Abstract
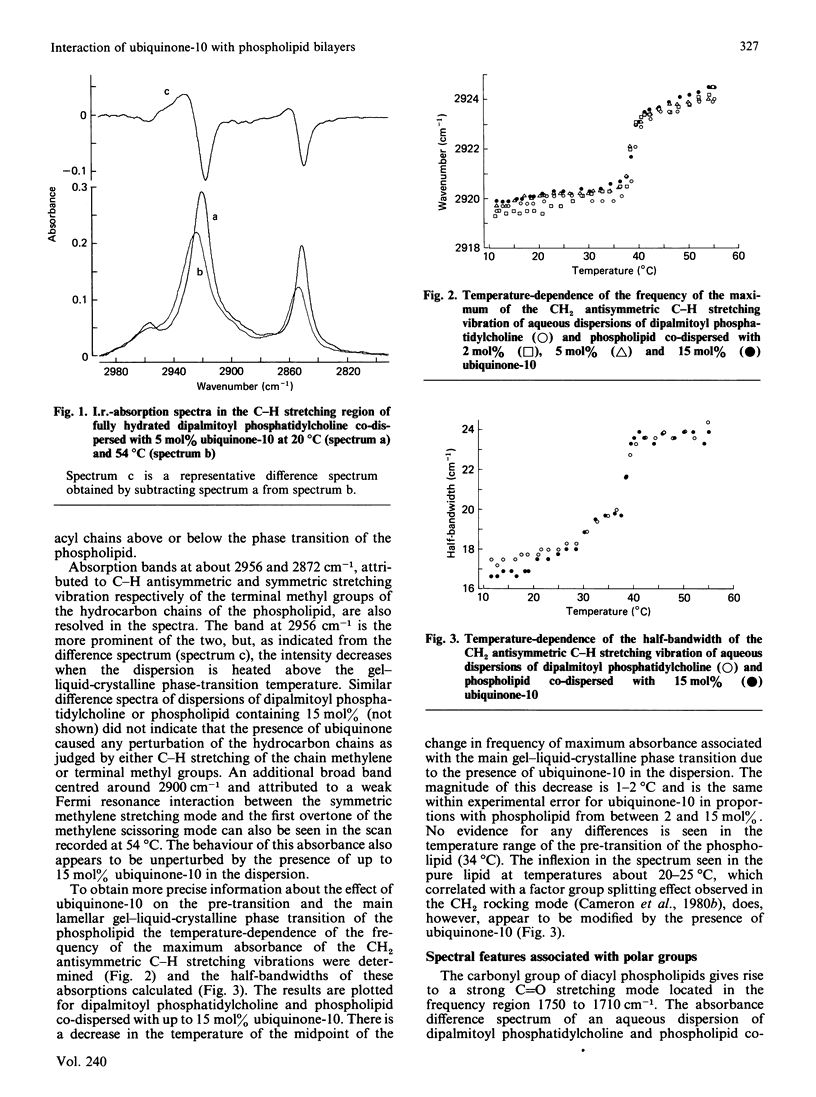
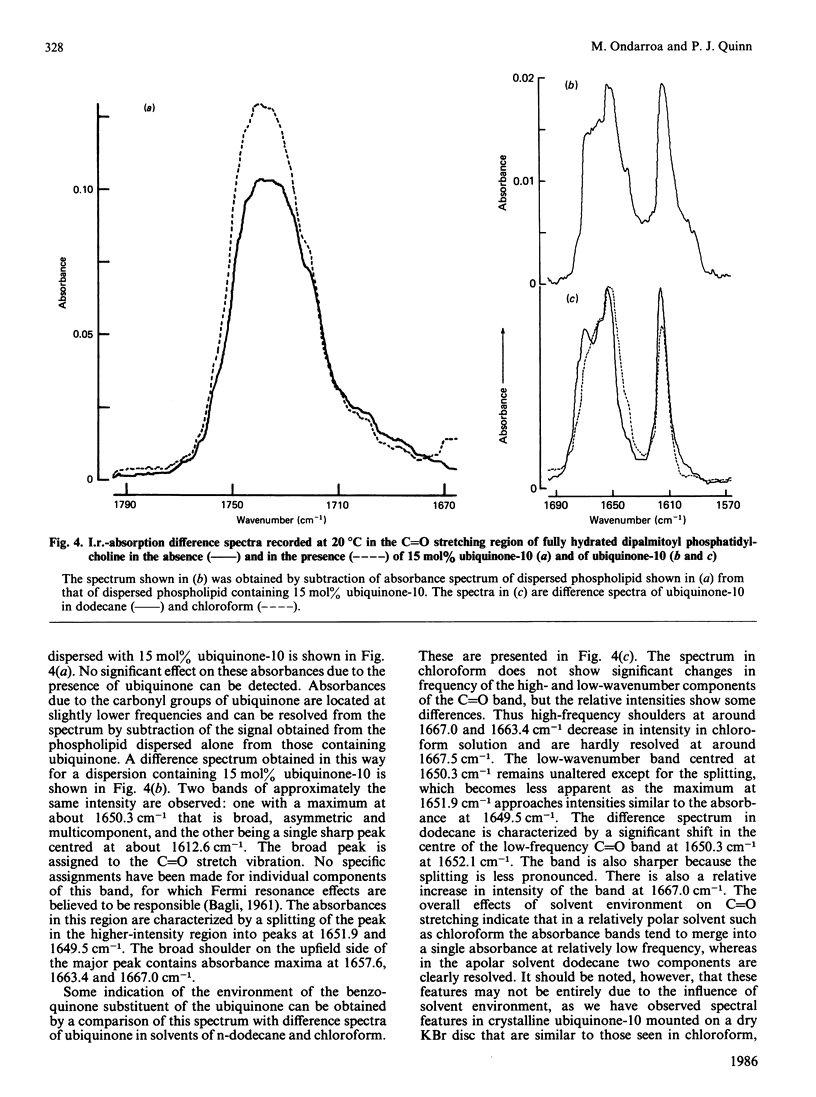
The interaction between 1,2-dipalmitoyl phosphatidylcholine and ubiquinone-10 in aqueous systems was studied by difference i.r. spectroscopy. Binary mixtures of the two lipids in proportions of 2, 5 and 15 mol% were investigated in the spectral regions reporting on the hydrocarbon chains of the phospholipid and the polar phosphate group. No spectral shifts or significant broadening of any absorbances due to the phospholipid were detected at temperatures of 20 or 54 degrees C. Changes in the frequency of the maximum of the CH2 antisymmetric C-H stretching vibration with temperature indicated that the gel-to-liquid-crystal-line phase-transition temperature of the phospholipid was lowered by about 2 degrees C in the presence of between 2 and 15 mol% ubiquinone-10. Absorbance by the benzoquinone substituent of ubiquinone-10 was detected by spectral subtraction of dispersions of phospholipid alone. Bands due to C = O stretching and ester group vibrations of ubiquinone-10 in co-dispersion with phospholipid were compared with the same spectral region when ubiquinone-10 was dissolved in solvents or as a crystalline solid. Spectral changes could be detected when ubiquinone-10 in phospholipid was compared with solution in dodecane and chloroform. These may indicate that the benzoquinone ring system is located within a hydrocarbon domain in dispersions with dipalmitoyl phosphatidylcholine. It was concluded from the study that when ubiquinone-10 is co-dispersed with dipalmitoyl phosphatidylcholine in water the two lipids phase-separate. There is no evidence that ubiquinone-10 intercalates between phospholipid molecules, which undergo a gel-liquid-crystalline phase transition in only a slightly modified form. The data suggest that the benzoquinone substituent resides in a hydrophobic domain and that aggregates spanning the bilayer are a possible arrangement of the ubiquinone in the structure.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrondo J. L., Goñi F. M., Macarulla J. M. Infrared spectroscopy of phosphatidylcholines in aqueous suspension. A study of the phosphate group vibrations. Biochim Biophys Acta. 1984 Jun 6;794(1):165–168. doi: 10.1016/0005-2760(84)90310-2. [DOI] [PubMed] [Google Scholar]
