Abstract
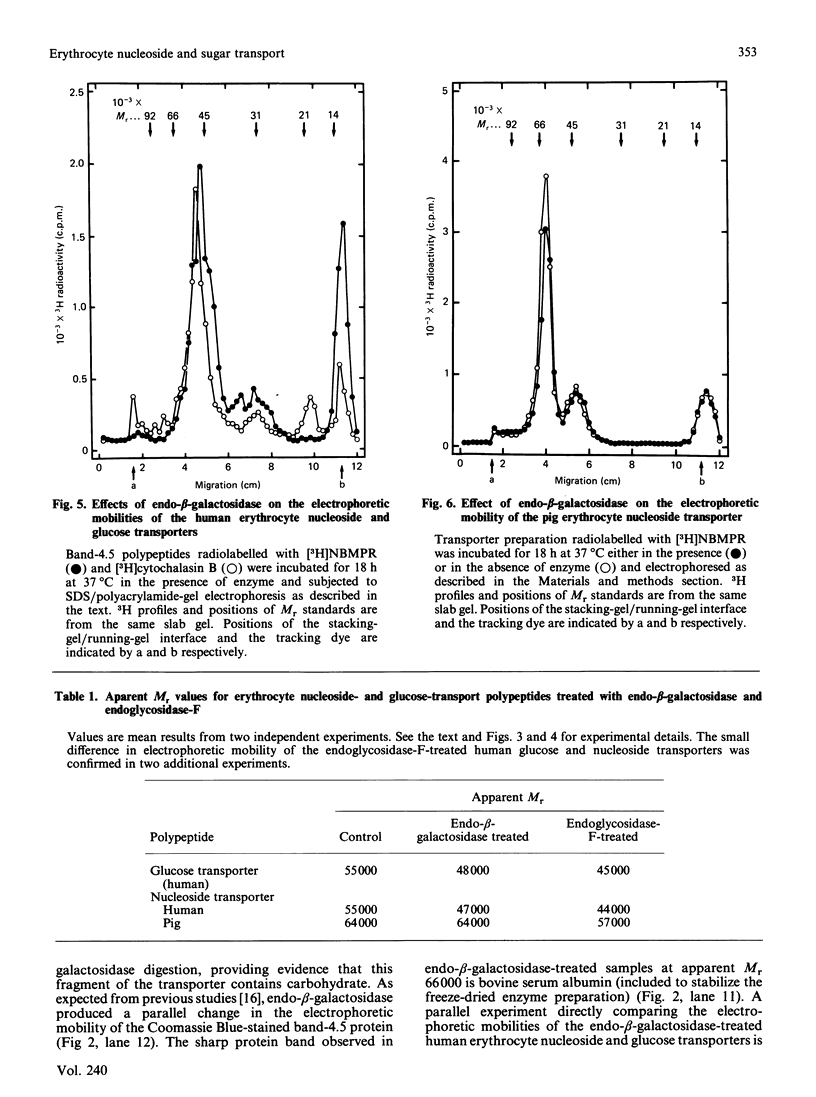
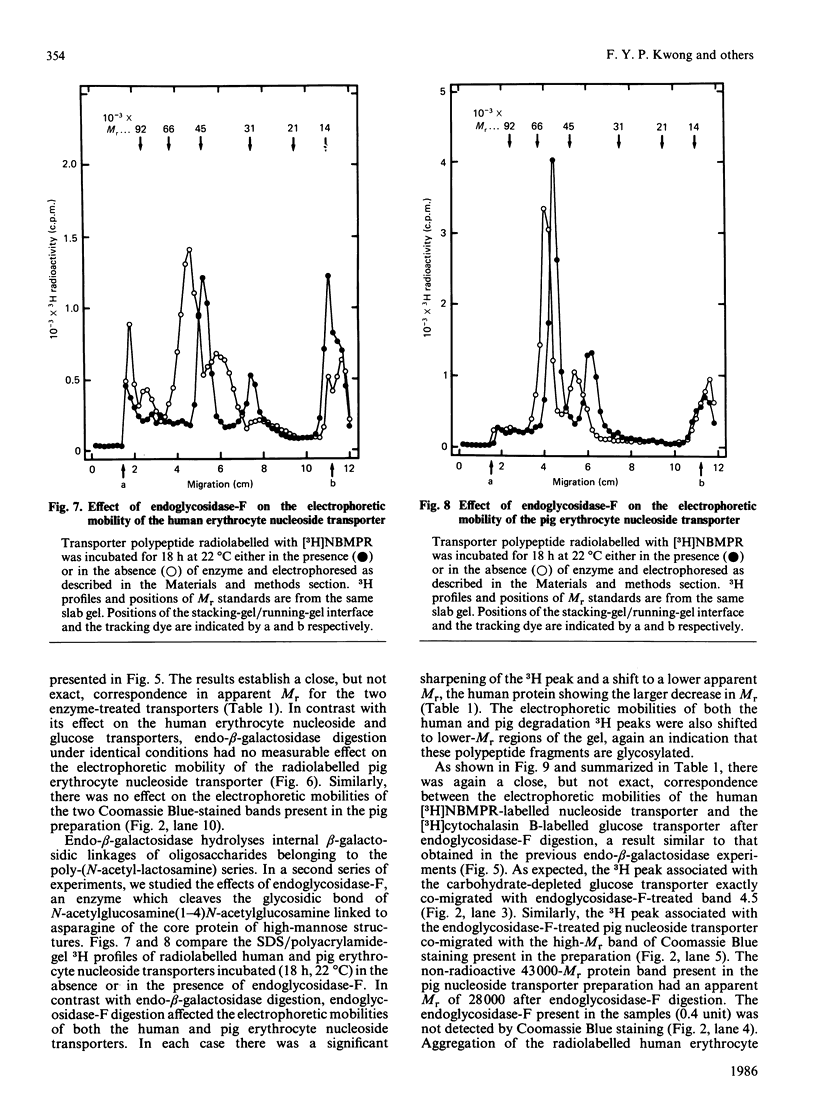
Nucleoside- and glucose-transport proteins isolated from human erythrocyte membranes were photoaffinity-labelled with [3H]nitrobenzylthioinosine and [3H]cytochalasin B, respectively, and subjected to endo-beta-galactosidase or endoglycosidase-F digestion. Without enzyme treatment the two radiolabelled transporters migrated on SDS/polyacrylamide gels with the same apparent Mr (average) of 55,000. Apparent Mr (average) values after endo-beta-galactosidase digestion were 47,000 and 48,000 for the nucleoside and glucose transporters respectively, and 44,000 and 45,000 respectively after endoglycosidase-F digestion. In contrast, endo-beta-galactosidase had no effect on the electrophoretic mobility of the nucleoside transporter isolated from pig erythrocytes. This transport system exhibited a higher Mr than the human protein, endoglycosidase-F treatment decreasing its apparent Mr (average) from 64,000 to 57,000. It is concluded that the human and pig erythrocyte nucleoside transporters are glycoproteins containing N-linked oligosaccharide. The data provide evidence of substantial carbohydrate and polypeptide differences between the human and pig erythrocyte nucleoside transporters, but evidence of molecular similarities between the human erythrocyte nucleoside and glucose transporters.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin S. A., Baldwin J. M., Lienhard G. E. Monosaccharide transporter of the human erythrocyte. Characterization of an improved preparation. Biochemistry. 1982 Aug 3;21(16):3836–3842. doi: 10.1021/bi00259a018. [DOI] [PubMed] [Google Scholar]
- Cairns M. T., Elliot D. A., Scudder P. R., Baldwin S. A. Proteolytic and chemical dissection of the human erythrocyte glucose transporter. Biochem J. 1984 Jul 1;221(1):179–188. doi: 10.1042/bj2210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Su C., Pessin J. E., Mora R., Gitomer W., Czech M. P. Photoaffinity labeling of the human erythrocyte D-glucose transporter. J Biol Chem. 1982 May 25;257(10):5419–5425. [PubMed] [Google Scholar]
- Gorga F. R., Baldwin S. A., Lienhard G. E. The monosaccharide transporter from human erythrocytes is heterogeneously glycosylated. Biochem Biophys Res Commun. 1979 Dec 14;91(3):955–961. doi: 10.1016/0006-291x(79)91972-7. [DOI] [PubMed] [Google Scholar]
