reoperations and/or who underwent radiotherapy were excluded to avoid confounding bias, patients with incomplete records or loss to follow-up were also excluded. According to our QST classification based on tumor origin as reported previously [11,12], all tumors were classified into three types: infrasellar/subdiaphragmatic CPs (Q-CPs), subarachnoidal CPs (S-CPs), and pars tuberalis CPs (T-CPs). The classification was analyzed with pre-operative MRI and confirmed intra-operatively, only T-CPs were enrolled and categorized based on histological results. For minimizing the possibility of bias regarding the surgical learning curve and oncological outcomes measured, all surgeries were performed by the team of chief surgeon (J.F.). Since the incidence rate of PCP is much lower than ACP, every PCP case selected was matched with 2∼3 ACP cases that operates at the same time period (within 3 months) for analysis. Thus, patients were divided into 2 groups: PCP group (24 cases) and ACP group (52 cases), and the mean follow-up duration of the entire cohort was 34.8 months (range 17–75 months). This study was approved by the Ethics Committee of Nanfang Hospital of Southern Medical University.
Neuroimaging and Surgical evaluation
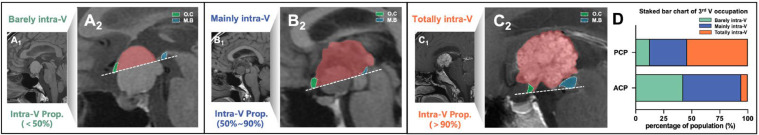
Before surgery, the duct-like recess is defined as a hollow tubular structure invaginated at the base of tumor that follows a diagonal trajectory towards the lesion's center through its midsagittal plane, which had been elaborated previously [8,9]. The 3rd V occupation of tumors were assessed based on the intra-ventricular proportion (IVP) of tumor volume, which is calculated as the percentage of the tumor mass above the reference dotted line that from ventral optic chiasm to ventral mammillary body in pre-operative midsagittal MRI into 3 types: Barely intra-ventricular type (IVP<50 %, Fig. 1A1-A2); Mainly intra-ventricular type (50 %≤IVP≤90 %, Fig. 1B1-B2) and Totally intra-ventricular type (IVP>90 %, Fig. 1C1-C2).
Fig. 1.
3rd V occupation of T-CPs based on intra-ventricular proportion of tumor mass.
Intra-ventricular occupation of tumor mass is calculated as the proportion of the tumor above the reference dotted line that from ventral O.C (green) to ventral M.B (blue) in midsagittal MRI. 3rd V occupation is categorized in 3 types: Barely intra-ventricular type (intra-ventricular proportion<50 %, A1-A2); Mainly intra-ventricular type (intra-ventricular proportion within 50-90 %, B1-B2) and Totally intra-ventricular type (intra-ventricular proportion>90 %, C1-C2). (D) Stacked bar chart of 3rd V occupation among T-type PCP and ACP group. 3rd V, third ventricle; Intra-V Prop., intra-ventricular proportion; M.B, mamillary body; O.C, optic chiasm.
The assessment of optic chiasm distortion was defined in detail as literatures described previously [7]. 6 patterns of optic chiasm distortions caused by T-CPs on preoperative T1-weighted midsagittal MRI were categorized here as: 1) normal or non-distorted; 2) compressed downward: the chiasm is being slightly pressed toward the sella turcica by the tumor; 3) compressed forward: the chiasm is being pushed against the tuberculum sellae by the CP; 4) stretched forward: the chiasm is being elongated along the anterior margin of the CP and displaced forward together with the communicating artery complex; 5) stretched upward: the chiasm is being tightened and elongated up to foramen of Monro by the tumor; and 6) stretched backward: the chiasm is being elongated and displaced toward the midbrain by the CP. Since the “stretched backward” pattern accounts for 0 % in T-CPs cohort here, the first 5 patterns were mainly analyzed (Fig. 2). Following the pre-operative radiological evaluation, all results were verified under surgical view to ensure accuracy or when sometimes anatomical landmarks were indiscernible in MRI.
Fig. 2.
Five main patterns of optic chiasm distortion caused by T-CPs identified by preoperative midsagittal MRI and corresponding intra-operative view.
(A1) Non-distorted or normal positioned O.C: Normal morphology in MRI and verified with surgical view (A2); (B1) Compressed downward O.C: Locates at the antero-inferior margin of the tumor due to its downward compressive force. A distorted O.C that bulged down could be seen under endoscopic view (B2); (C1) Compressed forward O.C: Displaced forward by the tumor and compressed against the tuberculum sellae. The O.C being pushed forward is visible after remove the tuberculum sellae (C2); (D1) Stretched forward O.C: Stretched along the anterior margin of the tumor. The O.C is bended forward by the suprasellar tumor under endoscopic view (D2); (E1) Stretched upward O.C: Stretched along the superior surface of the tumor. The O.C is bended upward and barely be seen since the tumor mass occupied the suprasellar cistern (E2). (F) Stacked bar chart of O.C distortion among T-type PCP and ACP group. O.C, optic chiasm; P.G, pituitary gland; TU, tumor.
Gross total resection (GTR) was defined as 100 % macroscopic tumor resection based on the surgical observation and post-operative radiological evidence, near-total resection (NTR) was defined as≥95 % but <100 % resection. Operating time was defined as time frame from the removal of tuberculum sellae bone to the skull base reconstruction process. For the tumor part with dense adhesions to the third ventricular floor, the involved tissue of the third ventricle floor was removed with the tumor to ensure complete resection. Finally, the tumor specimen of each case was sent for histological evaluation to identify the pathological type and investigate the tumor–third ventricle relationships. The videos, pre- and postoperative radiological assessment were assessed by three senior neurosurgeons who were blinded to the clinical outcome and blinded to each other.
