Abstract
There is increasing evidence that CD8 lymphocytes may represent targets for infection by human immunodeficiency virus type 1 (HIV-1) in vivo whose destruction may contribute to the loss of immune function underlying AIDS. HIV-1 may infect thymic precursor cells destined to become CD4 and CD8 lymphocytes and contribute to the numerical decline in both subsets on disease progression. There is also evidence for the induction of CD4 expression and susceptibility to infection by HIV-1 of CD8 lymphocytes activated in vitro. To investigate the relationship between CD8 activation and infection by HIV-1 in vivo, activated subsets of CD8 lymphocytes in peripheral blood mononuclear cells (PBMCs) of HIV-seropositive individuals were investigated for CD4 expression and HIV infection. Activated CD8 lymphocytes were identified by expression of CD69, CD71, and the human leukocyte antigen (HLA) class II, the β-chain of CD8, and the RO isoform of CD45. CD4+ and CD4− CD8 lymphocytes, CD4 lymphocytes, other T cells, and non-T cells were purified using paramagnetic beads, and proviral sequences were quantified by PCR using primers from the long terminal repeat region. Frequencies of activated CD8 lymphocytes were higher in HIV-infected study subjects than in seronegative controls, and they frequently coexpressed CD4 (mean frequencies on CD69+, CD71+, and HLA class II+ cells of 23, 37, and 8%, respectively, compared with 1 to 2% for nonactivated CD8 lymphocytes). The level of CD4 expression of the double-positive population approached that of mature CD4 lymphocytes. That CD4 expression renders CD8 cell susceptible to infection was indicated by their high frequency of infection in vivo; infected CD4+ CD8 lymphocytes accounted for between 3 and 72% of the total proviral load in PBMCs from five of the eight study subjects investigated, despite these cells representing a small component of the PBMC population (<3%). Combined, these findings provide evidence that antigenic stimulation of CD8 lymphocytes in vivo induces CD4 expression that renders them susceptible to HIV infection and destruction. The specific targeting of responding CD8 lymphocytes may provide a functional explanation for the previously observed impairment of cytotoxic T-lymphocyte (CTL) function disproportionate to their numerical decline in AIDS and for the deletion of specific clones of CTLs responding to HIV antigens.
Infection with human immunodeficiency virus type 1 (HIV-1) is associated with progressive destruction of CD4+ T-helper lymphocytes and the development of the profound immunodeficiency that underlies AIDS. The targeting of CD4 lymphocytes by HIV-1 is thought to result from expression of cell surface CD4 (11, 26, 31), the chemokine receptor CXCR4 (15), and (on activation) CCR5 (1, 13), which act as receptors for the attachment and entry of HIV-1. CD4 and CCR5 expression probably also accounts for the infection of macrophages and macrophage-derived cell types in vivo, although the effect that this has on the immune system is unresolved.
Whether HIV-1 targets other cells in the immune system and whether infection and direct or indirect destruction of CD4 lymphocytes entirely accounts for the immunodeficiency observed in AIDS have remained controversial. It is possible that HIV-1 may be able to infect other lymphoid cells through a CD4-independent mechanism (as has been suggested for the infection of astrocytes in the central nervous system), or it may target cells that transiently express CD4 during maturation or activation. Many investigators have provided evidence for the widespread infection in vitro and in vivo of human CD8+, cytotoxic T lymphocytes by HIV-1 (16, 30, 32, 34, 50, 54) or by the primate simian immunodeficiency virus (SIV) homologues SIVmac in sooty mangabeys (12) and SIVagm in African green monkeys (37). Infection of cells destined to become CD8 lymphocytes may occur through a conventional CD4-dependent mechanism during their maturation in the thymus, at the stage where CD4 is coexpressed with CD8 (12, 24). Destruction of thymic precursor cells may significantly deplete the pool of both CD4 and CD8 lymphocytes; infection at this stage may subsequently be manifested by the appearance of a population of HIV provirus-positive, antigen-naive CD8 lymphocytes in the peripheral circulation comprising cells that survived infection. This hypothesis is supported by the finding that implants of human thymic tissue containing HIV-1-infected double-positive (DP) thymocytes was followed by the appearance of infected single-positive CD8 lymphocytes in the peripheral circulation (9, 24, 28). The hypothesis of thymic infection is further supported by our recent observation of the distribution of proviral sequences in CD8 lymphocytes expressing the RA (naive) isoform of CD45 (32).
Very recently, isolation of HIV-1 from purified CD8 lymphocytes from two AIDS patients was described, which produced apparently CD8-tropic variants that could be propagated in transformed CD8 lymphocyte cultures or mitogen-stimulated cultures of peripheral blood mononuclear cells (PBMCs) (44). Their subsequent biological characterization led to the proposal that these variants infect CD8 lymphocytes through initial attachment to CD8 instead of CD4 (54). These isolates showed the additional peculiarity of not using CCR5, CXCR4, or other known coreceptors in mediating subsequent virus entry steps.
Apart from intrathymic and CD8-independent mechanisms for infection of CD8 lymphocytes, several investigators have demonstrated that in vitro activation of purified peripheral CD8 lymphocytes with mitogens, anti-CD3 and anti-CD28 antibodies, or antigen-pulsed autologous dendritic cells leads to de novo synthesis of CD4 expression, sufficient to confer susceptibility to infection with laboratory isolates of HIV-1 (16, 23, 50). However, it is presently unknown whether up-regulation or de novo synthesis of CD4 on CD8 lymphocytes also occurs in vivo. To investigate this issue and the possibility that CD4 expression on CD8 lymphocytes confers susceptibility to HIV infection in vivo, we have measured the expression of CD4 on activated and nonactivated CD8 lymphocytes recovered from peripheral blood of HIV-infected and uninfected individuals. In addition, the distribution of HIV-1 sequences in isolated cell subsets, in particular in relation to expression of CD4 on CD8 lymphocytes from eight HIV-seropositive patient PBMC samples, was assessed.
MATERIALS AND METHODS
Samples and clinical details of study subjects.
Volumes of 20 to 30 ml of whole blood anticoagulated with EDTA were obtained by venipuncture from 5 HIV-seronegative controls (S1 to S5) (3 male and 2 female; mean age, 27.6 years [range, 23 to 40 years]) and from 20 HIV-seropositive individuals attending the Regional Infectious Diseases Unit, Western General Hospital, Edinburgh, United Kingdom. Information on CD4 count, viral load, duration of therapy, and risk group for the HIV-seropositive individuals is presented in Table 1. HIV plasma virus levels were determined by a commercially available PCR assay (Roche Monitor, Lewes, East Sussex, United Kingdom).
TABLE 1.