- Asher I. M., Levin I. W. Effects of temperature and molecular interactions on the vibrational infrared spectra of phospholipid vesicles. Biochim Biophys Acta. 1977 Jul 4;468(1):63–72. doi: 10.1016/0005-2736(77)90151-1. [DOI] [PubMed] [Google Scholar]
- Cameron D. G., Casal H. L., Gudgin E. F., Mantsch H. H. The gel phase of dipalmitoyl phosphatidylcholine. An infrared characterization of the acyl chain packing. Biochim Biophys Acta. 1980 Mar 13;596(3):463–467. doi: 10.1016/0005-2736(80)90135-2. [DOI] [PubMed] [Google Scholar]
- Cameron D. G., Casal H. L., Mantsch H. H. Characterization of the pretransition in 1,2-dipalmitoyl-sn-glycero-3-phosphocholine by Fourier transform infrared spectroscopy. Biochemistry. 1980 Aug 5;19(16):3665–3672. doi: 10.1021/bi00557a005. [DOI] [PubMed] [Google Scholar]
- Cameron D. G., Casal H. L., Mantsch H. H. The application of fourier transform infrared transmission spectroscopy to the study of model and natural membranes. J Biochem Biophys Methods. 1979;1(1):21–36. doi: 10.1016/0165-022x(79)90043-5. [DOI] [PubMed] [Google Scholar]
- Cameron D. G., Mantsch H. H. The phase transition of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine as seen by Fourier transform infrared difference spectroscopy. Biochem Biophys Res Commun. 1978 Aug 14;83(3):886–892. doi: 10.1016/0006-291x(78)91478-x. [DOI] [PubMed] [Google Scholar]
- Casal H. L., Cameron D. G., Smith I. C., Mantsch H. H. Acholeplasma laidlawii membranes: a Fourier transform infrared study of the influence of protein on lipid organization and dynamics. Biochemistry. 1980 Feb 5;19(3):444–451. doi: 10.1021/bi00544a007. [DOI] [PubMed] [Google Scholar]
- Chapman D., Gómez-Fernández J. C., Goni F. M., Barnard M. Difference infrared spectroscopy of aqueous model and biological membranes using an infrared data station. J Biochem Biophys Methods. 1980 Jun;2(6):315–323. doi: 10.1016/s0165-022x(80)90048-2. [DOI] [PubMed] [Google Scholar]