Histopathologic assessment
As described in our previous studies [11,13,14], each specimen was histologically examined separately to provide a comprehensive synthesis of overall information regarding the relationships between the tumor and third ventricle. The antibodies used were to collagen I (1:300, Abcam); glial fibrillary acidic protein (GFAP) (1:200, Cell Signaling Technology, Danvers MA); Laminin beta 1 (1:400, Sigma, St. Louis, MO); pan Cytokeratin (Pan-CK) (1:300, Abcam, ab215838).
Variables and Follow-up
Preoperative assessment of visual disturbances [7] were classified as visual acuity loss in the worse-seeing eye according to the ranges defined in the clinical modification of the International Classification of Diseases by the World Health Organization: 1) normal vision, ≥0.8; 2) mild disturbance, <0.8-0.1; and 3) severe disturbance, ≤0.1. Endocrinological evaluation was evaluated before and after surgery as described previously [15]. Partial anterior hypopituitarism was defined as hormone deficiencies in one or two axes, and panhypopituitarism was defined as hormone deficiencies in three or more axes. Diabetes insipidus (DI) was diagnosed in patients who had polydipsia and polyuria with urine-specific gravity <1.005 and urine osmolality <300 mOsm/kg, as well as a positive response to desmopressin. New-onset DI was calculated based on the patients without DI preoperatively. Transient-DI was considered if DI recovered in the latest follow-up and those patients with DI who did not recover were categorized as permanent-DI.
Neuropsychological function was evaluated pre- and postoperatively as follows: the subjective daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS) [16], Everyday Memory Questionnaire-Revised (EMQ-R) [17] was used to evaluate self-reported memory difficulties, the cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA) [18], depression and anxiety were assessed by Hamilton Depression Rating Scales (HDRS) [19] and Hamilton Anxiety Rating Scales (HARS) [20], the quality of life was assessed with the anterior skull base quality-of-life questionnaire (ASBQ) [21] which consists of 35 questions and higher scores indicate a better QOL. Data were presented with ∆ values of different scales (∆= the difference of values between last follow-up and pre-operative period). Considered that the amount of body fat changes with age and less accuracy in during puberty, only adult patients are included (24 PCPs and 31 ACPs) when investigating central obesity. The weight and BMI were documented in different time spots (before surgery, 3,6,12 months after surgery and at last follow-up), the BMI in pre-operative period was considered as the baseline value and obesity was defined as BMI≥28.
Statistical analysis
Differences between groups were assessed by Chi-square test for categorical variables, and a two-tailed t test was used to compare continuous variables. Statistical analysis of the data was performed using IBM SPSS Statistics version 21, The results were considered significant at P<0.05.
Results
Clinical presentation
Compared with the ACPs, PCPs all occurred in adult patients (p<0.001) while it suggests a lack of correlation in gender. Severe disturbance of visual acuity showed significantly higher results in ACPs (69.2 %) than PCPs (37.5 %) (p<0.001). There was no statistical difference observed in other clinical symptoms including pituitary endocrinology, obesity, and headache (Table 1).
Table 1.
Clinical characteristics of patients with enrolled CPs before surgery.
| Variable | Categories | PCP (n=24) | ACP (n=52) | P Value |
|---|---|---|---|---|
| Age, n (%) | Adult | 24(100) | 31(59.6) | <0.001 |
| Children | 0(0) | 21(40.4) | ||
| Gender, n (%) | Female | 10(42.7) | 23(44.2) | 0.834 |
| Male | 14(58.3) | 29(55.8) | ||
| Headache, n (%) | Present | 8(33.3) | 14(26.9) | 0.567 |
| Obesity, n (%) | Present | 4(16.7) | 4(7.7) | 0.236 |
| Visual acuity, n (%) | Normal | 5(20.8) | 14(26.9) | <0.001 |
| Mild disturbance | 10(41.7) | 2(3.8) | ||
| Severe disturbance | 9(37.5) | 36(69.2) | ||
| Endocrinology, n (%) | Normal | 0(0) | 6(11.5) | 0.135 |
| Partial hypopituitarism | 17(70.8) | 37(71.2) | ||
| Pan-hypopituitarism | 7(29.2) | 9(17.3) | ||
| Tumor shape, n (%) | Round | 21(87.5) | 23(44.2) | <0.001 |
| Lobulated | 3(12.5) | 29(55.8) | ||
| Maximum diameter, n (%) | <3 cm | 10(41.7) | 12(23.1) | 0.097 |
| ≥3cm | 14(58.3) | 40(76.9) | ||
| PS involvement, n (%) | Lower portion visible | 14(58.3) | 16(30.8) | 0.022 |
| Completed infiltrated | 10(41.7) | 36(69.2) | ||
| Duct-like Recess, n (%) | Present | 5(20.8) | 0(0) | 0.002 |
| Hydrocephalus, n (%) | Present | 7(29.2) | 28(55.8) | 0.045 |
| Calcification, n (%) | Present | 2(8.3) | 43(82.7) | <0.001 |
| 3rd V occupation, n (%) | Barely intra-V | 3(12.5) | 22(42.3) | <0.001 |
| Mainly intra-V | 8(33.3) | 27(51.9) | ||
| Totally intra-V | 13(54.2) | 3(5.8) | ||
| Chiasm distortion, n (%) | Normal | 5(20.8) | 12(23.1) | 0.010 |
| Compressed downward | 3(12.5) | 0(0) | ||
| Compressed forward | 11(45.8) | 13(25.0) | ||
| Stretched forward | 3(12.5) | 22(42.3) | ||
| Stretched upward | 2(8.3) | 5(9.6) | ||
| Stretched backward | 0(0) | 0(0) |
Imaging findings and topographical characteristics
As listed in Table 1, the round tumor shape was predominant in 87.5 % of PCPs but only 44.2 % of ACPs (p<0.001). PCPs are less likely to have infiltrated PS than ACPs (43.5 % vs. 69.2 %, p=0.022), while the duct-like recess was only observed in PCPs (p=0.002). Nevertheless, hydrocephalus and calcification were significantly more observed in ACPs. No significant differences in tumor size were detected between groups. Regarding to 3rd V occupation, significant difference (p<0.001) was detected as the “Totally intra-V” type more commonly occurred in PCPs (54.2 %) than ACPs (5.8 %), whereas ACPs are mostly compose of “Mainly intra-V” (51.9 %) and “Barely intra-V” (42.3 %) type (Fig. 1D), which indicated the main body of PCPs are more likely to occupies the 3rd V cavity than ACPs. Another noteworthy difference was found in chiasm distortion pattern, “compressed forward” was the most common pattern in 45.8 % of the PCPs. The ACPs, by contrast, showed a highest association with “stretched forward” pattern in 42.5 % patients (detailed in Fig. 2F, p=0.01).