- Janmohamed N. S., Young J. D., Jarvis S. M. Proteolytic cleavage of [3H]nitrobenzylthioinosine-labelled nucleoside transporter in human erythrocytes. Biochem J. 1985 Sep 15;230(3):777–784. doi: 10.1042/bj2300777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Ng A. S. Identification of the adenosine uptake sites in guinea pig brain. J Neurochem. 1985 Jan;44(1):183–188. doi: 10.1111/j.1471-4159.1985.tb07129.x. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D., Ansay M., Archibald A. L., Harkness R. A., Simmonds R. J. Is inosine the physiological energy source of pig erythrocytes? Biochim Biophys Acta. 1980 Mar 27;597(1):183–188. doi: 10.1016/0005-2736(80)90162-5. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Extraction and partial purification of the nucleoside-transport system from human erythrocytes based on the assay of nitrobenzylthioinosine-binding activity. Biochem J. 1981 Jan 15;194(1):331–339. doi: 10.1042/bj1940331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. D., McManus T. J. Studies on the energy metabolism of pig red cells. I. The limiting role of membrane permeability in glycolysis. Biochim Biophys Acta. 1971 Jan 26;230(1):1–11. doi: 10.1016/0304-4165(71)90048-1. [DOI] [PubMed] [Google Scholar]
- Kim H. D., Watts R. P., Luthra M. G., Schwalbe C. R., Conner R. T., Brendel K. A symbiotic relationship of energy metabolism between a 'non-glycolytic' mammalian red cell and the liver. Biochim Biophys Acta. 1980 Feb 8;589(2):256–263. doi: 10.1016/0005-2728(80)90042-0. [DOI] [PubMed] [Google Scholar]
- Kwan K. F., Jarvis S. M. Photoaffinity labeling of adenosine transporter in cardiac membranes with nitrobenzylthioinosine. Am J Physiol. 1984 May;246(5 Pt 2):H710–H715. doi: 10.1152/ajpheart.1984.246.5.H710. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Crabb J. H., Ransome K. J. Endoglycosidase f cleaves the oligosaccharides from the glucose transporter of the human erythrocyte. Biochim Biophys Acta. 1984 Jan 25;769(2):404–410. doi: 10.1016/0005-2736(84)90324-9. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Scudder P., Uemura K., Dolby J., Fukuda M. N., Feizi T. Isolation and characterization of an endo-beta-galactosidase from Bacteroides fragilis. Biochem J. 1983 Aug 1;213(2):485–494. doi: 10.1042/bj2130485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M. M., Wu J. S., Lee C. M., Young J. D. Nucleoside transport. Photoaffinity labelling of high-affinity nitrobenzylthioinosine binding sites in rat and guinea pig lung. Biochem Biophys Res Commun. 1984 Jan 30;118(2):594–600. doi: 10.1016/0006-291x(84)91344-5. [DOI] [PubMed] [Google Scholar]