Clinical profile of HIV-seropositive study subjects, ranked from low to high CD4 count.
| Study subject | Sexa | Age (yr) | Risk groupb | CD4 count/μl | Viral load/ml | Duration of therapy (mo) |
|---|---|---|---|---|---|---|
| p1 | F | 41 | IVDU | 25 | 109,000 | NTc |
| p2 | M | 53 | IVDU | 35 | 83,100 | 24 |
| p3 | F | 34 | WSM | 87 | >750,000 | 2 |
| p4 | M | 44 | IVDU | 94 | 205,000 | 6 |
| p5 | F | 40 | IVDU | 104 | 14,800 | NT |
| p6 | F | 39 | WSM | 117 | <50 | 17 |
| p7 | F | 37 | WSM | 201 | 2,100 | NT |
| p8 | F | 43 | WSM | 207 | 105,000 | 12 |
| p9 | F | 38 | WSM | 236 | 101 | 14 |
| p10 | M | 37 | MSM | 252 | 1,030 | 3 |
| p11 | M | 48 | MSM | 325 | <50 | 31 |
| p12 | F | 35 | IVDU | 334 | 139,000 | NT |
| p13 | M | 64 | Needlestick | 334 | <400 | 7 |
| p14 | F | 33 | WSM | 399 | <50 | 25 |
| p15 | F | 25 | WSM | 420 | <50 | 11 |
| p16 | M | 37 | MSM | 430 | 20,500 | NT |
| p17 | M | 38 | MSM | 536 | 924 | 1 |
| p18 | F | 41 | WSM | 546 | <50 | 33 |
| p19 | M | 44 | IVDU | 713 | 41,400 | NT |
| p20 | M | 41 | IVDU | 826 | <50 | 12 |
M, male; F, female.
IVDU, intravenous-drug abuser; MSM, man who has sex with men; WSM, woman who has sex with men; needlestick, needlestick injury.
NT, no therapy given.
Immunomagnetic T-cell separation.
PBMCs were obtained from whole blood by density centrifugation over Lymphoprep separation medium (Nycomed Pharma AS). T lymphocytes were purified from the isolated PBMCs by negative selection using the pan-T-cell kit (Miltenyl Biotec) on an AutoMACS system, with all solutions kept on ice to help prevent capping and patching of antibodies on the surface of the cells and retard any possible cellular activation that was occurring. Analysis of the levels of activation of CD69 on T lymphocytes before and after immunomagnetic separations did not show any evidence for cellular activation occurring during the purification process (mean percent expression [n = 3]: prepurification CD4+ CD69+, 0.18%; prepurification CD8+ CD69+, 0.54%; postpurification CD4+ CD69+, 0.21%; postpurification CD8+ CD69+, 0.43%).
For quantification of HIV-1 proviral levels in different cell subsets, T lymphocytes were isolated as described above and the cells remaining in the column were eluted and are referred to as non-T cells, which would consist of B lymphocytes, dendritic cells, monocytes, and NK cells. Contaminating γδ-T-cell receptor (γδ-TCR)-expressing lymphocytes were removed using the γδ microbead kit (Miltenyl Biotec). From the remaining αβ-TCR+ T lymphocyte fraction, CD8+ and CD8− lymphocytes were isolated by positive selection using the CD8 multisort kit (Miltenyl Biotec). From the CD8+ fraction, the CD8 microbeads were enzymatically removed from the selected fraction according to the manufacturer's instructions. The CD8+ lymphocyte fraction was then further separated by positive selection using CD4 microbeads (Miltenyl Biotec) to give CD8+ CD4+ and CD8+ CD4− lymphocyte fractions. From the CD8− fraction, CD4+ lymphocytes were isolated by positive selection for CD4 using CD4 microbeads (Miltenyl Biotec) to give CD4+ CD8− and CD4− CD8− lymphocyte fractions. For the purpose of this study, the non-αβ-TCR-expressing lymphocytes and those lymphocytes which were CD4− CD8− were combined and are referred to as other T cells.
Flow cytometric analysis.
Flow cytometric analysis was carried out on an FACSCalibur flow cytometer (Becton Dickinson, Crawley, United Kingdom) by gating on lymphocytes based on their forward and side scatter parameters, with a minimum of 10,000 events collected. For analysis of CD4 expression on CD8 lymphocytes, further gates were placed around those CD8+ T cells based on the expression of activation markers and the level of CD4 expression was plotted. Monoclonal antibodies and isotype-matched mouse immunoglobulin controls (labeled with phycoerythrin [PE], PE-Cy5 [Cy5], and fluorescein isothiocyanate isomer 1 [FITC]) to the following cell surface markers were obtained: CD3-PE (clone UCHT1), CD4-Cy5 (clone MT310), CD8-FITC (clone DK25), CD14-PE (clone TUK4), CD45-FITC (clone T29/33), and pan-HLA class II–FITC (clone CR3/43) (Dako, Ely, United Kingdom); CD69-FITC (clone FN50) and CD71-FITC (clone M-A712) (BD Pharminigen, Crowley, United Kingdom); and CD8 β-chain–PE (clone 2ST8.5H7) (Coulter Immunotech, High Wycombe, Buckinghamshire, United Kingdom).
Detection and quantitation of HIV sequences.
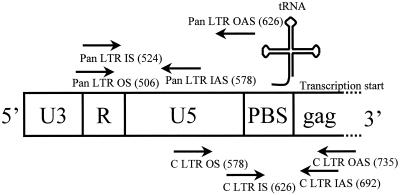
DNA was extracted from selected cell subsets using the QIAamp DNA Mini kit (Qiagen Ltd., Crawley, United Kingdom). HIV proviral sequences were quantified by limiting-dilution nested PCR using a nested set of highly conserved PCR primers from the complete long terminal repeat (LTR) region. Pan-LTR primers were 5′-GRAACCCACTGCTTAASSCTCAA-3′ (outer, sense), 5′-TGTTCGGGCGCCACTGCTAGAGA-3′ (outer, antisense), 5′-CTCAATAAAGCTTGCCTTGAG-3′ (inner, sense), and 5′-GAGGGATCTCTAGNYAVVAGAGT-3′ (inner, antisense) (5′ base positions 506, 626, 524, and 578 in the HXB2 genome, respectively). Complete LTR (C-LTR) primers were 5′-ACTCTGGTRNCTAGAGATCCCTC-3′ (outer, sense), 5′-GGCGTACTCACCAGTCGCCG-3′ (outer, antisense), 5′-TCTCTAGCAGTGGCGCCCGAAC-3′ (inner, sense), and 5′-TCAGCAAGCCGAGTCCTG-3′ (inner, antisense) (5′ base positions 578, 735, 626, and 692 in the HXB2 genome, respectively). The binding sites of the pan-LTR and C-LTR primers in the HIV-1 genome were chosen to allow detection of all cDNA transcripts and of completed proviral sequences, respectively (Fig. 1). Both primary and secondary reactions for pan-LTR and C-LTR primers were carried out using the following parameters: 94°C for 18 s, 55°C for 21 s, and 72°C for 1 min 30 s for 30 cycles followed by a final extension step of 72°C for 6 min. PCR amplicons were run at 150 V for 30 min on 2% (wt/vol) agarose gels containing 0.5 μg of ethidium bromide/ml and visualized under UV light. Quantification of the proviral load in each of the selected cell subsets was performed by limiting-dilution PCR as previously described (45).
FIG. 1.
