Abstract
Background
Size at birth, an indicator of intrauterine growth, has been studied extensively in relation to subsequent health, growth and developmental outcomes. Our umbrella review synthesises evidence from systematic reviews and meta-analyses on the effects of size at birth on subsequent health, growth and development in children and adolescents up to age 18, and identifies gaps.
Methods
We searched five databases from inception to mid-July 2021 to identify eligible systematic reviews and meta-analyses. For each meta-analysis, we extracted data on the exposures and outcomes measured and the strength of the association.
Findings
We screened 16 641 articles and identified 302 systematic reviews. The literature operationalised size at birth (birth weight and/or gestation) in 12 ways. There were 1041 meta-analyses of associations between size at birth and 67 outcomes. Thirteen outcomes had no meta-analysis.
Small size at birth was examined for 50 outcomes and was associated with over half of these (32 of 50); continuous/post-term/large size at birth was examined for 35 outcomes and was consistently associated with 11 of the 35 outcomes. Seventy-three meta-analyses (in 11 reviews) compared risks by size for gestational age (GA), stratified by preterm and term. Prematurity mechanisms were the key aetiologies linked to mortality and cognitive development, while intrauterine growth restriction (IUGR), manifesting as small for GA, was primarily linked to underweight and stunting.
Interpretation
Future reviews should use methodologically sound comparators to further understand aetiological mechanisms linking IUGR and prematurity to subsequent outcomes. Future research should focus on understudied exposures (large size at birth and size at birth stratified by gestation), gaps in outcomes (specifically those without reviews or meta-analysis and stratified by age group of children) and neglected populations.
PROSPERO registration number
CRD42021268843.
Keywords: Child Health, Child Development, Mortality, Mental health, Growth
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
It provides a comprehensive overview of reviews on the effects of size and gestation at birth on all subsequent health, growth and developmental outcomes in children.
It identifies outcomes with no meta-analyses and topics where there is a large, conclusive literature, and areas needing further or more conclusive research.
Introduction
Size at birth is affected both by in utero growth and by length of gestation. Researchers have been quantifying the relationship between size at birth and subsequent outcomes for over a century, resulting in a vast, nearly unmanageable, literature.1,3 A quick PubMed search on size at birth generates almost half-a-million articles (online supplemental material 1), shaped by contemporaneous topics or theories of interest and by prevailing measurement capabilities.
The observation that small neonates were at substantially higher risk of dying than larger babies was quantified by early studies which defined ‘prematurity’ as low birth weight (LBW).1 2 By the 1950s, prematurity was redefined using gestational age (GA) cut-offs; table 1 shows these and other definitions used as risk factors in our review. Research expanded from mortality outcomes to other potential consequences of being born with immature lung, neurological or immune-system development. At the other end of the size spectrum, macrosomia or high birth weight (HBW) was explored as a predictor of traumatic delivery or adverse growth outcomes. By the mid-1960, LBW, prematurity and intrauterine growth restriction (IUGR) were being distinguished, and modellers began looking at distributional components and developing population-specific and custom birthweight curves (late 1960s–1990s). The 1990s also saw the ‘developmental origins of disease’ theory, which suggested that small size at birth, quantified as LBW, increased disease risks in later life. This led to a burgeoning literature examining in utero shocks and their effects on cardiovascular and metabolic outcomes in adults and on early markers of these diseases in young children.1 2 Starting in 2013, the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21) used eight geographically diverse populations to develop global standard curves for fetal growth by sex and by GA.3
Table 1. Measurements and threshold used for size-at-birth definitions.
| Risk factors (exposures) | Measurement units and thresholds used in definitions |
| Continuous measures | |
| Gestational age (GA)* | The duration of gestation is usually reported in completed weeks with additional days, or in completed days. |
| Birth weight (BW)† | Weight at birth measured in gram or kg. Reported using birth weight thresholds below or as mean birth weight with standard deviation |
| Small size at birth | |
| Extremely preterm (EPT) | <28 gestational weeks |
| Very preterm (VPT) | <32 gestational weeks |
| Preterm (PT) | <37 gestational weeks |
| Extremely low birth weight (ELBW) | <1000 g |
| Very low birth weight (VLBW) | <1500 g |
| Low birth weight (LBW) | <2500 g |
| Small for gestational age (SGA) | <10th percentile of birth weight for GA |
| Intrauterine growth restriction (IUGR) | Defined in the footnotes of online supplemental material 3 tables 1 a-g |
| Large size at birth/post term | |
| Post term | >41 gestational weeks |
| High birth weight (HBW)/macrosomia | >4000 g |
| Large for gestational age (LGA) | >90th percentile of weight for GA |
GA is counted in calendar days from the first day of gestation, with the number of completed weeks calculated as the number of days divided by 7, presented as a whole integer plus a remainder, for example, day 258 is 36+6. Methods used to assess GA vary by study, which can affect reliability and comparability between studies. Methods using ultrasound assessment in the first trimester are most accurate.
Birth weight is the first weight of the fetus or neonate obtained after birth. For live births, birth weight should preferably be measured within the first hour of life before significant postnatal weight loss has occurred.
GAgestational age
Despite a large literature and eight previous umbrella reviews,4,11 there is no comprehensive summary of the main associations between size at birth and health, growth and developmental (including motor, cognitive and educational) outcomes, or of the literature gaps. Previous umbrella reviews (1) do not examine the full size-at-birth spectrum (neglecting larger neonates)45 7,10; (2) focus primarily on specific associations, for example, on the effects of LBW on mortality or chronic diseases11 or of preterm birth on developmental outcomes4 5; (3) limit reviews to young children or adults and neglecting older children; and most importantly, to our knowledge, only one umbrella review (4) examines size for GA stratified by gestation, making it difficult to elucidate the relative importance of IUGR versus prematurity.
Our umbrella review aims to serve as a primary source of up-to-date compiled evidence on the effect of the full range of size-at-birth measures on a wide range of subsequent child and adolescent well-being outcomes.
Our umbrella review objectives are to (1) identify systematic reviews on the effects of size at birth on health (including mortality, acute ill health, lung-related ill health, chronic ill health and mental health), growth, developmental outcomes in children and adolescents; (2) map the evidence from reviews with meta-analyses, highlighting the magnitude, direction and consistency of the associations; (3) indicate evidence gaps; in addition, (4) we will suggest approaches needed for future empirical studies and meta-analyses.
Methods
We conducted an umbrella review, gathering information from existing systematic reviews and meta-analyses which examined the effects of size at birth on health, growth and developmental outcomes in children up to 18 years of age.
We systematically searched MEDLINE, Embase, ERIC and Cochrane Library databases for articles published until 15 July 2021, without restricting on date, language or location. The search was limited to peer-reviewed systematic reviews or meta-analyses. Key search concepts included (“birth weight” OR “gestational age” OR “intrauterine growth restriction” OR “prematurity”) AND (“systematic review” OR “meta-analysis”). To maximise the eligible reviews, we did not limit the outcomes or the study population. We also hand-searched the reference lists of the eight identified umbrella reviews to ensure we did not miss any reviews. The full search strategy and the steps for data extraction are included in online supplemental material 2.
In Online supplemental material 3 tables 1 a-g, we mapped the evidence on the effects of 12 different size-at-birth risk factors on a wide range of outcomes, grouped in seven themes: mortality and hospitalisation (theme a); neonatal and early childhood acute ill health (theme b); allergies and lung-related ill health (theme c); chronic ill health (theme d); behavioural and mental health (theme e); growth and nutrition (theme f); and developmental (motor, cognitive and educational) (theme g). The 7 themes had 67 subthemes. The subthemes in the behavioural and mental health themes (theme g) were grouped based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM5), classifications.12
The direction of the association was indicated using different colours in online supplemental material 3 tables 1 a-g with dark blue denoting a harmful effect, yellow denoting no statistically significant effect, and green denoting a beneficial effect.
Results
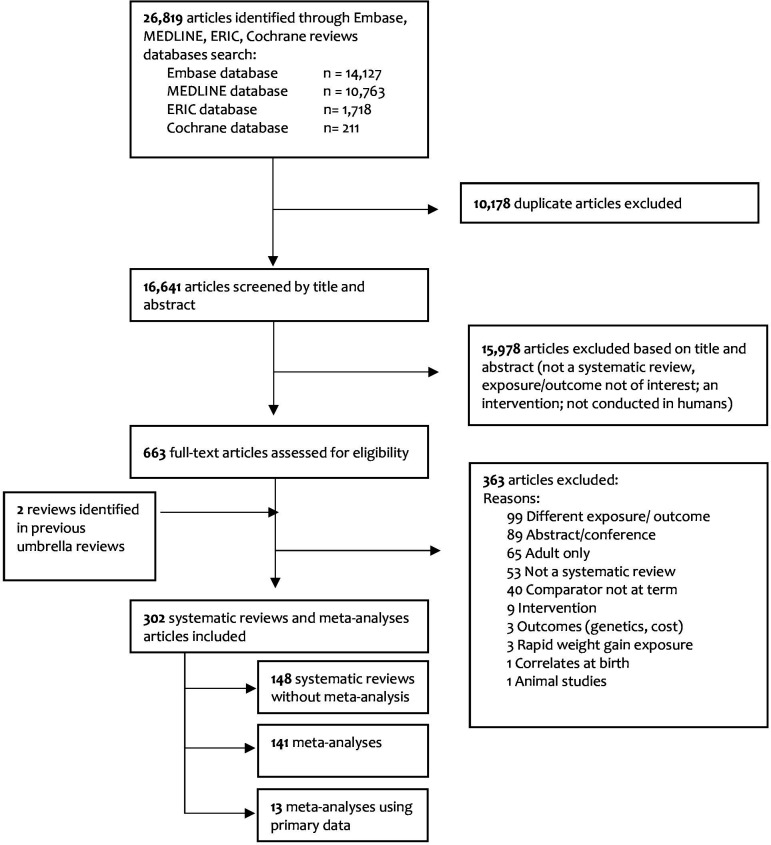
We screened 16641 articles and identified 367 systematic reviews, of which 65 focused on outcomes in adults. This left 302 eligible systematic reviews of outcomes in children or in children and adults: 148 without meta-analyses, 141 with meta-analysis and 13 with meta-analyses of primary data (figure 1). Studies were published between 1989 and 2021.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart for study selection.