Intraoperative findings
The GTR rate was not significantly different between the PCP and ACP groups (95.8 % vs 96.2 %, p = 0.95), the operating time was also equivalent between groups. Unlike the predominantly heterogeneous mixed consistency (67.3 %) among ACPs, half of the PCPs had heterogeneous solid components (p<0.001). Interestingly, most of the other PCPs with cystic or mixed consistency are characterised as unicystic components instead of multicystic tumor with “motor-oil” content that often seen in ACPs (data not shown). In line with the imaging study, the intra-operative findings demonstrated that, in contrast to ACPs, PCPs did not exhibit subdiaphragmatic invasion (p=0.05). Notably, the specific distortion pattern of chiasm and its relation to the perforating microvessels of the tumor were all stereoscopically visible via EEA (Fig. 2). Since majority (66.7 %) of the PCPs were “Totally intra-V” or “Mainly intra-V” type as the IVP ≥50 %, the chiasm-tuberculum distance were usually very narrow (Fig. 3D), the tumors were deep-seated within the 3rd V chamber and occasionally extend to or beyond the foramen of Monro (Fig. 3F-G). Precise separation of the tumor generally starts from the infundibular recess (Fig. 3E) and tumor capsule could usually be easily stripped away from the 3rd V wall through a cleavage pial plane, sometimes additional supra-chiasmatic trans-lamina terminalis corridor may combined for the total removal. Unlike PCPs, ACPs seldom develops as“Totally intra-V” type but usually extends at suprasellar cistern even down to subdiaphragmatic space. The chiasm-tuberculum distance is widened by the suprasellar part of the CPs, thus the primary task is the tumor separation from the perforating arteries and chiasm (Fig. 4D-F). Afterwards the widened chiasm–pituitary corridor enables en-bloc resection of the tumor within 3rd V. Although more than half of the PS were preserved in both groups, but no significant difference was observed.
Fig. 3.
Illustrative cases of PCP with radiological findings and intra-operative observation.
(A-C) Pre-operative CT and MRI of a “Totally intra-V” type PCP. The O.C remained with normal morphology and tumor occupies the 3rd V chamber without calcification; (D) Under EEA, normal positioned O.C is confirmed. Note the chiasm-tuberculum distance is narrow and the tumor mass is hidden above the 3rd V floor; (E) The tumor is dissected precisely from the infundibular recess; (F) Intraventricular tumor is easily stripped away from the 3rd V wall through a cleavage pial plane (white dotted line); (G) Choroid plexus (pentagram) and each foramen of Monro (asterisk) are visible when tumor is removed en bloc; (H) Overall view after tumor removed totally; (I-J) MRI obtained 4 months after surgery. 3rd V, third ventricle; O.C, optic chiasm; O.N, optic nerve; P.S, pituitary stalk; P.G, pituitary gland; TU, tumor.
Fig. 4.
Illustrative cases of ACP with radiological findings and intra-operative observation.
(A-C) Pre-operative CT and MRI of a “Mainly intra-V” type ACP. MRI indicates O.C as “stretched forward” pattern and CT scan reveals the calcification; (D) Under EEA, O.C that bended forward by the suprasellar tumor is confirmed. (E) Precisely handling of the perforating arteries that supplying the suprasellar part of tumor; (F) Blunt dissecting the suprasellar part from the O.C along the plane in between (white dotted line); (G) Intraventricular tumor is easily stripped away from the 3rd V wall through a cleavage pial plane (white dotted line); (H) Overall view after tumor removed totally; (I-J) MRI obtained 2 weeks after surgery. 3rd V, third ventricle; M.B, mamillary body; O.C, optic chiasm; O.N, optic nerve; P.S, pituitary stalk; P.G, pituitary gland; P.CoA, posterior communicating artery; TU, tumor.
Histological relationships between the tumor and the third ventricle
Immunofluorescence staining on portions of tumor contacting with the third ventricle floor, a layer of gliosis with various thickness was observed between the tumor and the third ventricle floor (Fig. 5 A1–B3). Additionally, the morphological patterns between the tumor and the third ventricle floor in each group were also investigated. ACPs always forms a finger-like bulge in the third ventricle floor, with the absence of the pia mater and the development of reactive gliosis between the tumor and the neural tissue of the third ventricle floor. In contrast, basal membranes of tumor epithelium and reactive gliosis were separated by a layer of collagen fibers in most of the PCPs, which formed a continuous smooth plane between the tumor and the ventricle floor (Fig. 5C1–D3).
Fig. 5.
Histological observation in tumor–ventricle floor interface and post-operative weight gain in PCPs/ACPs.
Immunofluorescent staining shows a layer of gliosis (GFAP positive) with various thickness between the tumor (Pan-CK positive) and the neural tissue of the third ventricle floor in PCP (A1–A3) and ACP (B1–B3). PCP in (C1–C3) shows that the basal membranes of tumor epithelium (Pan-CK positive) and reactive gliosis were separated by a layer of fibers (collagen positive, white arrow). ACP in (D1–D3) indicates a finger-like structure in which the tumor forms a finger-like bulge into the third ventricle floor, with the absence of the pia mater (Laminin β1 positive) at some areas (yellow arrowhead) and the development of gliosis between the tumor and the neural tissue of the third ventricle floor. Scale bar =200 μm. (E–F) The trend and comparison of obesity rate from the pre-operative period to the last follow-up. (G) shows the changes of BMI in groups. The BMI in pre-operative period is considered as the baseline value, △BMI in (H) indicates the difference between the peak value and the baseline (*p < 0.05). △BMI in (I) indicates the difference between the latest value (documented in the last follow-up) and the baseline (**p < 0.01). 3VF, third ventricle floor; Tu, tumor; mo, months; pre-op, pre-operative period; F-U, follow-up.