Binding sites of the pan-LTR and C-LTR primers used for detection of proviral LTR sequences. Numbers in parentheses indicate the 5′ base positions in the HXB2 genome. Abbreviations: PBS, primer binding site; OS, outer, sense; IS, inner, sense; IAS, inner, antisense; OAS, outer, antisense.
RESULTS
Purity of immunomagnetically isolated T lymphocytes.
T lymphocytes were separated from PBMCs by negative selection by incubation with a cocktail of immunomagnetically labeled antibodies to B-lymphocyte, dendritic cell, monocyte, and NK cell markers. Labeled cells were then passed through a separation column held in a magnetic field, with phenotypically unchanged T lymphocytes passing straight through. Between 1.3 and 9.8 million T lymphocytes were isolated from PBMCs of the uninfected and HIV-infected study subjects, of which at least 94.4% (HIV-negative subjects) to 96.1% (HIV-positive subjects) of the total sorted T cells expressed CD3 and CD45 (Table 2). The proportions of cells recovered that expressed CD4 were approximately the same numbers when compared to previously determined CD4 absolute counts (Table 1). The frequency of monocyte (CD45+ CD14+) contamination was less than 2%. Isolated T lymphocytes were subsequently further purified by immunomagnetic isolations to obtain CD4-expressing CD8 lymphocytes and other relevant T-cell subsets.
TABLE 2.
Purity of isolated T lymphocytes from PBMC samples obtained from HIV-seronegative and -seropositive individualsa
| Study subject | % of lymphocytes:
|
|||
|---|---|---|---|---|
| CD14+ CD45+ | CD3+ CD45+ | CD3+ CD4+ | CD3+ CD8+ | |
| Seronegative | ||||
| S1 | 0.1 | 96.4 | 53.4 | 43.6 |
| S2 | 0.1 | 95.4 | 65.1 | 30.5 |
| S3 | 1.8 | 96.5 | 62.2 | 33.8 |
| S4 | 0.1 | 94.4 | 69.4 | 26.1 |
| S5 | 1.9 | 96.2 | 40.7 | 53.3 |
| Mean | 0.8 | 95.8 | 58.2 | 37.5 |
| Seropositive | ||||
| p4 | 0.2 | 96.1 | 16.7 | 76.8 |
| p6 | 0.8 | 97.1 | 13.3 | 74.6 |
| p11 | 0.9 | 97.5 | 35.6 | 66.8 |
| p15 | 0.2 | 99.1 | 63.1 | 36.6 |
| p17 | 0.4 | 99.4 | 51.5 | 47.8 |
| Mean | 0.5 | 97.8 | 36.1 | 60.5 |
HIV-seropositive individuals were ranked from low to high CD4 count. A minimum of 10,000 events were collected for each analysis.
Activation markers on CD4 and CD8 lymphocytes.
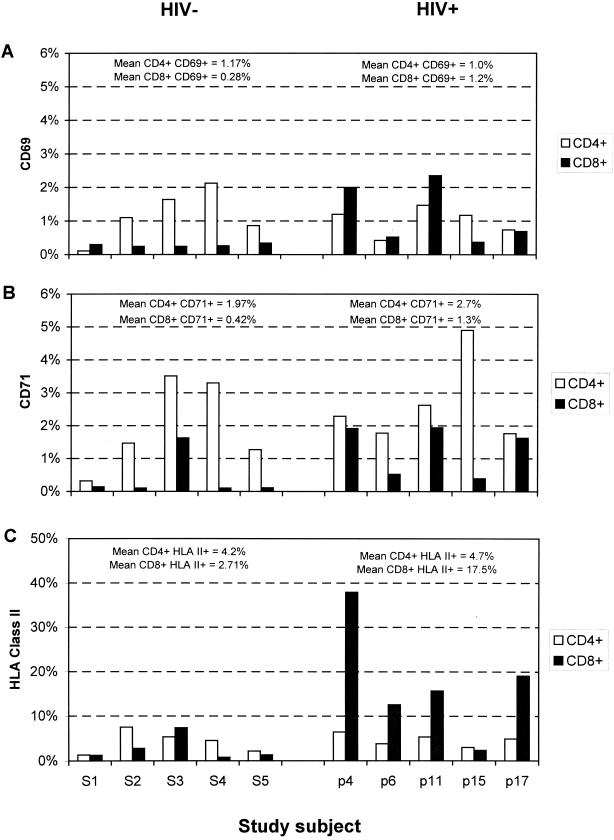
Expression of the cell surface markers CD69, CD71, and HLA class II was used to identify activated CD4 and CD8 lymphocytes (Fig. 2). HIV-positive study subjects showed higher frequencies of activated CD8 lymphocytes (mean values [range] of 1.2% [0.4 to 2.4%], 1.3% [0.4 to 1.9%], and 17.5% [2.4 to 37.9%] for the three markers, respectively) than the HIV-negative controls (0.28% [0.2 to 0.3%], 0.42% [0.1 to 1.6%], and 2.7% [0.8 to 7.4%], respectively). Frequencies of activated CD4 lymphocytes were comparable between the two study groups (CD69, 1.0% [HIV positive] compared with 1.2% [HIV negative]; CD71, 4.7% [HIV positive] and 4.2% [HIV negative]; and HLA class II, 4.7% [HIV positive] and 4.2% [HIV negative]).
FIG. 2.
Expression of the activation markers CD69 (A), CD71 (B), and HLA class II (C) on CD4 and CD8 lymphocytes from HIV-seronegative (S1 to S5) and HIV-seropositive (p4 to p17) individuals. HIV-seropositive individuals were ranked from low CD4 count (p4) to high CD4 count (p17); mean values for expression of each activation marker on CD4 and CD8 lymphocytes in the seronegative and seropositive groups are indicated above the histograms. A minimum of 7,500 lymphocytes from each individual were collected and analyzed.
CD4 expression on activated and nonactivated CD8 lymphocytes.
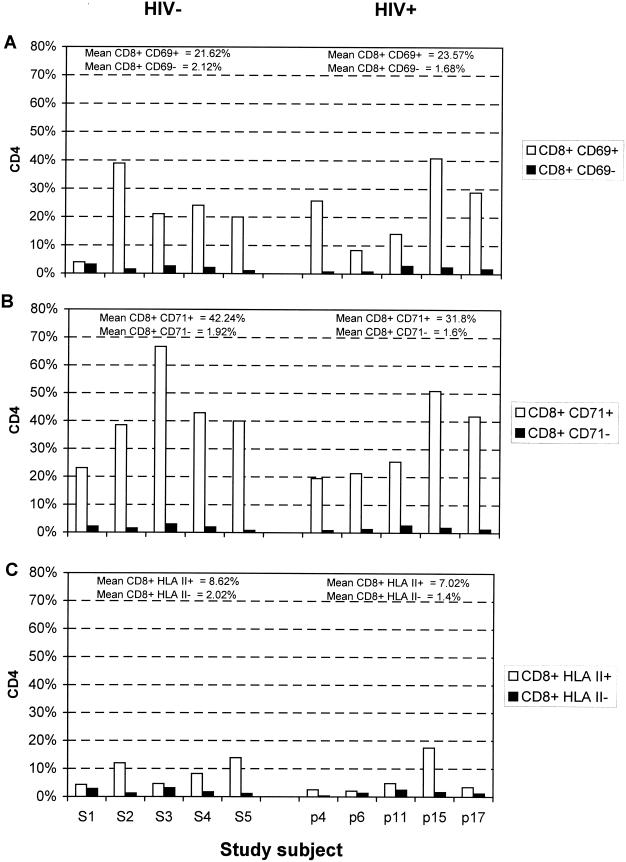
Expression of CD4 on activated and nonactivated CD8 lymphocytes was determined by three-color flow cytometry (Fig. 3). CD4 expression was more frequent on activated cells, irrespective of the activation marker used and the HIV status of the study subject. For example, 21.6% (mean for HIV-negative subjects) and 23.6% (mean for HIV-positive subjects) of the CD69+ CD8 lymphocyte population expressed CD4, compared with 2.1 and 1.7%, respectively, in CD69− CD8 lymphocytes. An even greater (20-fold) difference in frequency of CD4 expression was observed between CD71+ and CD71− cells in both study groups. The proportion of CD8 lymphocytes expressing CD4 varied between the activation markers, with lower frequencies of CD4 expression observed on the HLA class II+ lymphocytes.