- Cortijo M., Alonso A., Gomez-Fernandez J. C., Chapman D. Intrinsic protein-lipid interactions. Infrared spectroscopic studies of gramicidin A, bacteriorhodopsin and Ca2+-ATPase in biomembranes and reconstituted systems. J Mol Biol. 1982 Jun 5;157(4):597–618. doi: 10.1016/0022-2836(82)90501-0. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Ernster L. Enzyme topology of intracellular membranes. Annu Rev Biochem. 1977;46:201–262. doi: 10.1146/annurev.bi.46.070177.001221. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M., Bertoli E., Parenti-Castelli G., Fato R., Mascarello S., Lenaz G. Incorporation of ubiquinone homologs into lipid vesicles and mitochondrial membranes. Arch Biochem Biophys. 1981 Aug;210(1):21–32. doi: 10.1016/0003-9861(81)90159-4. [DOI] [PubMed] [Google Scholar]
- Futami A., Hurt E., Hauska G. Vectorial redox reactions of physiological quinones. I. Requirement of a minimum length of the isoprenoid side chain. Biochim Biophys Acta. 1979 Sep 11;547(3):583–596. doi: 10.1016/0005-2728(79)90035-5. [DOI] [PubMed] [Google Scholar]
- Katsikas H., Quinn P. J. Fluorescence probe studies of the distribution of ubiquinone homologues in bilayers of dipalmitoylglycerophosphocholine. Eur J Biochem. 1983 Apr 5;131(3):607–612. doi: 10.1111/j.1432-1033.1983.tb07306.x. [DOI] [PubMed] [Google Scholar]
- Katsikas H., Quinn P. J. The distribution of ubiquinone-10 in phospholipid bilayers. A study using differential scanning calorimetry. Eur J Biochem. 1982 May;124(1):165–169. doi: 10.1111/j.1432-1033.1982.tb05920.x. [DOI] [PubMed] [Google Scholar]
- Katsikas H., Quinn P. J. The interaction of coenzyme Q with dipalmitoylphosphatidylcholine bilayers. FEBS Lett. 1981 Oct 26;133(2):230–234. doi: 10.1016/0014-5793(81)80512-1. [DOI] [PubMed] [Google Scholar]
- Katsikas H., Quinn P. J. The polyisoprenoid chain length influences the interaction of ubiquinones with phospholipid bilayers. Biochim Biophys Acta. 1982 Jul 28;689(2):363–369. doi: 10.1016/0005-2736(82)90270-x. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Durrani A. A., Chapman D. A difference infrared spectroscopic study of gramicidin A, alamethicin and bacteriorhodopsin in perdeuterated dimyristoylphosphatidylcholine. Biochim Biophys Acta. 1984 Jan 11;769(1):49–56. doi: 10.1016/0005-2736(84)90008-7. [DOI] [PubMed] [Google Scholar]