Post-operative Complications and Hypothalamic Outcomes
The improved visual outcome of the entire cohort occurred more commonly in PCPs than ACPs (62.5 % vs 29.6 %, p = 0.011). In the aspects of neuropsychological outcomes, we observed that in the daytime sleepiness and memory questionnaire, the PCPs showed better outcomes than the ACPs groups (p < 0.001, Table 2). The proportion of obesity increased significantly at 12 moths in PCPs and 6 months in ACPs after surgery when compared with pre-operative period (p<0.01, Fig. 5E), and kept stable thereafter till the last follow-up (Fig. 5F). The average BMI showed no significant difference between 2 groups in each time spot (Fig. 5G). Meanwhile, significant changes were observed in the difference of BMI between the peak value to the baseline (p < 0.05, Fig. 5H) and the latest value to the baseline (p < 0.01, Fig. 5I), indicating PCPs might suffered a more moderate postoperative weight gain among adult-onset patients.
Table 2.
Intra-operative findings and surgical outcomes of recruited CP patients via EEA.
| Variable | Categories | PCP (n=24) | ACP (n=52) | P Value |
|---|---|---|---|---|
| Resection extent, n (%) | GTR | 23(95.8) | 50(96.2) | 0.947 |
| NTR | 1(4.2) | 2(3.8) | ||
| Operating time, n (%) | ≥3h | 7(29.2) | 15(28.8) | 0.977 |
| <3h | 17(70.8) | 37(71.2) | ||
| Tumor consistency, n (%) | Cystic | 2(8.3) | 16(30.8) | <0.001 |
| Solid | 12(50.0) | 1(1.9) | ||
| Mixed | 10(41.7) | 35(67.3) | ||
| PS status, n (%) | Preserved | 15(62.5) | 28(53.8) | 0.479 |
| Sacrificed | 9(37.5) | 24(46.2) | ||
| Subdiaphragmatic invasion, n (%) | Present | 0(0) | 8(15.4) | 0.050 |
| Endocrinology, n (%) | ACTH deficiency | 13(54.2) | 37(71.2) | 0.147 |
| TSH deficiency | 19(79.2) | 46(88.5) | 0.284 | |
| GN deficiency | 22(95.8) | 41(78.8) | 0.059 | |
| GH deficiency | 8(33.3) | 20(38.5) | 0.667 | |
| Panhypopituitarism | 14(58.3) | 35(67.3) | 0.447 | |
| Deterioration of pituitary function① | Present | 30(69.8) | 34(77.3) | 0.427 |
| New-onset DI② | Present | 17(94.4) | 44(89.8) | 0.555 |
| Post-op DI, n (%) | NO-DI | 1(4.2) | 5(9.6) | 0.804 |
| Transient-DI | 9(37.5) | 17(32.7) | ||
| Permanent-DI | 14(58.3) | 30(57.7) | ||
| Visual outcome, n (%) | Improved | 15(62.5) | 14(26.9) | 0.011 |
| Unchanged | 7(29.2) | 32(61.5) | ||
| Worsened | 2(8.3) | 6(11.5) | ||
| Headache outcome, n (%) | Improved | 17(70.8) | 33(63.5) | 0.529 |
| Unchanged | 7(29.2) | 19(36.5) | ||
| Post-op CSF leak, n (%) | Present | 1(4.2) | 2(3.8) | 0.947 |
| Recurrence, n (%) | Present | 0(0) | 2(3.8) | 0.330 |
| Hypothalamic dysfunction, n (%) | Gain of BMI (%) | 1.63±3.04 | 5.52±5.99 | 0.004 |
| ∆ASBQ | 0.28±0.40 | 0.24±0.27 | 0.654 | |
| ∆HARS | 1.71±1.63 | 2.08±1.82 | 0.400 | |
| ∆HDRS | 1.83±1.37 | 2.17±2.16 | 0.482 | |
| ∆ESS | 1.21±2.06 | 3.25±2.15 | <0.001 | |
| ∆MoCA | 0.92±1.02 | 1.21±1.21 | 0.304 | |
| ∆EMQ-R | 1.63±2.73 | 5.05±3.39 | <0.001 |
① : % means “% of Pre-op patients without panhypopituitarism.” ②: % means “% of Pre-op normal.”
Discussion
Topographical characteristics and clinical presentation
Currently, the EEA is favored as the approach of choice for most CPs at many institutions [[22], [23], [24], [25]]. and several authors have described the major differences between PCPs and ACPs (Table 3). However, controversy remains in EEA for CPs with predominantly 3rd V occupation, especially in PCPs which is deemed typically develop at the infundibulum or within the 3rd V. But sometimes when CPs entirely fills the 3rd V cavity, the chiasm-tuberculum distance exhibited to be widened by the suprasellar tumor component, which instead facilitates the co-axial corridor to the 3rd V via EEA (Fig. 4). Therefore, a ratio of intra-ventricle to extra-ventricle part of tumor occupation is thought to be a useful factor when evaluating the topographical characteristics and indication of EEA. Although CPs that predominantly occupying the 3rd V has been intensively described as “strictly intraventricular” in Pascual criteria [26] or “Type IV intraventricular” proposed by Kassam et al. [10], rare reports have mentioned the ratio of intra-ventricular occupation in CPs when compared with “not-strictly intraventricular”, “secondarily intraventricular” or “trans-infundibular” types. In present study, calculation of intra-ventricular proportion has served a great reference for the understanding of CP topography. In accord with previous perspectives, our data confirmed that the main body of PCPs are more likely to occupies the 3rd V cavity than ACPs and rarely with subdiaphragmatic invasion. Interestingly, it was discovered that 12.5 % of the PCPs displayed predominantly extra-ventricular topography (Barely intra-ventricular type), for which EEA is strongly recommended. But as to the “Totally intra-ventricular type” (Intra-V Prop.>90 %), a transcallosal or frontal-transventricular approach could also be considered as straightforward alternatives if necessary. In 1981, Aguado et al. [27] described that the pars tuberalis is perforated by subarachnoid channels. In consistent with our surgical observation, bundles of dense arachnoid trabeculae were frequently observed surrounding the infundibulo-tuberal area, which tends to form a firm barrier preventing the tumor from extending ventrally. Besides the distinct genetic origins, the heterogeneity of topographical features in PCPs might be explained as its originating site at the uppermost area of the pars tuberalis and dense trabecular barrier at the point of infundibulo-tuberal area.