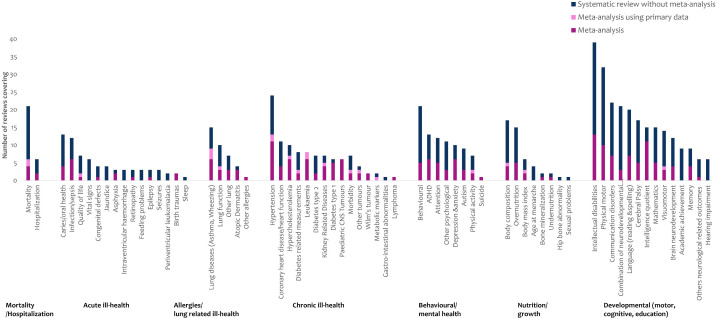
We identified 7 themes and 67 subthemes of outcomes. Of the 67 subthemes, 13 were systematically reviewed without a meta-analysis (via 29 reviews)13,41 (figure 2). Out of the 141 reviews with meta-analyses, 52 had a high-quality appraisal score, 61 medium and 28 low (online supplemental material 4a). Most of the meta-analyses (100 of 141) assessed publication bias (online supplemental material 4b).
Figure 2. Themes and subthemes identified in 302 reviews.
Online supplemental material 3 tables 1 a-g shows the associations grouped by themes and subthemes. A total of 1041 associations were summarised from the 150 studies with meta-analyses (including those with primary data): 772 with small size at birth as risk factor (including extremely preterm, very preterm, preterm, extremely low birth weight (ELBW), very low birth weight (VLBW), LBW and small for gestational age (SGA)), 144 with large size at birth/post-term (including post-term, HBW and large for gestation age (LGA)) and 125 with size as a continuous risk factor (weight and gestation). Only 85 of 1041 associations used SGA or LGA as risk factors. Of the 1041 associations, 225 focused on children under 5, 487 focused on children under 18, and 329 focused on mixed children and adults. The magnitude, direction and consistency of these associations are presented in online supplemental material 3 tables 1 a-g online supplemental material 3with a detailed narrative summary to explain the results by theme.
The main manuscript contains table 2 as an example of online supplemental table 1 f showing the associations between size at birth and nutrition and growth outcomes. Table 3 shows a subset of seven reviews which measured size for GA stratified by gestation, including four reviews missing from online supplemental material 3 tables 1 a-g because they included only stratified exposures.42,45
Table 2. Associations between size at birth and nutrition and growth outcomes.
| Ref | Exposures (size at birth) | Population | Outcomes | Effect size (CI), direction of the association | |||||||||||
| Small | Cont | Large | |||||||||||||
| EPT (<28 weeks) | ELBW (<1000 g) | VPT (<32 weeks) | VLBW (<1500 g) | PT (<37 weeks) | LBW (<2500 g) | SGA (<10th percentile) | BW (cont.) | GA (cont.) | Post term (>41 weeks) | HBW (>4000 g) | LGA (>90th percentile) | ||||
| Body composition | |||||||||||||||
| 155 | X | Infants | Length (cm) | MD=−3.71 (−4.60 to –2.81) | |||||||||||
| 85 | X | 11 years | Height (cm) | z-score difference=−0.92 (−0.03), p<0.001 | |||||||||||
| 155 | X | Infants | Weight (kg) | MD=−0.59 (−0.75 to –0.44) | |||||||||||
| 85 | X | 11 years | Weight (kg) | z-score difference=−0.61 (0.18), p<0.001 | |||||||||||
| 155 | X | Infants | Head circumference (cm) | MD=−1.03 (−1.52 to –0.54) | |||||||||||
| 85 | X | 11 years | Head circumference (cm) | z-score difference=−1.52 (0.44), p<0.001 | |||||||||||
| 85 | X | 11 years | Body surface area | z-score difference=−0.10 (−0.01), p<0.001 | |||||||||||
| 155 | X | Infants | Total body fat (%) | MD=3.06 (0.25 to 5.88) | |||||||||||
| 156 | X | 4–7 years | Total body fat (%) | SMD=−3.05 (−8.73 to 2.62) | |||||||||||
| 155 | X | Infants | Fat mass (kg) | MD=−0.05 (−0.09 to –0.01) | |||||||||||
| 155 | X | Infants | Fat-free mass (kg) | MD=−0.46 (−0.64 to –0.27) | |||||||||||
| 156 | X | 4–7 years | Fat mass index | SMD=−1.31 (−5.42 to 2.81) | |||||||||||
| 156 | X | 4–7 years | Childhood Trunk Fat Index | SMD=1.03 (−1.64 to 3.71) | |||||||||||
| 157 | ** | At birth | Cord blood adiponectin concentrations | SMD=−1.14 (−2.15 to –0.12) | |||||||||||
| 157 | * | At birth | Cord blood adiponectin concentrations | SMD=−1.93 (−4.093 to –0.022) | |||||||||||
| 157 | X | At birth | Cord blood adiponectin concentrations | SMD=−0.383 (−0.744 to –0.022) | |||||||||||
| 158 | X | 0.5 hours–11 days | Total body water (%) | MD=4.40 (2.83 to 5.96) | |||||||||||
| 158 | X | 6 hours–7 days | Total body water (%) | β=−1.44 (−0.63 to –2.24) per week | |||||||||||
| 158 | X | 0.5 hours–11 days | Total body water (%) | MD=−5.23 (−4.54 to –5.91) | |||||||||||
| Bone mineralisation | |||||||||||||||
| 159 | X | 10 years | Bone mass content | β=0.02 (0.01 to 0.04) | |||||||||||
| 159 | X | 10 years | Bone mass density | β=0.01 (−0.01 to 0.03) | |||||||||||
| BMI | |||||||||||||||
| 84 | X | 6–32 years | BMI (kg/m2) | MD=−0.50 (−1.10 to 0.09) | |||||||||||
| 84 | X | 5–30 years | BMI (kg/m2) | MD=−0.30 (−0.54 to –0.05) | |||||||||||
| 84 | X | 4.5–35.7 years | BMI (kg/m2) | MD=−0.13 (−0.40 to 0.14) | |||||||||||
| 84 | X | <10 years | BMI (kg/m2) | MD=−0.70(−1.13 to –2.28) | |||||||||||
| 84 | X | <19 years | BMI (kg/m2) | MD=5.20 (−3.82 to 14.21) | |||||||||||
| 84 | X | 10–19 years | BMI (kg/m2) | MD=−0.25 (−0.76 to 0.26) | |||||||||||
| 91 | XGA | 16.0–46.9 years | BMI (kg/m2) | β=0.52 (0.20 to 0.84)/kg increase | |||||||||||
| 91 | GA | 16.0–46.9 years | BMI (kg/m2) | β=0.51 (−0.08 to 1.11)/kg increase | |||||||||||
| 91 | X | 16.0–46.9 years | BMI (kg/m2) | β=0.52 (0.17 to 0.86)/kg increase | |||||||||||
| 77 | T | 0–2 years | BMI trajectory: class 2 (rapid growth to 2 years) | aOR=2.02 (1.49 to 2.74) | |||||||||||
| 77 | T | 0–6 years | BMI trajectory: class 3 (persistent rapid growth to 6 years) | aOR=1.89 (0.42 to 8.49) | |||||||||||
| 77 | ◊ | 0–2 years | BMI trajectory: class 2 (rapid growth) | aOR=1.48 (1.05 to 2.10) | |||||||||||
| 77 | ◊ | 0–6 years | BMI trajectory: class 3 (persistent rapid growth) | aOR=0.78 (0.10 to 6.45) | |||||||||||
| 77 | X | 0–2 years | BMI trajectory: class 2 (rapid growth) | aOR=0.81 (0.68 to 0.96) | |||||||||||