FIG. 3.
Comparison of frequencies of CD4 expression on activated and nonactivated CD8 lymphocytes identified by CD69 (A), CD71 (B), and HLA class II (C) from HIV-seronegative (S1 to S5) and HIV-seropositive (p4 to p17) individuals. Mean values for CD4 expression on each activated and nonactivated lymphocyte subset are indicated above the histograms. A minimum of 7,500 lymphocytes from each individual were collected and analyzed.
To investigate whether DP lymphocytes were CD4 lymphocytes with aberrant CD8 αα-homodimer expression (8, 20, 21, 39), the presence of CD8 αβ-heterodimers (found on true CD8 lymphocytes) was detected by flow cytometry for CD8 α-chain- and β-chain-specific antibody (Table 3). Although the individual measurements were subject to stochastic sampling errors, there was no evidence for an excess of CD4 expression on CD8 α-chain-positive lymphocytes over that found on lymphocytes expressing the CD8 β-chain (n = 7; mean values of 2.76% [α-chain] and 2.77% [β-chain]). These results indicate that CD8 β-chain-expressing lymphocytes are capable of expressing CD4 and exclude the possibility that CD4 lymphocytes were expressing the CD8 αα homodimer.
TABLE 3.
Comparison of the percent expression of CD4 on CD8α+ or CD8β+ lymphocytes from seven HIV-seropositive individuals, ranked from low to high CD4 counta
| Study subject | % of lymphocytes expressing CD4
|
|
|---|---|---|
| CD8α+ lymphocytes | CD8β+ lymphocytes | |
| p1 | 0.91 | 1.52 |
| p5 | 6.80 | 6.67 |
| p10 | 1.21 | 1.72 |
| p12 | 3.49 | 3.68 |
| p13 | 3.73 | 4.00 |
| p16 | 0.46 | 0.24 |
| p18 | 2.75 | 1.59 |
| Mean | 2.76 | 2.77 |
A minimum of 25,000 αβ-TCR+ lymphocytes from each individual sample were analyzed.
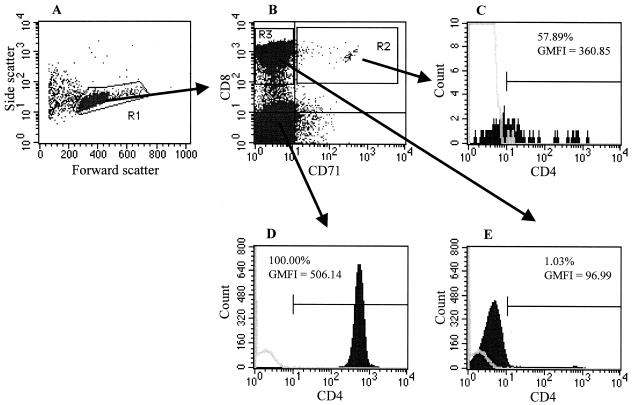
To further confirm that our results were due to CD4 expression on true CD8 lymphocytes, CD4 and CD71 expression on CD8 β-chain-expressing lymphocytes was analyzed and compared with the expression of CD4 on CD4 lymphocytes. The amount of CD4 expressed on the CD4+ fraction of CD71+ (activated) CD8 β-chain-expressing lymphocytes was similar to that on CD4 lymphocytes (Fig. 4) (geometric mean fluorescence intensity [GMFI] of CD4 expression on CD8β+ CD71+ = 360.85; GMFI on CD3+ CD4+ = 506.14). CD4 expression on activated CD8 lymphocytes identified by other activation markers (CD69 and HLA class II) was similar to that observed on the CD71+ cells; levels of CD4 expression were also comparable between activated CD8 lymphocytes from HIV-seropositive and -seronegative individuals (data not shown).
FIG. 4.
Flow cytometric analysis of CD4 expression on activated and nonactivated CD8 lymphocytes. (A) Forward and side scatter plots of immunomagnetically isolated T lymphocytes (purity of T cell isolation: CD3+ αβ-TCR+, 94.69%; CD3+ γδ-TCR+, 2.18%). (B) Dual-parameter plot of CD8 and CD71 expression from the R1 gate highlighting the activated CD8 lymphocytes (CD71+, R2 gate) and nonactivated CD8 lymphocytes (CD71−, R3 gate). (C) Histogram of CD4 expression from CD71+ CD8 lymphocytes (R3 gate). (D) Histogram of CD4 expression from CD8− lymphocytes from panel B. (E) Histogram of CD4 expression from CD71− CD8 lymphocytes (R2 gate). The frequency of CD4-expressing cells is shown in the histograms; the GMFI of CD4 expression in CD4+ populations is indicated below this frequency. Open histograms refer to the binding of an isotype-matched control antibody. A minimum of 25,000 CD8β+ lymphocytes from each individual were collected and analyzed.
CD45 isoform expression on CD4+ CD8 lymphocytes.
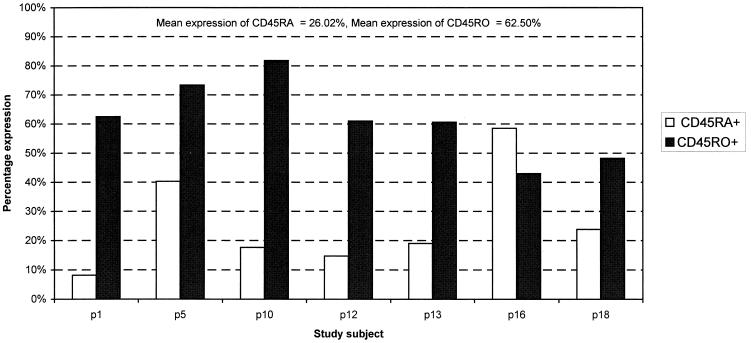
To determine whether expression of CD4 on CD8 β-chain-expressing lymphocytes occurred on immature thymocytes recently released into the periphery from the thymus or on activated mature lymphocytes, we compared expression of naive (CD45RA) and memory (CD45RO) cell markers on CD4-expressing CD8 lymphocytes. Immature thymocytes are antigen inexperienced and therefore exclusively express the CD45RA isoform. In contrast, mature lymphocytes responding to antigen would up-regulate the CD45RO isoform. By flow cytometry using antibodies to CD45RA, CD45RO, and CD4, we found that CD8 lymphocytes in all samples examined expressed high levels of CD45RO in conjunction with CD45RA (Fig. 5) (n = 7; mean percent CD45RO+ = 61.50% [range, 42.99 to 81.82%], mean percent CD45RA+ = 26.02% [range, 8.16 to 58.50%]). The expression of CD45RO rules out the possibility that the CD4−-expressing CD8 lymphocytes are immature thymocytes.