- Pennock J. F., Neiss G., Mahler H. R. Biochemical studies on the developing avian embryo. 5. Ubiquinone and some other unsaponifiable lipids. Biochem J. 1962 Dec;85(3):530–537. doi: 10.1042/bj0850530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan C. I., Heron C. The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Evidence for stoicheiometric association. Biochem J. 1978 Sep 15;174(3):783–790. doi: 10.1042/bj1740783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. N., Boardman N. K. The link between charge separation, proton movement and ATPase reactions. FEBS Lett. 1975 Dec 1;60(1):1–6. doi: 10.1016/0014-5793(75)80405-4. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., DeGrip W. J., Sanches R. Fourier transform infrared study of photoreceptor membrane. I. Group assignments based on rhodopsin delipidation and reconstitution. Biochim Biophys Acta. 1980 Mar 13;596(3):338–351. doi: 10.1016/0005-2736(80)90121-2. [DOI] [PubMed] [Google Scholar]
- Stidham M. A., McIntosh T. J., Siedow J. N. On the localization of ubiquinone in phosphatidylcholine bilayers. Biochim Biophys Acta. 1984 Dec 18;767(3):423–431. doi: 10.1016/0005-2728(84)90040-9. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Nagoaka S., Yu L., King T. E. Evidence of ubisemiquinone radicals in electron transfer at the cytochromes b and c1 region of the cardiac respiratory chain. Arch Biochem Biophys. 1980 Oct 1;204(1):59–70. doi: 10.1016/0003-9861(80)90007-7. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Yu L. Isolation and properties of a mitochondrial protein that converts succinate dehydrogenase into succinate-ubiquinone oxidoreductase. Biochemistry. 1980 Jul 22;19(15):3579–3585. doi: 10.1021/bi00556a025. [DOI] [PubMed] [Google Scholar]
- Zhu Q. S., Berden J. A., De Vries S., Slater E. C. On the role of ubiquinone in the respiratory chain. Biochim Biophys Acta. 1982 Apr 19;680(1):69–79. doi: 10.1016/0005-2728(82)90317-6. [DOI] [PubMed] [Google Scholar]