| 77 | X | 0–6 years | BMI trajectory: class 3 (persistent rapid growth) | aOR=0.48 (0.15 to 1.53) | |||||||||||
| 77 | T | 0–2 years | BMI trajectory: class 2 (rapid growth) | aOR=0.98 (0.86 to 1.12) | |||||||||||
| 77 | T | 0–6 years | BMI trajectory: class 3 (persistent rapid growth) | aOR=1.62 (0.88 to 2.99) | |||||||||||
| Undernutrition | |||||||||||||||
| 160 | X | 12–60 months | Wasting (weight for length/height for age <2 z-scores) | OR=1.55 (1.21 to 1.97) | |||||||||||
| 160 | X | 12–60 months | Wasting (weight for length/height for age <2 z-scores) | OR=2.68 (2.23 to 3.21) | |||||||||||
| 160 | X | 12–60 months | Wasting (weight for length/height for age<2 z-scores) | OR=2.36 (2.14 to 2.60) | |||||||||||
| 160 | X | 12–60 months | Stunting (length/height for age<2 z-scores) | OR=1.69 (1.48 to 1.93) | |||||||||||
| 160 | X | 12–60 months | Stunting (length/height for age<2 z-scores) | OR=2.92 (2.56 to 3.33) | |||||||||||
| 160 | X | 12–60 months | Stunting (length/height for age<2 z-scores) | OR=2.32 (2.12 to 2.54) | |||||||||||
| 160 | X | 12–60 months | Underweight (weight for age less than 2 z-scores) | OR=1.66 (1.42 to 1.95) | |||||||||||
| 160 | X | 12c60 months | Underweight (weight for age less than 2 z-scores) | OR=3.48 (3.14 to 3.87) | |||||||||||
| 160 | X | 12–60 months | Underweight (weight for age less than 2 z-scores) | OR=2.96 (2.61 to 3.36) | |||||||||||
| Overnutrition | |||||||||||||||
| 161 | X | 0–18 years | Overweight | OR=0.60 (0.54 to 0.67) | |||||||||||
| 161 | X | 1–75 years | Overweight | β=0.34 (0.28 to 0.40)/kg increase | |||||||||||
| 161 | X | 0–18 years | Overweight | OR=1.76 (1.65 to 1.87) | |||||||||||
| 156 | X | 6–14 years | Obesity | OR=1.19 (1.13 to 1.26) | |||||||||||
| 162 | ◊ | 3–18 years | Obesity | OR=0.87 (0.69 to 1.08) | |||||||||||
| 162 | X | 1–17 years | Obesity | OR=0.61 (0.46 to 0.80) | |||||||||||
| 162 | X | <6 years | Obesity | OR=0.61 (0.43 to 0.88) | |||||||||||
| 162 | X | 6–13 years | Obesity | OR=0.54 (0.32 to 0.90) | |||||||||||
| 162 | X | 13–17 years | Obesity | OR=0.74 (0.37 to 1.49) | |||||||||||
| 163 | X | 7–11 years | Obesity | β=0.649/kg increase | |||||||||||
| 162 | ◊ | 1–16 years | Obesity | OR=2.23 (1.91 to 2.61) | |||||||||||
| 162 | X | 0–17 years | Obesity | OR=2.07 (1.91 to 2.24) | |||||||||||
| 162 | X | <6 years | Obesity | OR=2.10 (1.93 to 2.29) | |||||||||||
| 162 | X | 6–13 years | Obesity | OR=1.76 (1.36 to 2.20) | |||||||||||
| 162 | X | 13–17 years | Obesity | OR=2.58 (1.56 to 4.26) | |||||||||||
Exposures: EPT (<28 weeks), ELBW (<1000 g), VPT (<32 weeks), VLBW (<1500 g), PT (<37 weeks), LBW (<2500 g), SGA (<10th percentile), post term (>41 weeks), HBW (>4000 g) and LGA (>90th percentile).
Symbols in exposures: X, as defined in exposure; XGA, adjusted and unadjusted for GA; GA, BW adjusted for GA; **, SGA <3rd, 5th and 10th percentile/value×by SD for GA; *, SGA <3rd percentile/value×by SD for GA; ◊, reference category 2500–4000 g; T, reference category GA 37≤term≤41.
Outcomes:  , harmful effect;
, harmful effect;  , no effect;
, no effect;  , beneficial effect; italic, calculation/post review.
, beneficial effect; italic, calculation/post review.
aORadjusted ORBMIbody mass indexBWbirth weightBW (cont.)birth weight continuousELBWextremely low birth weightEPTextremely pretermGAgestational ageGA (cont.)gestational age continuousHBWhigh birth weightLBWlow birth weightLGAlarge for gestational ageMDmean differencePTpretermSGAsmall for gestational ageSMDstandardised mean differenceVLBWvery low birth weightVPTvery preterm
Table 3. Association between maturity and SGA/IUGR combinations and different outcomes.
| Ref | Outcomes | Population | Exposures | Reference | Effect size (CI), direction of association | ||||||
| PTSGA | PTAGA | TIUGR | TSGA | TLBW | TAGA | TNBW | T | ||||
| 48 | Neonatal mortality | ≤28 days | <34 | X | OR=56.97 (11.1 to 291.7) | ||||||
| 48 | Neonatal mortality | ≤28 days | <34 | X | OR=74.9 (32.6 to 171.7) | ||||||
| 48 | Neonatal mortality | ≤28 days | 34–36 | X | OR=19.88 (8.3 to 47.5) | ||||||
| 48 | Neonatal mortality | ≤28 days | 34–36 | X | OR=3.18 (1.0 to 10.7) | ||||||
| 48 | Neonatal mortality | ≤28 days | X | X | OR=2.23 (1.2 to 4.10) | ||||||
| 46 | Neonatal mortality | <28 days | X | X | RR=15.42 (9.11 to 26.1) | ||||||
| 46 | Neonatal mortality | <28 days | X | X | RR=8.05 (3.88 to 16.72) | ||||||
| 46 | Neonatal mortality | <28 days | X | X | RR=2.44 (1.67 to 3.57) | ||||||
| 46 | Early neonatal mortality | <7 days | X | X | RR=17.19 (9.57 to 30.91) | ||||||
| 46 | Early neonatal mortality | <7 days | X | X | RR=7.59 (3.38 to 17.08) | ||||||
| 46 | Early neonatal mortality | <7 days | X | X | RR=2.76 (1.82 to 4.18) | ||||||
| 46 | Late neonatal mortality | 8–28 days | X | X | RR=17.37 (10.27 to 29.37) | ||||||
| 46 | Late neonatal mortality | 8–28 days | X | X | RR=5.60 (2.75 to 11.43) | ||||||
| 46 | Late neonatal mortality | 8–28 days | X | X | RR=2.45 (1.7 to 3.51) | ||||||
| 46 | Postneonatal mortality | 29–365 days | X | X | RR=5.22 (2.8 to 9.64) | ||||||
| 46 | Postneonatal mortality | 29–365 days | X | X | RR=2.72 (1.5 to 4.79) | ||||||
| 46 | Postneonatal mortality | 29–365 days | X | X | RR=1.98 (1.39 to 2.81) | ||||||
| 46 | Infant mortality | <365 days | X | X | RR=9.24 (4.33 to 19.71) | ||||||
| 46 | Infant mortality | <365 days | X | X | RR=5.30 (2.39 to 11.76) | ||||||
| 46 | Infant mortality | <365 days | X | X | RR=2.28 (1.52 to 3.41) | ||||||
| 160 | Wasting | 12–60 months | X | X | aOR=4.19 (2.90 to 6.05) | ||||||
| 160 | Wasting | 12–60 months | X | X | aOR=1.96 (1.46 to 2.63) | ||||||
| 160 | Wasting | 12–60 months | X | X | aOR=2.52 (2.27 to 2.80) | ||||||