FIG. 5.
Analysis of the levels of expression of CD45RA (naive) and CD45RO (memory) cell markers on CD8 β-chain-positive lymphocytes expressing CD4 molecules. A minimum of 15,000 CD8β+ lymphocytes from each individual were collected and analyzed.
Detection of HIV proviral sequences in isolated cell subsets.
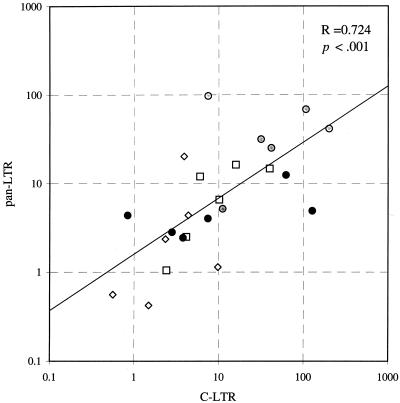
T lymphocytes were isolated from PBMC samples by immunomagnetic negative selection, and CD4 and CD8 lymphocytes were separated from T-lymphocyte samples by positive selection for CD4 and CD8, respectively. The isolation procedure for CD8 lymphocytes produced less than 0.5% contamination with CD4 lymphocytes (n = 7; range, 0.3 to 0.5%). This allowed the calculation of the proviral load found in the CD8+ lymphocyte fractions due to “worst-case” CD4 contamination (see below). Further immunomagnetic separations on the CD8 lymphocytes based on the expression of CD4 were carried out to give the CD8+ CD4+ and CD8+ CD4− fractions. HIV proviral sequences were detected using two sets of highly conserved primers directed against the LTR region that allowed detection of incomplete and complete HIV transcripts, as previously described (32). Proviral loads in CD4, CD4+ CD8, and CD4− CD8 lymphocytes using the two sets of primers were comparable, with no evidence for a significant excess of sequences detected by the pan-LTR primers when six HIV-seropositive samples were analyzed (Fig. 6) (Spearman correlation coefficient [r] = 0.724; P < 0.001). Using the C-LTR primers and assuming the worst-case scenario for CD4 lymphocyte contamination of the isolated CD8 lymphocyte fractions, <3.1% of the detected proviral load could be attributed to CD4 lymphocyte contamination in the CD4+ CD8 lymphocyte fractions (for samples p2, p7, p9, p14, and p20) and in the CD4− CD8 fractions (samples p2 and p8). CD4 lymphocyte contamination was greater than 10% in the remaining two samples (p19, 32% in the CD4+ CD8 lymphocyte fraction; p3, 12% in the CD4− CD8 fraction), and these were omitted from subsequent analysis.
FIG. 6.
Correlation between proviral loads in different lymphocyte subsets measured by the C-LTR primers (x axis) and the pan-LTR primers (y axis); the regression line and Spearman's correlation coefficient are shown. Symbols: ○, CD4 lymphocyte; ●, CD4+ CD8 lymphocyte; □, other T cell; ⧫, non-T cell.
Contribution of individual cell subsets to total proviral load.
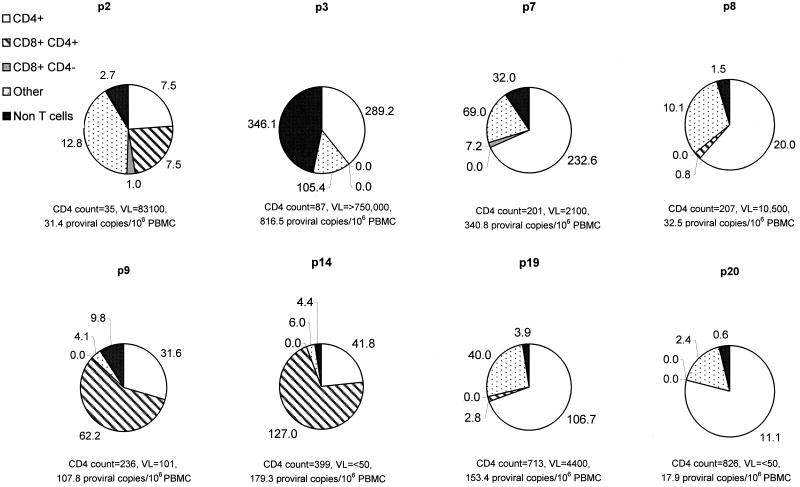
HIV sequences in DNA samples from each of the purified cell subsets from eight HIV-seropositive individuals were quantified using C-LTR primers, and the contribution of each subset to the total proviral load detected in PBMCs was calculated (Fig. 7). LTR sequences could be detected in all samples of CD4 lymphocytes and contributed 22 to 69% of the total PBMC proviral load. Proviral sequences were detected in five of eight samples of CD4+ CD8 lymphocytes and at lower frequencies in two of eight samples of CD4− CD8 lymphocytes. The contribution of CD8 lymphocyte infection to overall proviral load in PBMCs varied considerably between individuals; in two cases, the majority of the infected PBMCs comprised CD4+ CD8 lymphocytes (p9 and p14). There was no association between CD4+ or CD4− CD8 lymphocyte infection and CD4 count. Frequencies of HIV infection in the different PBMC subsets did not correlate with disease status, risk group, or antiviral therapy (Table 1).
FIG. 7.
Contribution to overall proviral load of CD4 lymphocytes, CD4+ and CD4− CD8 lymphocytes, other T cells, and non-T cells (expressed as copies per 106 PBMCs) in peripheral blood samples from eight HIV-seropositive individuals. HIV-seropositive individuals were ranked from low CD4 count (p2) to high CD4 count (p20). VL, circulating viral load (RNA copies per milliliter of plasma).
DISCUSSION
CD4 expression on activated CD8 lymphocytes.
This study is the first to demonstrate high frequencies of CD4 expression on the activated subset of CD8 lymphocytes in vivo. This was achieved by comparison of frequencies of CD4 expression on CD8 lymphocytes colabeled with the activation markers CD69, CD71, and HLA class II, as well as the different isoforms of CD45. CD4 expression was detected on approximately 20 to 40% of CD8 lymphocytes identified as activated by expression of CD69 or CD71 and on a significant proportion of the much large number of HLA class II-positive cells (Fig. 3). Quantitative analysis of CD4 expression on DP lymphocytes indicated a level of CD4 expression comparable to that of CD4 lymphocytes (GMFI values of 360 and 500, respectively [Fig. 4]). These levels of expression were greater than those observed on CD8 lymphocytes activated in vitro through mitogens, anti-CD3/anti-CD28, or, more recently, pulsing of CD8 lymphocytes with antigen-bearing dendritic cells (16, 23, 50). It is possible that the greater CD4 expression detected in vivo in this study may result from differences in the cellular milieu in which CD8 activation takes place and/or differences between antigenic and nonphysiological methods for T-cell stimulation.