Table 3.
Literature review on the major differences of PCPs and ACPs.
| Authors, year [Ref] | n (PCP/ACP) | Source | Major features considered |
|---|---|---|---|
| Sartoretti-Schefer et al. 1997 [5] | 15/25 | Non-contrast T1-MRI | Hypointense cyst (PCP) / hyperintense cyst (ACP) |
| Tumor shape | Mostly spherical (PCP) / mostly lobulated (ACP) | ||
| Encasement of vessels | Rare (PCP) / common (ACP) | ||
| Lee et al. 2016 [29] | 8/30 | T1 bright signal intensity | Rare (PCP) / common (ACP) |
| Calcification on CT | Common (PCP) / rare(ACP) | ||
| Hölsken et al. 2016 [41] | 35/80 | DNA methylation | Upregulation of Wnt- and SHH signaling in ACP |
| Gene mutation | BRAFV600E (PCP) / CTNNB1 (ACP) | ||
| Feng et al. 2018 [42] | 119/622 | Hypothalamus/pituitary disorder | Significantly higher in PCP than ACP |
| Central diabetes insipidus | Significantly higher in PCP than ACP | ||
| Chen et al. 2018 [43] | 40/157 | Inflammatory markers | Higher neutrophil count and NLR in PCP Higher platelet count and PNI in ACP |
| Teng et al. 2022 [44] | 44/53 | Maximum diameter | Significantly lower in PCP than ACP |
| CNN models | ResNet50 achieves highest AUC to diagnose PCP/ACP | ||
| Guo et al. 2023 [45] | 125/617 | Diabetes insipidus | More in PCP than ACP |
| Hyperprolactinemia | More in PCP than ACP | ||
| Adrenocortical hypofunction | Less in PCP than ACP |
Abbreviation: AUC, area under the receiver operating characteristic curves; CNN, convolutional neural network; NLR, Neutrophil lymphocyte ratio; PNI, Prognostic nutritional index.
In a thorough research from Pascual et al. [7], mild-to-moderate visual impairment to cases with severe visual impairment was about 1:5 in the stretched types, indicating the worst visual symptom. Our results were congruent with this as stretch types, severe visual disturbance and less visual improvements are more frequently observed in ACPs. Thus, weeks of target therapy may be a better alternative than immediate surgery if PCPs are considered with moderate vision disturbance. Recently, Pascual et al. proposed that the duct-like recess represents an invaluable sign for accurately diagnosing strictly 3rd V PCPs [8,9]. and our findings supported this as well. This may be explained as the tumor bulging from the bottom of the 3rd V floor and forming a dimple-like sign that resembles an apple stem due to the less inflammatory thickening of the intra-arachnoidal segment in PCPs. Moreover, we founded that the calcification of PCPs doesn't commonly locates at the pars tuberalis region (where tumor originates) in most PCPs. Combined with clinical findings that accord with earlier investigations [5,28,29], our data demonstrate convincingly that tumor topography differs between PCPs and ACPs, suggesting that these findings may result in distinct clinical characteristics that of a highly valuable guideline for diagnosis. Together with cell-free DNA analysis of circulating mutant BRAF as noninvasive detection in patient cohort, the precise identification of PCPs for subsequent target therapy may become possible.
Operating notes
Although cycles of vemurafenib–cobimetinib treatment showed durable partial or better responses in PCPs [3], urgent operative intervention is still necessary when obstructive hydrocephalus or severe visual decline occurs in PCPs. For CPs that arises from pars tuberalis, the chiasm–pituitary corridor is the basic route enough for most cases. When dealing with PCPs that almost hidden above the 3rd V floor, the chiasm-tuberculum distance is usually narrow for manipulating. Together with our experience that extensively described previously [13], CPs with predominantly ventricular involvement are separated from the 3rd V floor by the intact pia mater at most of the tumor–brain interface. Therefore, carefully identifying the cleavage plane in between and blunt resection is critical to ensure the minimal injury to the hypothalamus. Sometimes when tumor reach or beyond the foramen of Monro, a supraoptic and infraoptic approach might be combined to avoiding severe hypothalamic injury resulting from blunt traction [30]. Interestingly, a smaller deficient TVF was usually observed in PCPs owing to its higher IVP, which is consistent with our previous observation [13]. For ACPs with predominant suprasellar occupation, commonly the chiasm-tuberculum distance is wide enough to access. Thus, precisely handling of the perforating arteries that covers on the suprasellar part of tumor is the primary task. Usually, partial blood supply of the CPs originates from the secondary branches of the anterior choroidal artery. Considering that the intraoperative injury and dense adherence to the chiasm are the predictors for a worse visual outcome, tumor debulking to decompress the chiasm is of the top priority. Under EEA, surgeons have a superior view for exploring the tumor blood supply, therefore further protecting the hypothalamus by highly selective devascularization.