| 160 | Stunting | 12–60 months | X | X | aOR=4.51 (3.42 to 5.93) | ||||||
| 160 | Stunting | 12–60 months | X | X | aOR=1.93 (1.71 to 2.18) | ||||||
| 160 | Stunting | 12–60 months | X | X | aOR=2.43 (2.22 to 2.66) | ||||||
| 160 | Undernutrition | 12–60 months | X | X | aOR=5.35 (4.39 to 6.53) | ||||||
| 160 | Undernutrition | 12–60 months | X | X | aOR=2.07 (1.76 to 2.44) | ||||||
| 160 | Undernutrition | 12–60 months | X | X | aOR=3.17 (2.78 to 3.62) | ||||||
| 174 | Motor | <7 years | X | X | aSMD=−0.15 (−0.40 to 0.09) | ||||||
| 174 | Motor | <7 years | X | X | aSMD=−0.23 (−0.42 to –0.03) | ||||||
| 174 | Motor | <7 years | X | X | aSMD=−0.007 (−0.08 to 0.06) | ||||||
| 174 | Cognitive | <7 years | X | X | aSMD=−0.17 (−0.29 to –0.05) | ||||||
| 174 | Cognitive | <7 years | X | X | aSMD=−0.14 (−0.24 to –0.05) | ||||||
| 174 | Cognitive | <7 years | X | X | aSMD=−0.02 (−0.10 to 0.06) | ||||||
| 174 | Language | <7 years | X | X | aSMD=−0.02 (−0.23 to 0.19) | ||||||
| 174 | Language | <7 years | X | X | aSMD=−0.03 (−0.12 to 0.06) | ||||||
| 172 | Cerebral palsy | Neonates | X | X | OR=2.34 (1.43 to 3.82) | ||||||
| 42 | Neonatal mortality | Neonates | X | X | OR=4.11 (3.70 to 4.56) | ||||||
| 42 | Non-neurological neonatal morbidity | Neonates | X | X | OR=2.98 (1.58 to 5.61) | ||||||
| 42 | Neonatal morbidity: neurological | Neonates | X | X | OR=2.12 (1.56 to 2.91) | ||||||
| 43 | Morbidly composite | 1–18 years | X | X | OR=1.49 (1.02 to 2.1) | ||||||
| 43 | Morbidly composite | 1–18 years | X | X | OR=0.98 (0.87 to 1.10) | ||||||
| 43 | Learning difficulties or learning disabilities | 12 months–18 years | X | X | OR=2.03 (1.65 to 2.50) | ||||||
| 43 | Obesity | 2–18 years | X | X | OR=0.94 (0.59 to 1.49) | ||||||
| 43 | Obesity | 6–11 years | X | X | OR=0.90 (0.50 to 1.64) | ||||||
| 43 | Hypertension | 3–16 years | X | X | OR=0.98 (0.8 to 1.12) | ||||||
| 44 | Neurodevelopmental scores (high scores) | 40 weeks–10 years | X | X | Largest SMD=−0.32 (−0.38 to –0.25) | ||||||
| 44 | Neurodevelopmental scores (low scores) | 40 weeks–10y ears | X | X | Smallest SMD=−0.31 (−0.38 to –0.25) | ||||||
| 45 | Cognitive score | 0.16–10.0 years | X | XI | X | SMDH=−0.39 (−0.50 to –0.28) | |||||
| 45 | Cognitive score | 0.16–10.0 years | X | X | SMDH=−0.34 (−0.45 to –0.22) | ||||||
| 45 | Cognitive score | 2.0–9.5 years | X | I | X | SMDH=−0.58 (−0.82 to –0.35) | |||||
| 45 | Borderline intellectual impairment | Child | X | X | OR=1.75 (1.50 to 2.04) | ||||||
| 84 | Systolic blood pressure | Child/adult | X | X | MD=2.00 (0.21 to 3.78) | ||||||
| 84 | Systolic blood pressure | Child/adult | X | X | MD=1.46 (0.13 to 2.79) | ||||||
| 84 | Diastolic blood pressure | Child/adult | X | X | MD=1.39 (0.00 to 2.78) | ||||||
| 84 | Diastolic blood pressure | Child/adult | X | X | MD=1.22 (0.19 to 2.25) | ||||||
| 84 | High-density lipoprotein | Child/adult | X | X | MD=0.03 (−0.04 to 0.10) | ||||||
| 84 | High-density lipoprotein | Child/adult | X | X | MD=0.01 (−0.04 to 0.07) | ||||||
| 84 | Low-density lipoprotein | Child/adult | X | X | MD=0.67 (0.38 to 0.97) | ||||||
| 84 | Low-density lipoprotein | Child/adult | X | X | MD=0.13 (−0.03 to 0.29) | ||||||
| 84 | Triglyceride | Child/adult | X | X | MD=0.00 (−0.07 to 0.06) | ||||||
| 84 | Triglyceride | Child/adult | X | X | MD=−0.04 (−0.09 to 0.02) | ||||||
| 84 | Insulin | Child/adult | X | X | MD=−1.65 (−3.39 to 0.10) | ||||||
| 84 | Insulin | Child/adult | X | X | MD=−1.07 (−2.29 to 0.15) | ||||||
| 84 | BMI | Child/adult | X | X | MD=−0.38 (−0.98 to 0.22) | ||||||
| 84 | BMI | Child/adult | X | X | MD=0.06 (−0.34 to 0.46) | ||||||
| 87 | Systolic blood pressure | 11.3–41.3 years | X | X | SMD=0.41 (0.12 to 0.70) | ||||||
| 87 | Systolic blood pressure | 11.3–41.3 years | X | X | SMD=0.31 (−0.33 to 0.95) | ||||||
| 87 | Diastolic blood pressure | 11.3–41.3 years | X | X | SMD=0.28 (0.05 to 0.51) | ||||||
| 87 | Diastolic blood pressure | 11.3–41.3 years | X | X | SMD=0.09 (−0.08 to 0.26) | ||||||
| 87 | Serum creatinine | 17.6–22.9 years | X | X | SMD=0.18 (−0.24 to 0.59) | ||||||
| 87 | Serum creatinine | 17.6–22.9 years | X | X | SMD=0.02 (−0.32 to 0.35) | ||||||
 , harmful effect from high to lower risks;
, harmful effect from high to lower risks;  , no effect high to lower risk.
, no effect high to lower risk.
Symbols inexposures: X, as defined in exposure; XI, SGA and IUGR (defined in reference 45); I, IUGR (defined in reference 45).
(45) IUGR is defined as antenatal evidence of growth restriction by abnormal middle cerebral artery pulsatility index and umbilical artery pulsatility index, or late onset verified by ultrasound or clinically, or ultrasound and clinical evaluation, or third trimester serial ultrasound.
AGAappropriate for gestational ageBMIbody mass indexIUGRintrauterine growth restrictionLBWlow birth weightMDmean differenceNBWnormal body weightPTpretermRRrelative riskSGAsmall for gestational ageSMDstandardised mean differenceSMDHstandardized mean difference for heteroscedastic population variancesTterm
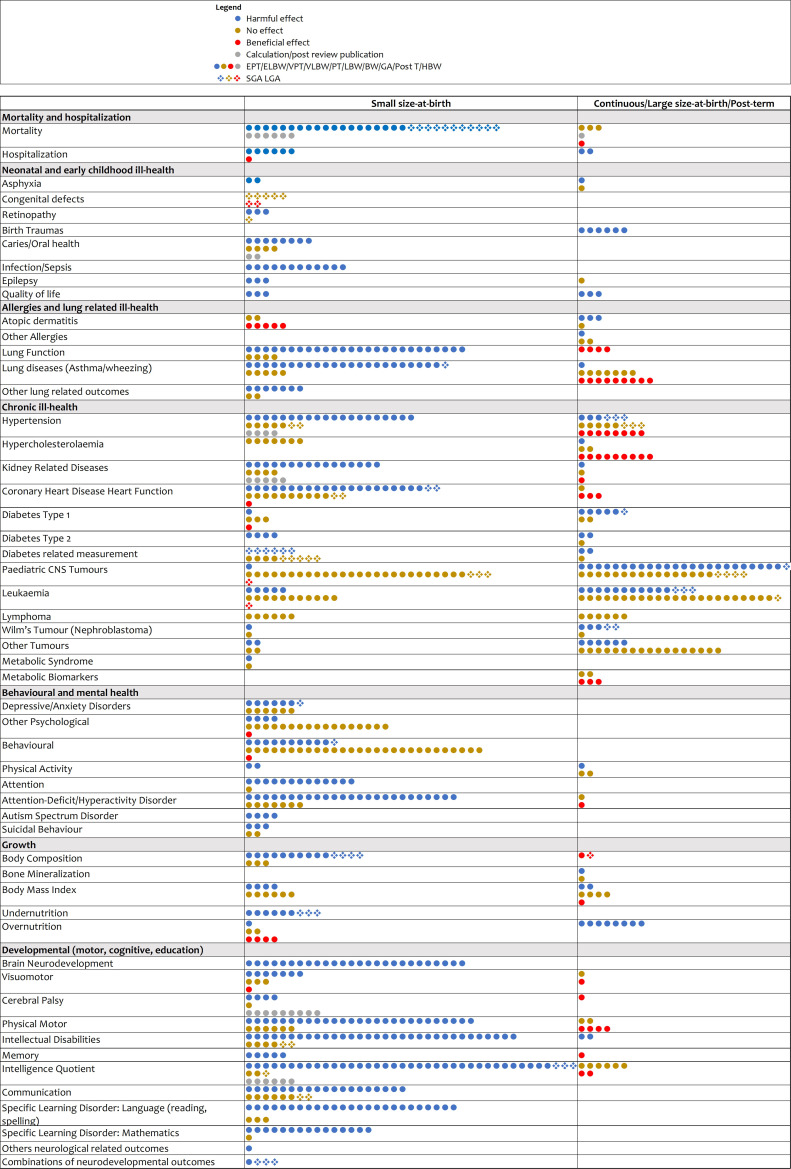
Figure 3 summarises findings on the direction of the association by subtheme of online supplemental material 3 tables 1 a-g .46,195 Except for a few subthemes like undernutrition, most studies were conducted in high-income countries (online supplemental material 5).
Figure 3. Summary of the associations presented in online supplemental table 1a–g. BW, birth weight; ELBW, extremely low birth weight; EPT, extremely preterm; GA, gestational age; HBW, high birth weight; LBW, low birth weight; LGA, large for gestational age; PT, preterm; SGA, small for gestational age; VLBW, very low birth weight; VPT, very preterm.