To exclude the possibility that the DP lymphocytes identified represented aberrant CD8 α-chain expression on CD4 lymphocytes (8, 20, 36, 39), we demonstrated approximately equal frequencies of CD4 expression on lymphocytes expressing the α- and β-chains of CD8, demonstrating that CD4 expression was confined to true CD8 lymphocytes (20). Similarly, although CD69 is expressed on recent thymic emigrants (49), they differ from CD8 lymphocytes activated by antigenic stimulation by the absence of the expression of the RO isoform of CD45 (47). High-level expression of CD45RO on the DP lymphocytes examined in this study provided clear evidence that they represented activated lymphocytes rather than recent thymic emigrants. The levels of CD45RA and CD45RO found on DP lymphocytes in vivo was similar to that found in in vitro studies (16), with dual expression of CD45RA and CD45RO previously associated with cellular activation (27).
Taken together, these findings indicate that de novo CD4 expression is a component of the phenotypic change that occurs in CD8 lymphocytes on cellular activation and is one that may play a functional role in subsequent cellular interactions. In the future, culture systems that more fully replicate the physiological conditions of CD8 activation in vivo may provide information on the functional significance of CD4 up-regulation. This issue is particularly relevant for HIV-infected individuals, in whom increased frequencies of activated lymphocytes, particularly cytotoxic T cells, accompany infection and disease progression (Fig. 2) (2–4, 6,7, 17, 19, 41).
Susceptibility of CD8 lymphocytes to HIV infection.
This study documents the specific association of HIV infection with the activated subset of CD8 lymphocytes in vivo. Although DP CD8 lymphocytes numerically represent a minor subset of CD8 lymphocytes (accounting for only 0.25 to 7% of CD8 lymphocytes found in peripheral blood) (Table 3), greater proviral loads were found in this subset compared with CD4− CD8 lymphocytes in six of the eight study subjects (Fig. 7). It is possible that the HIV-infected CD4− CD8 lymphocytes detected in this study correspond to the infected CD45RA+ CD8 lymphocytes reported in our previous study (32). Those cells had been initially purified using negative selection for CD8, a procedure which would have removed the CD8 DP population analyzed in the present study. It is therefore possible that two populations of HIV-infected CD8 lymphocytes may be present in the peripheral circulation, one associated with naive CD8 lymphocytes infected previously in the thymus and one associated with activated, DP CD8 lymphocytes after antigenic exposure.
In common with our previous work on CD8 lymphocyte infection (30, 32), we acknowledge that while magnetic bead separation can produce highly purified subsets of lymphocytes suitable for PCR-based detection of HIV proviral sequences, considerable care has to be taken to ensure that the proviral sequences detected in CD8 lymphocytes did not originate from low frequencies of contaminating CD4 lymphocytes or other cell types. As with the previous study (32), a combination of purity measurements with calculations of the worst-case contribution to proviral load of contaminating CD4 lymphocytes indicated that proviral sequences detected in at least seven of the nine immunomagnetically purified subsets of CD8 lymphocytes could not have originated from contaminating CD4 cells. Similarly, the purity of T cells isolated by the pan-T negative selection method and the lack of significant monocyte contamination (Table 2) provided evidence against a significant contribution to proviral load by other cell types in the PBMC population.
The expression of high levels of CD4 of activated CD8 lymphocytes detected in this study potentially confers a phenotype susceptible to infection with HIV. Productive infection would be further favored by the activation status of the CD8 lymphocytes, which provides a suitable internal cellular environment for completion of reverse transcription, integration, and viral gene expression (10, 14, 43, 46, 48, 51, 52), as well as up-regulating expression of the chemokine receptor, CCR5 (23), whose expression is required for entry of the majority of HIV variants in vivo (5, 35).
Apart from our direct evidence for extensive infection of DP CD8 lymphocytes in vivo, the hypothesis that activated CD8 lymphocytes may be susceptible to HIV infection is further supported by observations of productive infection of mitogen- or anti-CD3, anti-CD28-stimulated CD8 lymphocytes in vitro in a CD4-dependent manner (16, 23, 50). Furthermore, this in vitro susceptibility was observed despite the induction of generally lower levels of CD4 expression than observed on activated CD8 lymphocytes in vivo (see above). A direct cytopathic effect of HIV on CD8 lymphocytes as they become activated may therefore contribute to the decline in numbers and the even more marked functional impairment of CD8 lymphocytes observed upon disease progression.
Clinical significance of CD8 lymphocyte infection.
Whether direct infection of CD8 lymphocytes contributes to the immunodeficiency observed in AIDS remains to be demonstrated, and there are a number of other mechanisms for the observed numerical decline and functional impairment of CD8 lymphocytes observed on disease progression. These include indirect mechanisms such as the loss of CD4 lymphocyte helper function leading to impaired clonal expansion and function of CD8 lymphocytes on antigenic exposure (22). CD8 lymphocytes may also become more susceptible to apoptosis from alterations in the cytokine milieu in lymphoid tissue, bystander effects from neighboring productively infected CD4 lymphocytes, or toxicity from the release of HIV-encoded proteins such as gp120 or Tat (18, 29).
There is also evidence from both the SIV-macaque model of HIV infection (12) and examination of thymic tissue implanted into SCID mice (9, 24, 25, 28) for extensive infection of T lymphocyte precursors in the thymus. Infection of DP cells during maturation may profoundly reduce the supply of T cells into the peripheral circulation and cause a marked decline in circulating numbers of the naive (CD45RA+) subsets of CD4 and CD8 lymphocytes during natural infection or in the SCID-hu mouse animal model (9, 42, 53). Abortive, nonproductive, or noncytopathic infection of DP cells in the thymus also leads to the subsequent release of HIV or SIV provirus-positive CD4 and CD8 lymphocytes into the peripheral circulation (9, 12, 24). Infection during thymic development may therefore account for the detection of infected naive CD45RA+ CD4 lymphocytes in peripheral blood (38), as well as our recent evidence for measurable frequencies of quiescently infected CD45RA+ CD8 lymphocytes in the peripheral circulation of a proportion of HIV-seropositive individuals (32). Infection of CD4− CD8 lymphocytes found in three individuals in the present study may similarly represent the outcome of previous thymic infection.