- Tse C. M., Belt J. A., Jarvis S. M., Paterson A. R., Wu J. S., Young J. D. Reconstitution studies of the human erythrocyte nucleoside transporter. J Biol Chem. 1985 Mar 25;260(6):3506–3511. [PubMed] [Google Scholar]
- Wu J. S., Jarvis S. M., Young J. D. The human erythrocyte nucleoside and glucose transporters are both band 4.5 membrane polypeptides. Biochem J. 1983 Sep 15;214(3):995–997. doi: 10.1042/bj2140995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. S., Kwong F. Y., Jarvis S. M., Young J. D. Identification of the erythrocyte nucleoside transporter as a band 4.5 polypeptide. Photoaffinity labeling studies using nitrobenzylthioinosine. J Biol Chem. 1983 Nov 25;258(22):13745–13751. [PubMed] [Google Scholar]
- Wu J. S., Young J. D. Photoaffinity labelling of nucleoside-transport proteins in plasma membranes isolated from rat and guinea-pig liver. Biochem J. 1984 Jun 1;220(2):499–506. doi: 10.1042/bj2200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M., Belt J. A., Gati W. P., Paterson A. R. Identification of the nucleoside transporter in cultured mouse lymphoma cells. Photoaffinity labeling of plasma membrane-enriched fractions from nucleoside transport-competent (S49) and nucleoside transport-deficient (AE1) cells with [3H]nitrobenzylthioinosine. J Biol Chem. 1984 Jul 10;259(13):8363–8365. [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M., Clanachan A. S., Henderson J. F., Paterson A. R. Nitrobenzylthioinosine: an in vivo inhibitor of pig erythrocyte energy metabolism. Am J Physiol. 1986 Jul;251(1 Pt 1):C90–C94. doi: 10.1152/ajpcell.1986.251.1.C90. [DOI] [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M. Nucleoside transport in animal cells. Biosci Rep. 1983 Apr;3(4):309–322. doi: 10.1007/BF01122895. [DOI] [PubMed] [Google Scholar]
- Young J. D., Paterson A. R., Henderson J. F. Nucleoside transport and metabolism in erythrocytes from the Yucatan miniature pig. Evidence that inosine functions as an in vivo energy substrate. Biochim Biophys Acta. 1985 Oct 17;842(2-3):214–224. doi: 10.1016/0304-4165(85)90205-3. [DOI] [PubMed] [Google Scholar]
- Zeidler R. B., Metzler M. H., Moran J. B., Kim H. D. The liver is an organ site for the release of inosine metabolized by non-glycolytic pig red cells. Biochim Biophys Acta. 1985 Mar 8;838(3):321–328. doi: 10.1016/0304-4165(85)90229-6. [DOI] [PubMed] [Google Scholar]