Small size at birth (extremely preterm, very preterm, preterm, late preterm, ELBW, VLBW, LBW, SGA and IUGR) associations comprised most of the outcomes assessed (32 of 50) (online supplemental material 3 tables 1 a-g and figure 3). Seventeen of the 32 outcomes had been identified previously in eight published umbrella reviews as being associated with size at birth: mortality,1146,48 50 dental caries,856,59 infection,1150 52 60,63 quality of life,4 5 65 atopic dermatitis,5 11 67 68 lung function,45 11 70,73 asthma/wheezing,1152 73,80 including hypertension,411 84,88 94 type 2 diabetes type,9 11 113 114 physical activity,6 143 144 undernutrition,11 160 attention-deficit/hyperactivity disorder,45 140,142 149 cerebral palsy,5170,173 neurodevelopmental,45 164,167 motor development,4 5 146 147 168 intellectual disabilities1011 138 139 141 146 148 151 174 177 179 181,184 and IQ.1011 141 142 146 177 181,183 185 Unlike most previous umbrella reviews, we mapped the specific associations between different small size-at-birth risk factors and specific detailed outcomes. We also identified 15 subthemes which were consistently associated with small size at birth that had not been included in previous umbrella reviews of associations with hospitalisation,52 asphyxia,54 retinopathy,55 epilepsy,64 other lung related measurements,51 82 83 kidney related diseases,8587 105,107 attention,138139 146,148 autism spectrum disorder,140 152 153 body composition,85155,158 working memory,138 141 146 182 communication,138148 174 183 190,192 educational outcomes language learning disorder,138 141 184 190 191 193 194 mathematics learning disorder,138 141 173 184 193 non-right handedness195 and combined neurological measurements.176 We found two subthemes (hypercholesterolaemia84 and lymphoma128) which consistently showed no association. We also identified 16 associations with mixed evidence of association: congenital defects,53 coronary heart disease heart function,101 102 type 1 diabetes,108,111 diabetes-related measurement,84 115 paediatric central nervous system tumours,116,120 leukaemia,121 122 124 126 127 Wilms’ tumour,129 other tumours,130 metabolic syndrome,132 depressive/anxiety disorders,133,138 other psychological,132 135 139 adverse behaviours,138140,142 suicidal behaviour,154 body mass index,77 84 overnutrition156 161 162 and visuomotor.146 147 168
Large size at birth/post-term/continuous measurement of birth weight and GA were consistently associated with 11 subthemes: increased risk of hospitalisation,49 birth trauma,49 atopic dermatitis,69 lung function,70 body composition,158 overnutrition,161,163 cerebral palsy,170 Wilms’ tumour,112 129 intellectual disabilities,151 and decreased quality of life66 and working memory.182 Meta-analyses showed mixed evidence for 24 subthemes.
In table 3, only 11 reviews and 73 meta-analyses within these compared risks by size for GA stratified by gestation. Four reviews46 48 160 174 (37 meta-analyses) compared term SGA, preterm SGA and preterm- appropriate for gestational age (AGA) to term-AGA babies. These ideal comparisons elucidated the relative magnitude of the effect of SGA matching on preterm/term status and the relative magnitude of the effect of GA matching on AGA status.
Discussion
This umbrella review provides the most recent synthesis of evidence from multiple fields exploring associations of size at birth with a wide range of subsequent health, growth and developmental outcomes in children under 18. This umbrella review summarised 302 reviews and mapped the magnitude and consistency of 1041 meta-analyses (from 150 reviews). The umbrella review also showed 73 meta-analyses (from 11 reviews) which compared risks by size for gestational age, stratified by preterm and term. We revealed gaps in research and an absence of meta-analyses for some exposures and outcomes. We elucidated analytical and measurement approaches which, if replicated, could better reveal the relative importance of preterm and IUGR (SGA) in the aetiology of adverse outcomes in children.
Our findings indicate some of the potential mechanisms underlying the associations. There is a body of theory seeking to distinguish the causes and the consequences of prematurity from those of IUGR.46 196 197 Prematurity and fetal growth restriction are influenced by some similar factors, many of them maternal, such as weight, height, weight gain during pregnancy, smoking and age among others. Preterm delivery interrupts in utero development of neurological, immunological and lung function.198 199 By contrast, poor fetal intrauterine growth, reflected in IUGR (SGA), links to subsequent metabolic and growth issues reflected in undernutrition and poorer cognitive development,200 201 while rapid in utero growth, reflected by LGA, links to subsequent obesity and cancers. Analyses such as those shown in table 3, distinguishing the co-occurrence of preterm and SGA from the occurrence of preterm alone or SGA alone, and comparing these to term AGA babies, enable greater understanding of the relative importance of the prematurity and IUGR (and their respective causes) in the causation of specific adverse outcomes. This review suggests that prematurity mechanisms are the key aetiologies linked to mortality and cognitive development, while IUGR mechanisms are the key ones linked to underweight and stunting. Improved understanding of the relationship of these two different aetiologies to subsequent adverse outcomes will ensure we develop more appropriate interventions to address these risk factors and are better able to track intervention impacts.
It was not feasible in this discussion to explore all the potential reasons why mixed or contradictory effects were observed for each of the subthemes. Key reasons for why mixed estimates of effect were seen could include the number of included studies, the search strategy and inclusion/exclusion criteria, the constituent study designs and heterogeneity. Other potential reasons for inconsistent associations include the population used for the exposure (grouping extremely preterm with preterm), the comparator used (grouping normal birth weight with HBW as a comparator for LBW), the age of the child at assessment (allowing more or less time for a disease, such as type 2 diabetes, to develop), measurement practices in older versus newer reviews, and whether or not sex or other variables were adjusted for (female babies are appropriate for GA at a lower birth weights than male babies and could be misclassified if sex was not adjusted for).
By way of example of how the results have varied by review, we unpacked meta-analysis of the association between LBW and type 1 diabetes. The earliest review, by Harder and colleagues, included eight papers and suggested a protective effect (0.82), but had a confidence interval (CI) that overlapped 1 (95% CI 0.54 to 1.23).109 However, this review compared LBW to babies born at 2500+ g, including HBW infants. The next review, by Cardwell and colleagues, used a more appropriate normal (2500–4000 g) comparator and included many more studies (29 studies of which five were cohorts).111 They showed no association (OR=0.98, 95% CI 0.84 to 1.13), with high heterogeneity observed, although a meta-analysis of the cohorts showed a protective effect (OR=0.79, 95% CI 0.67 to 0.92).111 The most recent meta-analysis by Haiyan Wang and colleagues, focused only on six cohort studies and by virtue of having less heterogeneity and a larger sample size, they established that LBW appears to protect against type 1 diabetes compared with normal birth weight (HR 0.78, 95 % CI 0.69 to 0.88).110 By contrast there was only one systematic review of the effects of prematurity (Li and colleagues108) which included 18 studies and showed prematurity increased the risk of type 1 diabetes (OR=1.17, 95% CI 1.10 to 1.25) for high-quality studies.
Although we assessed review quality, we aimed to be comprehensive and so extracted data regardless of quality. This meant we included 28 reviews with low critical appraisal scores which might explain some of the mixed direction of effects observed. Thus, when exploring the association presented, it is important to consider the quality of the meta-analysis. For example, low-quality review on extremely preterm and ELBW and mortality showed very small neonates had a reduced prevalence of mortality compared with larger babies,47 an anomalous finding which probably stemmed from selection and publication bias favouring reports of very small surviving babies.
The evolution of our understanding of the relationships between size at birth and various outcomes in children is inextricably linked to improvements in measurement and in theory, as well as to disease burden and priority health topics. For example, literature on effects of small size at birth on adult health burgeoned after the ‘developmental origins of disease’ theory.1 2 Our review identified several gaps in relation to the risk factors, outcomes and populations studied. Very few meta-analyses examined outcomes linked to the effect of LGA and SGA or of the different combinations of gestation and size for GA at birth. For some subtheme outcomes (cognitive and motor), very small size at birth was the exposure measured rather than LBW or prematurity. Most of the systematic reviews were from high-income countries, reflecting a general bias in research.202 We also identified 14 subtheme outcomes missing meta-analyses. Older age children are rarely a priority population for studies of mortality or acute ill health, but this neglect may be because they generally have fewer ill-health outcomes and so are more difficult to study.
Strengths and limitations
Our review synthesised an enormous literature and was comprehensive, not restricting on outcome, year or language. It assessed methodological quality using a critical appraisal tool, showed gaps and focused on children up to 18, thereby bridging a gap between studies focused on young children and those focused on adults. Its limitations are its reliance on published systematic reviews, particularly those with meta-analyses. Our approach missed single studies not included in previous reviews and topics without systematic reviews. We did not do additional meta-analyses nor did we recalculate effect sizes, so we include three reviews with inconsistent data presented in abstract, figures and results.87 124 159 Moreover, while we did not restrict on language, we used English search terms and did not search non-English databases, for example, Chinese literature. As part of the umbrella review, we did not assess methods of the selected papers. In meta-analyses where we did not detect an association, we did not conduct further examination by assessing the confidence intervals.
Recommendations/conclusion
Our umbrella review compiled evidence from 1041 associations and showed the strength of evidence. It also alluded to potential mechanisms, enabling us to identify areas where we can appropriately target or track interventions aimed at improving outcomes in LBW/preterm or HBW children.
To improve future research and evidence on the mechanisms involved, we highlight the need to
Address gaps in the range of risk factors explored by including the whole spectrum of size and maturity where possible, including (1) splitting preterm into subgroups based on maturity, for example, extremely preterm, very preterm and moderate or late preterm; (2) considering all the combinations of size for GA (adjusted for preterm/term/post-term, specifically focusing on SGA and LGA); and (3) excluding HBW, post-term and LGA from the comparator when examining small size at birth (LBW, preterm and AGA). The latter recommendation is made because when the comparator is ‘anyone not SGA’, then the relative risk of SGA may be underestimated because the comparator lumps low-risk AGA babies with higher-risk LGA ones.