However, our findings of even more extensive infection of CD8 lymphocytes after cellular activation indicate an additional assault on the CD8 lymphocyte population. In particular, the association of susceptibility and cellular activation observed in in vitro studies suggests that CD8 lymphocytes recruited to respond to foreign antigen during HIV infection would be specifically targeted for HIV infection and destruction. The process of specific deletion of responding CD8 lymphocytes would produce a potentially devastating impairment of cytotoxic T-lymphocyte function, disproportionate to the decline in circulating CD8 lymphocyte number and potentially a major contribution to the overall immunosuppression seen in HIV infection. Destruction of antigen-responding CD8 lymphocytes may underlie the previously observed rapid disappearance of HIV-reactive clones of CD8 lymphocytes that occurs during acute infection with HIV but not other viruses (40) and following adoptive transfer of CD8 lymphocytes into SCID mice or humans infected with HIV, particularly in the absence of escape mutations in target epitopes (33).
In the future, it will be important to functionally characterize HIV isolates infecting different lymphocyte subsets in vivo. While the bulk of the evidence available to date indicates that infection of CD8 lymphocytes is restricted to a CD4-dependent pathway either on activation or in the thymus, apparently CD8-tropic isolates of HIV-1 that use the CD8 surface molecule as a receptor have recently been described (44, 54). Indeed, until CD4 dependence can be demonstrated for naturally occurring isolates of HIV-1 infecting CD8 lymphocytes, the preferential distribution of proviral sequences in the DP CD8 lymphocytes observed in this study might conceivably result simply from the requirement of HIV for cellular activation for productive infection and therefore be independent of the observed up-regulation not only of CD4 but of the coreceptor CCR5, which is apparently also not used for virus entry by these isolates (54). Whether these apparently CD4-independent viruses are widely distributed in HIV-1-infected individuals or whether HIV variants normally use cell surface-expressed CD4 to enter CD8 lymphocytes remains to be determined.
ACKNOWLEDGMENTS
We thank the patients and staff at the Regional Infectious Disease Unit for providing samples for analysis and Caroline Wilson for providing clinical information for the study subjects.
Grant support for this study was provided by the Medical Research Council (G9632414).
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1 alpha, MIP-1 beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Bass H Z, Hardy W D, Mitsuyasu R T, Wang Y X, Cumberland W, Fahey J L. Eleven lymphoid phenotypic markers in HIV infection: selective changes induced by zidovudine treatment. J Acquir Immune Defic Syndr. 1992;5:890–897. [PubMed] [Google Scholar]
- 3.Bass H Z, Nishanian P, Hardy W D, Mitsuyasu R T, Esmail E, Cumberland W, Fahey J L. Immune changes in HIV-1 infection: significant correlations and differences in serum markers and lymphoid phenotypic antigens. Clin Immunol Immunopathol. 1992;64:63–70. doi: 10.1016/0090-1229(92)90060-2. [DOI] [PubMed] [Google Scholar]
- 4.Benito J M, Zabay J M, Gil J, Bermejo M, Escudero A, Sanchez E, Fernandez-Cruz E. Quantitative alterations of the functionally distinct subsets of CD4 and CD8 T lymphocytes in asymptomatic HIV infection: changes in the expression of CD45RO, CD45RA, CD11b, CD38, HLA-DR, and CD25 antigens. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:128–135. doi: 10.1097/00042560-199702010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Bleul C C, Wu L J, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouscarat F, Levacher M, Landman R, MuffatJoly M, Girard P M, Saimot A C, Brunvezinet F, Sinet M. Changes in blood CD8+ lymphocyte activation status and plasma HIV RNA levels during antiretroviral therapy. AIDS. 1998;12:1267–1273. doi: 10.1097/00002030-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Brinchmann J E, Rosok B I, Spurkland A. Activation and proliferation of CD8+ T cells in lymphoid tissues of HIV-1-infected individuals in the absence of the high-affinity IL-2 receptor. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:332–338. doi: 10.1097/00042560-199812010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Brod S A, Purvee M, Benjamin D, Hafler D A. Frequency analysis of CD4+CD8+ T cells cloned with IL-4. Cell Immunol. 1990;125:426–436. doi: 10.1016/0008-8749(90)90096-a. [DOI] [PubMed] [Google Scholar]
- 9.Brooks D G, Kitchen S G, Kitchen C M, Scripture-Adams D D, Zack J A. Generation of HIV latency during thymopoiesis. Nat Med. 2001;7:459–464. doi: 10.1038/86531. [DOI] [PubMed] [Google Scholar]
- 10.Chun T W, Chadwick K, Margolick J, Siliciano R F. Differential susceptibility of naive and memory CD4(+) T cells to the cytopathic effects of infection with human immunodeficiency virus type 1 strain LAI. J Virol. 1997;71:4436–4444. doi: 10.1128/jvi.71.6.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 12.Dean G A, Reubel G H, Pedersen N C. Simian immunodeficiency virus infection of CD8+ lymphocytes in vivo. J Virol. 1996;70:5646–5650. doi: 10.1128/jvi.70.8.5646-5650.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y X, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4(+) cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 14.Farnet C M, Bushman F D. HIV-1 cDNA integration: requirement of HMG I(Y) protein for function of preintegration complexes in vitro. Cell. 1997;88:483–492. doi: 10.1016/s0092-8674(00)81888-7. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 16.Flamand L, Crowley R W, Lusso P, ColombiniHatch S, Margolis D M, Gallo R C. Activation of CD8(+) T lymphocytes through the T cell receptor turns on CD4 gene expression: implications for HIV pathogenesis. Proc Natl Acad Sci USA. 1998;95:3111–3116. doi: 10.1073/pnas.95.6.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giorgi J V, Ho H N, Hirji K, Chou C C, Hultin L E, O'Rourke S, Park L, Margolick J B, Ferbas J, Phair J P. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ CD38- CD8+ cells is associated with subsequent stable CD4+ cell levels. The Multicenter AIDS Cohort Study Group. J Infect Dis. 1994;170:775–781. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 18.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, Brien W A O, Verdin E. Apoptosis of CD8(+) T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 19.Ho H N, Hultin L E, Mitsuyasu R T, Matud J L, Hausner M A, Bockstoce D, Chou C C, O'Rourke S, Taylor J M G, Giorgi J V. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–3079. [PubMed] [Google Scholar]
- 20.Hori T, Paliard X, de Waal Malefijt R, Ranes M, Spits H. Comparative analysis of CD8 expressed on mature CD4+ CD8+ T cell clones cultured with IL-4 and that on CD8+ T cell clones: implication for functional significance of CD8 beta. Int Immunol. 1991;3:737–741. doi: 10.1093/intimm/3.7.737. [DOI] [PubMed] [Google Scholar]