Preserving the hypothalamic-pituitary axes is crucial in maintaining endocrinological function. In T-CPs included here, the tumor commonly grows vertically and expands the PS from above. Coinciding with previous studies [2,8], our results confirmed that more PS were visible at the lower portion in PCPs, which resulted in higher PS preservation rate after tumor resection. The infiltrated PS is more frequently seen in ACPs, sometimes the PS is still identifiable on the surface of tumor and could be preserved. But when the PS is almost unidentifiable and the capsule is extremely thin, the PS need be sacrificed to achieve a radical resection. Our overall PS preservation rate (56.6%) is comparable or greater than that reported in previous literatures [31,32]. Notably, only CPs arises from pars tuberalis which more invasive to PS and infundibulum were included in present study, whereas the PS preservation in other studies were mainly based on unclassified CP. Thus, it's believed that if with experienced hand, the PS could be maximally preserved under EEA even in trans-infundibular variety. Based on our data, tumor can sometimes grow along the stalk through the diaphragmatic foramen down to the sella turcica, but only observed in ACPs. It is noteworthy that the intrasellar part of tumor always concealed behind the anterior lobe, ignorance of this lesion will significantly increase the possibility of recurrence and difficulties in reoperation. Accordingly, pituitary transposition technique should be combined to achieve tumor exposure, optimal protection of the anterior lobe and complete resection [[33], [34], [35]].
Hypothalamic outcomes and tumor/3rd V floor relationship
It's been revealed that the pia mater overlays at most of the tumor–brain interface in CPs with ventricular extension, except at the antero-basal border of tumor contacting the 3rd V floor. As commonly seen in ACPs, tumor protrudes into the neural layer of the 3rd V floor with a band of reactive gliosis in various thickness instead of the pia mater. Therefore, hypothalamic injury inevitably occurs in most cases which may resulting in long-term consequences including central obesity and neuropsychological issues. Here, single-institution case series analysis was conduct to eliminate bias of inclusion indication criteria and surgical skills. In our data refers to the central obesity in adult-onset CPs, we demonstrated the change proportion of obesity, BMI, and BMI change, showed the strongest postoperative weight gain occurred at 6-12 months following surgery and thereafter kept stable. At each time spot, although no differences were observed in proportion of obesity and BMI between groups, but it can be found that the proportion of obesity and BMI were even slightly higher in PCPs at early stages (pre-operative period and 3 months postoperatively). However, the BMI differences of peak and latest value to the baseline of each patient were significantly higher in ACPs, suggesting a more severe augment of BMI postoperatively. Together with our data from neuropsychological follow-up, PCPs also has much better outcomes in sleep disturbances and memory defects, which indicates a less invasiveness to the hypothalamus. In line with our findings, some studies have reported that the PCPs has a more favorable prognosis than ACPs [36,37], while others reported that there was no significant difference between them [6,38]. In 1995, Crotty et al. [6] observed that differs from ACPs, no infiltration of the adjacent brain tissue was observed by any PCPs. Similarly, based on its presumed lower tendency to infiltrate the adjacent hypothalamic tissue or to induce a strong gliotic and inflammatory responses, some hypothesized that PCPs are more amenable to total removal than ACPs [39,40]. When investigating the relationships of the tumor to the 3rd V floor, the finger-like pattern relationships has been considered as the invasive pathological features in ACP variant. Even while the gliosis layer that separated the tumor from the adjacent neural tissue may provide a safe dissection plane, it is still challenging to remove the tumor without damaging the adjacent neural tissue when the 3rd V floor is extremely thin and adheres to the lesion tightly in ACPs. Here, our histological results provide a straightforward distinction that a layer of collagen fibers exists between the surface of tumor epithelium and reactive gliosis in most PCPs. During operation, this layer could serve as a clear cleavage plane for dissection and therefore maximize the hypothalamic protection as well. Hence, these findings might be considered as contributing to the better outcomes of central obesity and neuropsychological function, potentially explains the presumed less-aggressive behavior of PCPs [39].
Limitations
The intrinsic retrospective aspect of this study, which relies on the accuracy of clinical records on it. The fact that patients frequently get postoperative cortisol replacement therapy raises the possibility of bias in weight gain. Additionally, a subset of patients could only complete the neuropsychological questionnaires via telephone or online testing, which may be less informative than offline research.
Conclusion
For CPs that arises from pars tuberalis, PCPs are different from ACPs regards the topographical characteristics, clinical features, operative techniques and outcomes. On the ground of its less invasiveness to the third ventricle floor and better hypothalamic outcomes, PCPs are suggested more amenable to total removal if surgery is necessary. Understanding the differential diagnosis of the two subtypes is critical for target therapy planning or surgical strategy.
Funding
This work was supported by the National Natural Science Foundation of China (82400913, 82103033 and 82201516), the GuangDong Basic and Applied Basic Research Foundation (2023A1515111042, 2023A1515010150, 2024A04J5181 and 2024A1515030284), the Science and Technology Projects in Guangzhou (SL2022A04J02034 and SL2023A04J02597), the President Foundation of Nanfang Hospital, Southern Medical University (2021C032 and 2022A002), the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2023J008) and the Science and Technology Project of GanZhou (2022-RC1346).
Data availability
The datasets included in this study are available upon request by contact with the corresponding author.
CRediT authorship contribution statement
Le Yang: Data curation, Funding acquisition, Writing – original draft. Yi Liu: Formal analysis, Investigation, Methodology. ChaoHu Wang: Methodology, Project administration, Software. ZhanPeng Feng: Software, Validation, Visualization. Lei Yu: Data curation, Funding acquisition, Resources. Jun Pan: Project administration, Writing – review & editing. JunXiang Peng: Validation. Jing Nie: Data curation, Methodology. MingFeng Zhou: Data curation, Formal analysis, Software. YiChao Ou: Formal analysis, Visualization. Tao Liu: Data curation, Resources, Visualization. Songtao Qi: Conceptualization, Writing – review & editing. Jun Fan: Conceptualization, Supervision, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Contributor Information
Le Yang, Email: yl201509@sina.com.
Yi Liu, Email: liuyi818@smu.edu.cn.
ChaoHu Wang, Email: 1024596076@qq.com.
ZhanPeng Feng, Email: feng3388836@smu.edu.cn.
Lei Yu, Email: battikindy@163.com.