Conduct further research on understudied exposures (ie, large size at birth/post-term) or outcomes (eg, current research on LGA is largely limited to outcomes of growth, diabetes or cancer) and on inconclusive areas (for small size these include coronary heart disease and heart function indicators, congenital defects, overweight, leukaemia, paediatric central nervous system tumours, type 1 diabetes, and adverse behavioural and visuomotor outcomes). For large size at birth, there are numerous areas with inconclusive results. There is also a need to conduct meta-analyses on the 14 subthemes without one.
Address gaps in populations studied by further examining associations by different age groups and by sex, and by conducting additional research in low-income and middle-income countries for specific subtopics, particularly where risks may differ because of differences in access to treatment and preventive measures, or to differing epigenetic and environmental exposures.
Conduct theme-based meta-analyses starting with subthemes that are inconsistent in the literature and with meta-analysis that have low-quality scores. Considering the different reasons for inconsistency indicated in the discussion, future research would benefit from subanalysis of the associations stratified by age at the occurrence of the outcome and by the sex of the child.
Acknowledging that both small and large size at birth contribute to multiple burdens of diseases, this study gives further evidence on the importance of correctly measuring size at birth in order to be able to intervene properly. Compiling this evidence allows researchers and policymakers to understand potential pathways for child survival and to further explore pathways for children to attain their full thriving potential. This study provides guidance to funders and researchers to help prioritise understanding of inconsistent evidence in the literature and to inform and prioritise points of interventions that contribute the most to disability-adjusted life years.
supplementary material
Acknowledgements
We thank Russell Burke for reviewing the search strategy. Diala Obeid for supporting in graphing the data.
The funder had no role in study design, data collection, data analysis, data interpretation, or writing.
Footnotes
Funding: This work was supported by the Nagasaki University 'Doctoral Program for World-leading Innovative and Smart Education' for Global Health, KYOIKU KENKYU SHIEN KEIHI, Ministry of Education, Culture, Sports, Science and Technology, Japan
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Contributor Information
Zeina Jamaluddine, Email: zeina.jamaluddine@lshtm.ac.uk.
Eman Sharara, Email: ess05@mail.aub.edu.
Vanessa Helou, Email: vth00@mail.aub.edu.
Nadine El Rashidi, Email: nadineelrashidi@gmail.com.
Gloria Safadi, Email: gs61@aub.edu.lb.
Nehmat El-Helou, Email: ne82@aub.edu.lb.
Hala Ghattas, Email: hg15@aub.edu.lb.
Miho Sato, Email: mihos@nagasaki-u.ac.jp.
Hannah Blencowe, Email: hannah-jayne.blencowe@lshtm.ac.uk.
Oona M R Campbell, Email: oona.campbell@lshtm.ac.uk.
Data availability statement
Data are available in a public, open access repository.
References
- 1.Koch LA, Weymuller CA, James E. Reduction of mortality from premature birth: some practical measures. J Am Med Assoc. 1948;136:217–21. doi: 10.1001/jama.1948.02890210001001. [DOI] [PubMed] [Google Scholar]
- 2.Hughes MM, Black RE, Katz J. 2500-G low birth weight cutoff: history and implications for future research and policy. Matern Child Health J. 2017;21:283–9. doi: 10.1007/s10995-016-2131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the Intergrowth-21St project. Lancet. 2014;384:857–68. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 4.Kelly MM, Griffith PB. The influence of preterm birth beyond infancy: umbrella review of outcomes of adolescents and adults born preterm. J Am Assoc Nurse Pract. 2020;32:555–62. doi: 10.1097/JXX.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 5.Kelly MM, Griffith PB. Umbrella review of school age health outcomes of preterm birth survivors. J Pediatr Health Care. 2020;34:e59–76. doi: 10.1016/j.pedhc.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Aleksovska K, Puggina A, Giraldi L, et al. Biological determinants of physical activity across the life course: a "determinants of diet and physical activity" (DEDIPAC) umbrella systematic literature review. Sports Med Open. 2019;5:2. doi: 10.1186/s40798-018-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belbasis L, Stefanaki I, Stratigos AJ, et al. Non-genetic risk factors for cutaneous melanoma and keratinocyte skin cancers: an umbrella review of meta-analyses. J Dermatol Sci. 2016;84:330–9. doi: 10.1016/j.jdermsci.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Thang Le VN, Kim J-G, Yang Y-M, et al. Risk factors for early childhood Caries: an umbrella review. Pediatr Dent. 2021;43:176–94. [PubMed] [Google Scholar]
- 9.Bellou V, Belbasis L, Tzoulaki I, et al. Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PLoS One. 2018;13:e0194127. doi: 10.1371/journal.pone.0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sentenac M, Chaimani A, Twilhaar S, et al. The challenges of heterogeneity in gestational age and birthweight inclusion criteria for research synthesis on very preterm birth and childhood cognition: an umbrella review and meta-regression analysis. Paediatr Perinat Epidemiol. 2022;36:717–25. doi: 10.1111/ppe.12846. [DOI] [PubMed] [Google Scholar]
- 11.Belbasis L, Savvidou MD, Kanu C, et al. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. BMC Med. 2016;14:147. doi: 10.1186/s12916-016-0692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell CC. DSM-IV: diagnostic and statistical manual of mental disorders. JAMA. 1994;272:828. doi: 10.1001/jama.1994.03520100096046. [DOI] [Google Scholar]
- 13.Machado Júnior LC, Passini Júnior R, Rodrigues Machado Rosa I. Late prematurity: a systematic review. J Pediatr (Rio J) 2014;90:221–31. doi: 10.1016/j.jped.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Dong Y, Yu J-L. An overview of morbidity, mortality and long-term outcome of late preterm birth. World J Pediatr. 2011;7:199–204. doi: 10.1007/s12519-011-0290-8. [DOI] [PubMed] [Google Scholar]
- 15.Arpino C, Compagnone E, Montanaro ML, et al. Preterm birth and neurodevelopmental outcome: a review. Childs Nerv Syst . 2010;26:1139–49. doi: 10.1007/s00381-010-1125-y. [DOI] [PubMed] [Google Scholar]
- 16.Castro-Delgado OE, Salas-Delgado I, Acosta-Argoty FA, et al. Very low and extremely low birth weight. Pediatria (Santiago) 2016;49:23–30. doi: 10.1016/j.rcpe.2016.02.002. [DOI] [Google Scholar]
- 17.Paliwoda M, New K, Davies M, et al. Physiological vital sign ranges in newborns from 34 weeks gestation: a systematic review. Int J Nurs Stud. 2018;77:81–90. doi: 10.1016/j.ijnurstu.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Flamant C, Gascoin G. [Short-term outcome and small for gestational age newborn management]. Devenir Precoce et Prise en charge Neonatale Du Nouveau-NE petit pour L'Age Gestationnel. J Gynecol Obstet Biol Reprod (Paris) 2013;42:985–95. doi: 10.1016/j.jgyn.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Olusanya BO, Osibanjo FB, Mabogunje CA, et al. The burden and management of neonatal jaundice in Nigeria: a scoping review of the literature. Niger J Clin Pract. 2016;19:1–17. doi: 10.4103/1119-3077.173703. [DOI] [PubMed] [Google Scholar]
- 20.Milner KM, Neal EFG, Roberts G, et al. Long-term neurodevelopmental outcome in high-risk newborns in resource-limited settings: a systematic review of the literature. Paediatr Int Child Health. 2015;35:227–42. doi: 10.1179/2046905515Y.0000000043. [DOI] [PubMed] [Google Scholar]
- 21.Guillén Ú. Relationship between attrition and neurodevelopmental impairment rates in extremely preterm infants at 18 to 24 months: a systematic review. Arch Pediatr Adolesc Med . 2012;166:178. doi: 10.1001/archpediatrics.2011.616. [DOI] [PubMed] [Google Scholar]
- 22.Ancel PY. [Severe Sensorineural impairment in very premature infants: Epidemiological aspects]. handicap neuro-Sensoriel grave de L'Enfant grand premature. J Gynecol Obstet Biol Reprod (Paris) 2004;33:461–74. doi: 10.1016/S0368-2315(04)96559-3. [DOI] [PubMed] [Google Scholar]
- 23.Lorenz JM. Survival and long-term neurodevelopmental outcome of the extremely preterm infant. A systematic review. Saudi Med J. 2011;32:885–94. [PubMed] [Google Scholar]
- 24.Soleimani F, Zaheri F, Abdi F. Long-term neurodevelopmental outcomes after preterm birth. Iran Red Crescent Med J. 2014;16:e17965. doi: 10.5812/ircmj.17965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagliaro CL, Bühler KEB, Ibidi SM, et al. Dietary transition difficulties in preterm infants: critical literature review. J Pediatr (Rio J) 2016;92:7–14. doi: 10.1016/j.jped.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Formiga C, Linhares MBM. [Assessment of preterm children's early development]. Avaliacao do Desenvolvimento Inicial de Criancas Nascidas pre-Termo. Rev Esc Enferm USP. 2009;43:472–80. doi: 10.1590/S0080-62342009000200030. [DOI] [PubMed] [Google Scholar]