- 21.Jose B, Friedman S R, Neaigus A, Curtis R, Grund J P C, Goldstein M F, Ward T P, Jarlais D C D. Syringe-mediated drug-sharing (backloading)—a new risk factor for HIV among injecting drug users. AIDS. 1993;7:1653–1660. doi: 10.1097/00002030-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Kalams S A, Walker B D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitchen S G, Korin Y D, Roth M D, Landay A, Zack J A. Costimulation of naive CD8+ lymphocytes induces CD4 expression and allows human immunodeficiency virus type 1 infection. J Virol. 1998;72:9054–9060. doi: 10.1128/jvi.72.11.9054-9060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitchen S G, Uittenbogaart C H, Zack J A. Mechanism of human immunodeficiency virus type 1 localization in CD4-negative thymocytes: differentiation from a CD4-positive precursor allows productive infection. J Virol. 1997;71:5713–5722. doi: 10.1128/jvi.71.8.5713-5722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 27.LaSalle J M, Hafler D A. The coexpression of CD45RA and CD45RO isoforms on T cells during the S/G2/M stages of cell cycle. Cell Immunol. 1991;138:197–206. doi: 10.1016/0008-8749(91)90144-z. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Goldstein H, Baseler M, Adelsberger J, Golding H. Human immunodeficiency virus type 1 infection of mature CD3(hi)CD8(+) thymocytes. J Virol. 1997;71:6671–6676. doi: 10.1128/jvi.71.9.6671-6676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis D E, Tang D S N, Aduoppong A, Schober W, Rodgers J R. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol. 1994;153:412–420. [PubMed] [Google Scholar]
- 30.Livingstone W J, Moore M, Innes D, Bell J E, Simmonds P, Whitelaw J, Wyld R, Robertson J R, Brettle R P. Frequent infection of peripheral blood CD8-positive T-lymphocytes with HIV-1. Lancet. 1996;348:649–654. doi: 10.1016/s0140-6736(96)02091-0. [DOI] [PubMed] [Google Scholar]
- 31.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 32.McBreen S, Imlach S, Scott G R, Leen C, Bell J E, Simmonds P. Preferential infection of the CD45RA+ (naive) subset of CD8+ lymphocytes by human immunodeficiency virus type 1 in vivo. J Virol. 2001;75:4091–4102. doi: 10.1128/JVI.75.9.4091-4102.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKinney D M, Lewinsohn D A, Riddell S R, Greenberg P D, Mosier D E. The antiviral activity of HIV-specific CD8(+) CTL clones is limited by elimination due to encounter with HIV-infected targets. J Immunol. 1999;163:861–867. [PubMed] [Google Scholar]
- 34.Mercure L, Phaneuf D, Wainberg M A. Detection of unintegrated human immunodeficiency virus type-1 DNA in persistently infected CD8+ cells. J Gen Virol. 1993;74:2077–2083. doi: 10.1099/0022-1317-74-10-2077. [DOI] [PubMed] [Google Scholar]
- 35.Mo H M, Monard S, Pollack H, Ip J, Rochford G, Wu L J, Hoxie J, Borkowsky W, Ho D D, Moore J P. Expression patterns of the HIV type 1 coreceptors CCR5 and CXCR4 on CD4(+) T cells and monocytes from cord and adult blood. AIDS Res Hum Retroviruses. 1998;14:607–617. doi: 10.1089/aid.1998.14.607. [DOI] [PubMed] [Google Scholar]
- 36.Moebius U, Kober G, Griscelli A L, Hercend T, Meuer S C. Expression of different CD8 isoforms on distinct human lymphocyte subpopulations. Eur J Immunol. 1991;21:1793–1800. doi: 10.1002/eji.1830210803. [DOI] [PubMed] [Google Scholar]
- 37.Murayama Y, Mukai R, InoueMurayama M, Yoshikawa Y. An African green monkey lacking peripheral CD4 lymphocytes that retains helper T cell activity and coexists with SIVagm. Clin Exp Immunol. 1999;117:504–512. doi: 10.1046/j.1365-2249.1999.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostrowski M A, Chun T W, Justement S J, Motola I, Spinelli M A, Adelsberger J, Ehler L A, Mizell S B, Hallahan C W, Fauci A S. Both memory and CD45RA+/CD62L+ naive CD4+ T cells are infected in human immunodeficiency virus type 1-infected individuals. J Virol. 1999;73:6430–6435. doi: 10.1128/jvi.73.8.6430-6435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paliard X, Malefijt R W, de Vries J E, Spits H. Interleukin-4 mediates CD8 induction on human CD4+ T-cell clones. Nature. 1988;335:642–644. doi: 10.1038/335642a0. [DOI] [PubMed] [Google Scholar]
- 40.Pantaleo G, Soudeyns H, Demarest J F, Vaccarezza M, Graziosi C, Paolucci S, Daucher M, Cohen O J, Denis F, Biddison W E, Sekaly R P, Fauci A S. Evidence for rapid disappearance of initially expanded HIV-specific CD8(+) T cell clones during primary HIV infection. Proc Natl Acad Sci USA. 1997;94:9848–9853. doi: 10.1073/pnas.94.18.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plaeger S, Bass H Z, Nishanian P, Thomas J, Aziz N, Detels R, King J, Cumberland W, Kemeny M, Fahey J L. The prognostic significance in HIV infection of immune activation represented by cell surface antigen and plasma activation marker changes. Clin Immunol. 1999;90:238–246. doi: 10.1006/clim.1998.4646. [DOI] [PubMed] [Google Scholar]
- 42.Roederer M, Dubs J G, Anderson M T, Raju P A, Herzenberg L A. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roederer M, Raju P A, Mitra D K, Herzenberg L A. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J Clin Invest. 1997;99:1555–1564. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saha K, Zhang J, Zerhouni B. Evidence of productively infected CD8+ T cells in patients with AIDS: implications for HIV-1 pathogenesis. J Acquir Immune Defic Syndr. 2001;26:199–207. doi: 10.1097/00042560-200103010-00001. [DOI] [PubMed] [Google Scholar]
- 45.Simmonds P, Balfe P, Peutherer J F, Ludlam C A, Bishop J O, Leigh Brown A J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990;64:864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spina C A, Prince H E, Richman D D. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steffens C M, Al-Harthi L, Shott S, Yogev R, Landay A. Evaluation of thymopoiesis using T cell receptor excision circles (TRECs): differential correlation between adult and pediatric TRECs and naive phenotypes. Clin Immunol. 2000;97:95–101. doi: 10.1006/clim.2000.4938. [DOI] [PubMed] [Google Scholar]
- 48.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanhecke D, Leclercq G, Plum J, Vandekerckhove B. Characterization of distinct stages during the differentiation of human CD69+CD3+ thymocytes and identification of thymic emigrants. J Immunol. 1995;155:1862–1872. [PubMed] [Google Scholar]
- 50.Yang L P, Riley J L, Carroll R G, June C H, Hoxie J, Patterson B K, Ohshima Y, Hodes R J, Delespesse G. Productive infection of neonatal CD8(+) T lymphocytes by HIV-1. J Exp Med. 1998;187:1139–1144. doi: 10.1084/jem.187.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 52.Zack J A, Haislip A M, Krogstad P, Chen I S Y. Incompletely reverse transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaunders J, Carr A, McNally L, Penny R, Cooper D A. Effects of primary HIV-1 infection on subsets of CD4+ and CD8+ T lymphocytes. AIDS. 1995;9:561–566. doi: 10.1097/00002030-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Gupta A, Dave R, Yimen M, Zerhouni B, Saha K. Isolation of primary HIV-1 that target CD8+ T lymphocytes using CD8 as a receptor. Nat Med. 2001;7:65–72. doi: 10.1038/83365. [DOI] [PubMed] [Google Scholar]