Jun Pan, Email: 1448875873@qq.com.
JunXiang Peng, Email: pjxlaozong@126.com.
Jing Nie, Email: 345654527@qq.com.
MingFeng Zhou, Email: zhoumingfengsmu@163.com.
YiChao Ou, Email: neurosurgery_oyc@163.com.
Tao Liu, Email: 605232496@qq.com.
Songtao Qi, Email: qisongtaonfyy@126.com.
Jun Fan, Email: kinvan2002@163.com.
References
- 1.Yamini B, Narayanan M. Craniopharyngiomas: an update. Expert. Rev. Anticancer Ther. 2006;6(Suppl 9):S85–S92. doi: 10.1586/14737140.6.9s.S85. [DOI] [PubMed] [Google Scholar]
- 2.Prieto R, Barrios L, Pascual JM. Papillary craniopharyngioma: a type of tumor primarily impairing the hypothalamus - a comprehensive anatomo-clinical characterization of 350 well-described cases. Neuroendocrinology. 2022;112:941–965. doi: 10.1159/000521652. [DOI] [PubMed] [Google Scholar]
- 3.Brastianos PK, Twohy E, Geyer S, et al. BRAF-MEK inhibition in newly diagnosed papillary craniopharyngiomas. N. Engl. J. Med. 2023;389:118–126. doi: 10.1056/NEJMoa2213329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brastianos PK, Taylor-Weiner A, Manley PE, et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat. Genet. 2014;46:161–165. doi: 10.1038/ng.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartoretti-Schefer S, Wichmann W, Aguzzi A, Valavanis A. MR differentiation of adamantinous and squamous-papillary craniopharyngiomas. AJNR Am. J. Neuroradiol. 1997;18:77–87. [PMC free article] [PubMed] [Google Scholar]
- 6.Crotty TB, Scheithauer BW, Young WF, et al. Papillary craniopharyngioma: a clinicopathological study of 48 cases. J. Neurosurg. 1995;83:206–214. doi: 10.3171/jns.1995.83.2.0206. [DOI] [PubMed] [Google Scholar]
- 7.Prieto R, Pascual JM, Barrios L. Optic chiasm distortions caused by craniopharyngiomas: clinical and magnetic resonance imaging correlation and influence on visual outcome. World Neurosurg. 2015;83:500–529. doi: 10.1016/j.wneu.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Pascual JM, Prieto R, Carrasco R, Barrios L. Duct-like diverticulum at the base of third ventricle tumors: a morphological signature diagnostic of papillary craniopharyngioma. Neurosurg. Rev. 2022;45:3361–3379. doi: 10.1007/s10143-022-01848-7. [DOI] [PubMed] [Google Scholar]
- 9.Pascual JM, Carrasco R, Barrios L, Prieto R. Duct-like recess in the infundibular portion of third ventricle craniopharyngiomas: an MRI sign identifying the papillary type. AJNR Am. J. Neuroradiol. 2022;43:1333–1340. doi: 10.3174/ajnr.A7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassam AB, Gardner PA, Snyderman CH, et al. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J. Neurosurg. 2008;108:715–728. doi: 10.3171/JNS/2008/108/4/0715. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Qi S-T, Wang C-H, et al. Pathological relationship between adamantinomatous craniopharyngioma and adjacent structures based on QST classification. J. Neuropathol. Exp. Neurol. 2018;77:1017–1023. doi: 10.1093/jnen/nly083. [DOI] [PubMed] [Google Scholar]
- 12.Fan J, Liu Y, Pan J, et al. Endoscopic endonasal versus transcranial surgery for primary resection of craniopharyngiomas based on a new QST classification system: a comparative series of 315 patients. J. Neurosurg. 2021;135:1298–1309. doi: 10.3171/2020.7.JNS20257. [DOI] [PubMed] [Google Scholar]
- 13.Fan J, Liu Y, Wang C, et al. Reinvestigating tumor-ventricle relationship of craniopharyngiomas with predominantly ventricular involvement: an endoscopic endonasal series based on histopathological assessment. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.740410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi S, Liu Y, Wang C, et al. Membrane structures between craniopharyngioma and the third ventricle floor based on the QST classification and its significance: a pathological study. J. Neuropathol. Exp. Neurol. 2020;79:966–974. doi: 10.1093/jnen/nlaa087. [DOI] [PubMed] [Google Scholar]
- 15.Peng J, Yang L, Pan J, et al. Clinical features and prognosis of pediatric infradiaphragmatic craniopharyngioma relative to the tumor inflammatory response. Pediatr. Res. 2021;89:1119–1125. doi: 10.1038/s41390-020-1013-4. [DOI] [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Royle J, Lincoln NB. The everyday memory questionnaire-revised: development of a 13-item scale. Disabil. Rehabil. 2008;30:114–121. doi: 10.1080/09638280701223876. [DOI] [PubMed] [Google Scholar]
- 18.Chen K-L, Xu Y, Chu A-Q, et al. Validation of the Chinese version of montreal cognitive assessment basic for screening mild cognitive impairment. J. Am. Geriatr. Soc. 2016;64:e285–e290. doi: 10.1111/jgs.14530. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 21.Gil Z, Abergel A, Spektor S, et al. Development of a cancer-specific anterior skull base quality-of-life questionnaire. J. Neurosurg. 2004;100:813–819. doi: 10.3171/jns.2004.100.5.0813. [DOI] [PubMed] [Google Scholar]
- 22.Dhandapani S, Singh H, Negm HM, et al. Endonasal endoscopic reoperation for residual or recurrent craniopharyngiomas. J. Neurosurg. 2017;126:418–430. doi: 10.3171/2016.1.JNS152238. [DOI] [PubMed] [Google Scholar]
- 23.Leng LZ, Greenfield JP, Souweidane MM, et al. Endoscopic, endonasal resection of craniopharyngiomas: analysis of outcome including extent of resection, cerebrospinal fluid leak, return to preoperative productivity, and body mass index. Neurosurgery. 2012;70:110–123. doi: 10.1227/NEU.0b013e31822e8ffc. discussion 123-124. [DOI] [PubMed] [Google Scholar]