- 27.Hussain SM, Ackerman IN, Wang Y, et al. Could low birth weight and preterm birth be associated with significant burden of hip osteoarthritis? A systematic review. Arthritis Res Ther . 2018;20:121. doi: 10.1186/s13075-018-1627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visser SSM, van Diemen WJM, Kervezee L, et al. The relationship between preterm birth and sleep in children at school age: a systematic review. Sleep Med Rev. 2021;57:101447. doi: 10.1016/j.smrv.2021.101447. [DOI] [PubMed] [Google Scholar]
- 29.Low EXS, Mandhari MNKA, Herndon CC, et al. Perinatal, and childhood risk factors for development of irritable bowel syndrome: a systematic review. J Neurogastroenterol Motil. 2020;26:437–46. doi: 10.5056/jnm20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juul F, Chang VW, Brar P, et al. Birth weight, early life weight gain and age at menarche: a systematic review of longitudinal studies. Obes Rev. 2017;18:1272–88. doi: 10.1111/obr.12587. [DOI] [PubMed] [Google Scholar]
- 31.James E, Wood CL, Nair H, et al. Preterm birth and the timing of puberty: a systematic review. BMC Pediatr. 2018;18:3. doi: 10.1186/s12887-017-0976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul A, Deans R, Viner R, et al. Pubertal development and sexuality in female adolescents born preterm: a review of the literature. Int J Adolesc Med Health. 2011;23:175–9. doi: 10.1515/ijamh.2011.040. [DOI] [PubMed] [Google Scholar]
- 33.Yadav S, Rustogi D. Small for gestational age: growth and puberty issues. Indian Pediatr. 2015;52:135–40. doi: 10.1007/s13312-015-0588-z. [DOI] [PubMed] [Google Scholar]
- 34.Farajdokht F, Sadigh-Eteghad S, Dehghani R, et al. Very low birth weight is associated with brain structure abnormalities and cognitive function impairments: a systematic review. Brain Cogn. 2017;118:80–9. doi: 10.1016/j.bandc.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Nadal S, Bosch L. Cognitive and learning outcomes in late preterm infants at school age: a systematic review. Int J Environ Res Public Health. 2020;18:74. doi: 10.3390/ijerph18010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieira MEB, Linhares MBM. Developmental outcomes and quality of life in children born preterm at preschool- and school-age. J Pediatr (Rio J) 2011;87:281–91. doi: 10.2223/JPED.2096. [DOI] [PubMed] [Google Scholar]
- 37.Moreira RS, Magalhães LC, Alves CRL. Effect of preterm birth on motor development, behavior, and school performance of school-age children: a systematic review. J Pediatr (Rio J) 2014;90:119–34. doi: 10.1016/j.jped.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Samra HA, McGrath JM, Wehbe M. An integrated review of developmental outcomes and late-preterm birth. J Obstet Gynecol Neonatal Nurs. 2011;40:399–411. doi: 10.1111/j.1552-6909.2011.01270.x. [DOI] [PubMed] [Google Scholar]
- 39.Chung EH, Chou J, Brown KA. Neurodevelopmental outcomes of preterm infants: a recent literature review. Transl Pediatr. 2020;9:S3–8. doi: 10.21037/tp.2019.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Holditch-Davis DL, Darcy-Mahoney A. Perinatal, neonatal, and family social factors predicting poor school outcome of low-birth-weight survivors: an integrative review. Adv Neonatal Care. 2015;15:38–47. doi: 10.1097/ANC.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 41.Englerova K, Takacs L. The effects of prenatal, perinatal and neonatal factors on academic performance in primary school age children. Prenatalni, Perinatalni a Neonatalni Faktory a Jejich Vliv NA Skolni Uspesnost U Deti Mladsiho Skolniho Veku. Ceska Gynekol. 2020;85:71–9. [PubMed] [Google Scholar]
- 42.Malin GL, Morris RK, Riley R, et al. When is birthweight at term abnormally low? A systematic review and meta-analysis of the association and predictive ability of current birthweight standards for neonatal outcomes. BJOG. 2014;121:515–26. doi: 10.1111/1471-0528.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malin GL, Morris RK, Riley RD, et al. When is birthweight at term (>=37 weeks' gestation) abnormally low? A systematic review and meta-analysis of the Prognostic and predictive ability of current birthweight standards for childhood and adult outcomes. BJOG: Int J Obstet Gy . 2015;122:634–42. doi: 10.1111/1471-0528.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arcangeli T, Thilaganathan B, Hooper R, et al. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet Gynecol . 2012;40:267–75. doi: 10.1002/uog.11112. [DOI] [PubMed] [Google Scholar]
- 45.Sacchi C, Marino C, Nosarti C, et al. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: a systematic review and meta-analysis. JAMA Pediatr. 2020;174:772–81. doi: 10.1001/jamapediatrics.2020.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katz J, Lee AC, Kozuki N, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382:417–25. doi: 10.1016/S0140-6736(13)60993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang B, Dai Y, Chen H, et al. Neonatal mortality in hospitalized Chinese population: a meta-analysis. Biomed Res Int. 2019;2019:7919501. doi: 10.1155/2019/7919501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchant T, Willey B, Katz J, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. 2012;9:e1001292. doi: 10.1371/journal.pmed.1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi AC, Mullin P, Prefumo F. Prevention, management, and outcomes of Macrosomia: a systematic review of literature and meta-analysis. Obstet Gynecol Surv. 2013;68:702–9. doi: 10.1097/01.ogx.0000435370.74455.a8. [DOI] [PubMed] [Google Scholar]
- 50.Shi T, Vennard S, Mahdy S, et al. Risk factors for RSV associated acute lower respiratory infection poor outcome and mortality in young children: a systematic review and meta-analysis. J Infect Dis. 2021 doi: 10.1093/infdis/jiaa751. [DOI] [PubMed] [Google Scholar]
- 51.Risnes KR, Vatten LJ, Baker JL, et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol. 2011;40:647–61. doi: 10.1093/ije/dyq267. [DOI] [PubMed] [Google Scholar]
- 52.Isayama T, Lewis-Mikhael A-M, O’Reilly D, et al. Health services use by late preterm and term infants from infancy to adulthood: a meta-analysis. Pediatrics. 2017;140:e20170266. doi: 10.1542/peds.2017-0266. [DOI] [PubMed] [Google Scholar]
- 53.Villamor-Martinez E, Kilani MA, Degraeuwe PL, et al. Intrauterine growth restriction and patent ductus Arteriosus in very and extremely preterm infants: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2019;10:58. doi: 10.3389/fendo.2019.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desalew A, Semahgn A, Tesfaye G. Determinants of birth asphyxia among newborns in Ethiopia: a systematic review and meta-analysis. Int J Health Sci (Qassim) 2020;14:35–47. [PMC free article] [PubMed] [Google Scholar]
- 55.Bahmani T, Karimi A, Rezaei N, et al. Retinopathy Prematurity: a systematic review and meta-analysis study based on neonatal and maternal risk factors. J Matern Fetal Neonatal Med. 2022;35:8032–50. doi: 10.1080/14767058.2021.1940938. [DOI] [PubMed] [Google Scholar]
- 56.Occhi-Alexandre IGP, Cruz PV, Bendo CB, et al. Prevalence of dental Caries in preschool children born preterm and/or with low birth weight: a systematic review with meta-analysis of prevalence data. Int J Paediatr Dent. 2020;30:265–75. doi: 10.1111/ipd.12610. [DOI] [PubMed] [Google Scholar]
- 57.Shi L, Jia J, Li C, et al. Relationship between preterm, low birth weight and early childhood Caries: a meta-analysis of the case-control and cross-sectional study. Biosci Rep. 2020;40 doi: 10.1042/BSR20200870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Wang J, Li Y-H, et al. Association of molar Incisor Hypomineralization with premature birth or low birth weight: systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;33:1700–8. doi: 10.1080/14767058.2018.1527310. [DOI] [PubMed] [Google Scholar]
- 59.Bensi C, Costacurta M, Belli S, et al. Relationship between preterm birth and developmental defects of enamel: a systematic review and meta-analysis. Int J Paediatr Dent. 2020;30:676–86. doi: 10.1111/ipd.12646. [DOI] [PubMed] [Google Scholar]
- 60.Belachew A, Tewabe T. Neonatal sepsis and its association with birth weight and gestational age among admitted neonates in Ethiopia: systematic review and meta-analysis. BMC Pediatr. 2020;20:55. doi: 10.1186/s12887-020-1949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Washam M, Woltmann J, Haberman B, et al. Risk factors for methicillin-resistant staphylococcus aureus Colonization in the neonatal intensive care unit: a systematic review and meta-analysis. Am J Infect Control. 2017;45:1388–93. doi: 10.1016/j.ajic.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 62.Shi T, Balsells E, Wastnedge E, et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: systematic review and meta-analysis. J Glob Health. 2015;5:020416. doi: 10.7189/jogh.05.020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson S, Mathews KH, Pulanic D, et al. Risk factors for severe acute lower respiratory infections in children - a systematic review and meta-analysis. Croat Med J. 2013;54:110–21. doi: 10.3325/cmj.2013.54.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W, Peng A, Deng S, et al. Do premature and postterm birth increase the risk of epilepsy? An updated meta-analysis. Epilepsy Behav. 2019;97:83–91. doi: 10.1016/j.yebeh.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 65.Petrou S, Krabuanrat N, Khan K. Preference-based health-related quality of life outcomes associated with preterm birth: a systematic review and meta-analysis. Pharmacoeconomics. 2020;38:357–73. doi: 10.1007/s40273-019-00865-7. [DOI] [PubMed] [Google Scholar]