- 24.Moussazadeh N, Prabhu V, Bander ED, et al. Endoscopic endonasal versus open transcranial resection of craniopharyngiomas: a case-matched single-institution analysis. Neurosurg. Focus. 2016;41:E7. doi: 10.3171/2016.9.FOCUS16299. [DOI] [PubMed] [Google Scholar]
- 25.Cavallo LM, Frank G, Cappabianca P, et al. The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J. Neurosurg. 2014;121:100–113. doi: 10.3171/2014.3.JNS131521. [DOI] [PubMed] [Google Scholar]
- 26.Pascual JM, Prieto R, Carrasco R, Barrios L. Displacement of mammillary bodies by craniopharyngiomas involving the third ventricle: surgical-MRI correlation and use in topographical diagnosis. J. Neurosurg. 2013;119:381–405. doi: 10.3171/2013.1.JNS111722. [DOI] [PubMed] [Google Scholar]
- 27.Aguado LI, Schoebitz K, Rodríguez EM. Intercellular channels in the pars tuberalis of the rat hypophysis and their relationship to the subarachnoid space. Cell Tissue Res. 1981;218:345–354. doi: 10.1007/BF00210349. [DOI] [PubMed] [Google Scholar]
- 28.Lee H-J, Wu C-C, Wu H-M, et al. Pretreatment diagnosis of suprasellar papillary craniopharyngioma and germ cell tumors of adult patients. AJNR Am. J. Neuroradiol. 2015;36:508–517. doi: 10.3174/ajnr.A4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craniopharyngiomas: radiological differentiation of two types - PubMed. https://pubmed.ncbi.nlm.nih.gov/27651864/. Accessed 7 Aug 2023.
- 30.Bao Y, Yang Y, Zhou L, et al. Endoscopic endonasal supraoptic and infraoptic approaches for complex “Parasuprasellar” lesions: surgical anatomy, technique nuances, and case series. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.847250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dho Y-S, Kim YH, Se Y-B, et al. Endoscopic endonasal approach for craniopharyngioma: the importance of the relationship between pituitary stalk and tumor. J. Neurosurg. 2018;129:611–619. doi: 10.3171/2017.4.JNS162143. [DOI] [PubMed] [Google Scholar]
- 32.Du C, Leng Y, Zhou Q, et al. Relationship between postoperative hypothalamic injury and water and sodium disturbance in patients with craniopharyngioma: a retrospective study of 178 cases. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.958295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigo V, Chang JE, Nunez MA, et al. Endoscopic endonasal transtuberculum approach for pediatric tuberoinfundibular craniopharyngioma: 2-dimensional operative video. Oper. Neurosurg. Hagerstown Md. 2023 doi: 10.1227/ons.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 34.Mohyeldin A, Hwang P, Grant GA, Fernandez-Miranda JC. Endoscopic endonasal surgery for giant pediatric craniopharyngioma. Neurosurg. Focus. Video. 2020;2:V8. doi: 10.3171/2020.4.FocusVid.19983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang B, Xie S, Huang G, et al. Clinical features and operative technique of transinfundibular craniopharyngioma. J. Neurosurg. 2019:1–10. doi: 10.3171/2019.3.JNS181953. [DOI] [PubMed] [Google Scholar]
- 36.Szeifert GT, Sipos L, Horváth M, et al. Pathological characteristics of surgically removed craniopharyngiomas: analysis of 131 cases. Acta Neurochir. (Wien) 1993;124:139–143. doi: 10.1007/BF01401137. [DOI] [PubMed] [Google Scholar]
- 37.Tavangar SM, Larijani B, Mahta A, et al. Craniopharyngioma: a clinicopathological study of 141 cases. Endocr. Pathol. 2004;15:339–344. doi: 10.1385/ep:15:4:339. [DOI] [PubMed] [Google Scholar]
- 38.Duff J, Meyer FB, Ilstrup DM, et al. Long-term outcomes for surgically resected craniopharyngiomas. Neurosurgery. 2000;46:291–302. doi: 10.1097/00006123-200002000-00007. discussion 302-305. [DOI] [PubMed] [Google Scholar]
- 39.Adamson TE, Wiestler OD, Kleihues P, Yaşargil MG. Correlation of clinical and pathological features in surgically treated craniopharyngiomas. J. Neurosurg. 1990;73:12–17. doi: 10.3171/jns.1990.73.1.0012. [DOI] [PubMed] [Google Scholar]
- 40.Petito CK, DeGirolami U, Earle KM. Craniopharyngiomas: a clinical and pathological review. Cancer. 1976;37:1944–1952. doi: 10.1002/1097-0142(197604)37:4<1944::aid-cncr2820370446>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Hölsken A, Sill M, Merkle J, et al. Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol. Commun. 2016;4:20. doi: 10.1186/s40478-016-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng Y, Ni M, Wang Y-G, Zhong L-Y. Comparison of neuroendocrine dysfunction in patients with adamantinomatous and papillary craniopharyngiomas. Exp. Ther. Med. 2019;17:51–56. doi: 10.3892/etm.2018.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M, Zheng S-H, Yang M, et al. The diagnostic value of preoperative inflammatory markers in craniopharyngioma: a multicenter cohort study. J. Neurooncol. 2018;138:113–122. doi: 10.1007/s11060-018-2776-x. [DOI] [PubMed] [Google Scholar]
- 44.Teng Y, Ran X, Chen B, et al. Pathological diagnosis of adult craniopharyngioma on MR images: an automated end-to-end approach based on deep neural networks requiring no manual segmentation. J. Clin. Med. 2022;11:7481. doi: 10.3390/jcm11247481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Y, Pei L, Li Y, et al. Characteristics and factors influencing hypothalamic pituitary dysfunction in patients with craniopharyngioma. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1180591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets included in this study are available upon request by contact with the corresponding author.