- 66.Luijk MPCM, Kocevska D, Tham EKH, et al. Gestational age at birth and sleep duration in early childhood in three population-based cohorts. Sleep Med X. 2019;1:100002. doi: 10.1016/j.sleepx.2019.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu T, Zhao J, Qu Y, et al. Association of very preterm birth with decreased risk of eczema: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;78:1142–8. doi: 10.1016/j.jaad.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 68.Panduru M, Salavastru CM, Panduru NM, et al. Birth weight and atopic dermatitis: systematic review and meta-analyis. Acta Dermatovenerol Croat. 2014;22:91–6. [PubMed] [Google Scholar]
- 69.Wooldridge AL, McMillan M, Kaur M, et al. Relationship between birth weight or fetal growth rate and postnatal allergy: a systematic review. J Allergy Clin Immunol. 2019;144:1703–13. doi: 10.1016/j.jaci.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 70.Doyle LW, Andersson S, Bush A, et al. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: a meta-analysis of individual participant data. Lancet Respir Med. 2019;7:677–86. doi: 10.1016/S2213-2600(18)30530-7. [DOI] [PubMed] [Google Scholar]
- 71.Ronkainen E, Dunder T, Peltoniemi O, et al. New BPD predicts lung function at school age: follow-up study and meta-analysis. Pediatr Pulmonol. 2015;50:1090–8. doi: 10.1002/ppul.23153. [DOI] [PubMed] [Google Scholar]
- 72.Kotecha SJ, Edwards MO, Watkins WJ, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax. 2013;68:760–6. doi: 10.1136/thoraxjnl-2012-203079. [DOI] [PubMed] [Google Scholar]
- 73.den Dekker HT, Sonnenschein-van der Voort AMM, de Jongste JC, et al. Early growth characteristics and the risk of reduced lung function and asthma: a meta-analysis of 25,000 children. J Allergy Clin Immunol. 2016;137:1026–35. doi: 10.1016/j.jaci.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 74.Been JV, Lugtenberg MJ, Smets E, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001596. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonnenschein-van der Voort AMM, Arends LR, de Jongste JC, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133:1317–29. doi: 10.1016/j.jaci.2013.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jaakkola JJK, Ahmed P, Ieromnimon A, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–30. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 77.Rzehak P, Wijga AH, Keil T, et al. Body mass index trajectory classes and incident asthma in childhood: results from 8 European birth cohorts--a global allergy and asthma European network initiative. J Allergy Clin Immunol. 2013;131:1528–36. doi: 10.1016/j.jaci.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Mebrahtu TF, Feltbower RG, Greenwood DC, et al. Birth weight and childhood wheezing disorders: a systematic review and meta-analysis. J Epidemiol Community Health. 2015;69:500–8. doi: 10.1136/jech-2014-204783. [DOI] [PubMed] [Google Scholar]
- 79.Xu X-F, Li Y-J, Sheng Y-J, et al. Effect of low birth weight on childhood asthma: a meta-analysis. BMC Pediatr. 2014;14:275. doi: 10.1186/1471-2431-14-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mu M, Ye S, Bai M-J, et al. Birth weight and subsequent risk of asthma: a systematic review and meta-analysis. Heart Lung Circ. 2014;23:511–9. doi: 10.1016/j.hlc.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 81.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91:334–9. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Course CW, Kotecha S, Kotecha SJ. Fractional exhaled nitric oxide in Preterm-born subjects: a systematic review and meta-analysis. Pediatr Pulmonol. 2019;54:595–601. doi: 10.1002/ppul.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kotecha S, Clemm H, Halvorsen T, et al. Bronchial hyper-responsiveness in preterm-born subjects: a systematic review and meta-analysis. Pediatr Allergy Immunol . 2018;29:715–25. doi: 10.1111/pai.12957. [DOI] [PubMed] [Google Scholar]
- 84.Andraweera PH, Condon B, Collett G, et al. Cardiovascular risk factors in those born preterm - systematic review and meta-analysis. J Dev Orig Health Dis. 2021;12:539–54. doi: 10.1017/S2040174420000914. [DOI] [PubMed] [Google Scholar]
- 85.Gilarska M, Raaijmakers A, Zhang Z-Y, et al. Extremely low birth weight predisposes to impaired renal health: a pooled analysis. Kidney Blood Press Res . 2019;44:897–906. doi: 10.1159/000502715. [DOI] [PubMed] [Google Scholar]
- 86.de Jong F, Monuteaux MC, van Elburg RM, et al. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–34. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heo JS, Lee JM. The long-term effect of preterm birth on renal function: a meta-analysis. Int J Environ Res Public Health. 2021;18:2951. doi: 10.3390/ijerph18062951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mu M, Wang S-F, Sheng J, et al. Birth weight and subsequent blood pressure: a meta-analysis. Arch Cardiovasc Dis. 2012;105:99–113. doi: 10.1016/j.acvd.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 89.Lawlor DA, Ebrahim S, Davey Smith G. Is there a sex difference in the association between birth weight and systolic blood pressure in later life? Findings from a meta-regression analysis. Am J Epidemiol. 2002;156:1100–4. doi: 10.1093/aje/kwf154. [DOI] [PubMed] [Google Scholar]
- 90.Schluchter MD. Publication bias and heterogeneity in the relationship between systolic blood pressure, birth weight, and catch-up growth - A meta analysis. J Hypertens. 2003;21:273–9. doi: 10.1097/00004872-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 91.Ashtree DN, McGuinness AJ, Plummer M, et al. Developmental origins of cardiometabolic health outcomes in twins: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2020;30:1609–21. doi: 10.1016/j.numecd.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Gamborg M, Byberg L, Rasmussen F, et al. Birth weight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific results from 20 Nordic studies. Am J Epidemiol. 2007;166:634–45. doi: 10.1093/aje/kwm042. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y, Li H, Liu S, et al. The associations of high birth weight with blood pressure and hypertension in later life: a systematic review and meta-analysis. Hypertens Res. 2013;36:725–35. doi: 10.1038/hr.2013.33. [DOI] [PubMed] [Google Scholar]
- 94.Kooiman J, Terstappen F, van Wagensveld L, et al. Conflicting effects of fetal growth restriction on blood pressure between human and rat offspring: a meta-analysis. Hypertension . 2020;75:806–18. doi: 10.1161/HYPERTENSIONAHA.119.14111. [DOI] [PubMed] [Google Scholar]
- 95.McNeill G, Tuya C, Smith WCS. The role of genetic and environmental factors in the association between birthweight and blood pressure: evidence from meta-analysis of twin studies. Int J Epidemiol. 2004;33:995–1001. doi: 10.1093/ije/dyh260. [DOI] [PubMed] [Google Scholar]
- 96.Huxley R, Owen CG, Whincup PH, et al. Birth weight and subsequent cholesterol levels: exploration of the "fetal origins" hypothesis. JAMA. 2004;292:2755–64. doi: 10.1001/jama.292.22.2755. [DOI] [PubMed] [Google Scholar]
- 97.Owen CG, Whincup PH, Odoki K, et al. Birth weight and blood cholesterol level: a study in adolescents and systematic review. Pediatrics. 2003;111:1081–9. doi: 10.1542/peds.111.5.1081. [DOI] [PubMed] [Google Scholar]
- 98.Lawlor DA, Owen CG, Davies AA, et al. Sex differences in the association between birth weight and total cholesterol. A meta-analysis. Ann Epidemiol. 2006;16:19–25. doi: 10.1016/j.annepidem.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 99.Würtz P, Wang Q, Niironen M, et al. Metabolic signatures of birthweight in 18 288 adolescents and adults. Int J Epidemiol. 2016;45:1539–50. doi: 10.1093/ije/dyw255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Montfoort N, Finken MJJ, le Cessie S, et al. Could Cortisol explain the association between birth weight and cardiovascular disease in later life? A meta-analysis. Eur J Endocrinol. 2005;153:811–7. doi: 10.1530/eje.1.02050. [DOI] [PubMed] [Google Scholar]
- 101.Telles F, McNamara N, Nanayakkara S, et al. Changes in the preterm heart from birth to young adulthood: a meta-analysis. Pediatrics. 2020;146:e20200146. doi: 10.1542/peds.2020-0146. [DOI] [PubMed] [Google Scholar]
- 102.Epure AM, Leyvraz M, Anker D, et al. Risk factors in the first 1000 days of life and carotid intima-media thickness in children: a systematic review and meta-analysis. Eur J Pediatr. 2019;178:1775 [Google Scholar]
- 103.Huxley R, Owen CG, Whincup PH, et al. Is birth weight a risk factor for ischemic heart disease in later life. Am J Clin Nutr. 2007;85:1244–50. doi: 10.1093/ajcn/85.5.1244. [DOI] [PubMed] [Google Scholar]
- 104.Wang S-F, Shu L, Sheng J, et al. Birth weight and risk of coronary heart disease in adults: a meta-analysis of prospective cohort studies. J Dev Orig Health Dis. 2014;5:408–19. doi: 10.1017/S2040174414000440. [DOI] [PubMed] [Google Scholar]
- 105.Goetschalckx E, Mekahli D, Levtchenko E, et al. Glomerular filtration rate in former extreme low birth weight infants over the full pediatric age range: a pooled analysis. Int J Environ Res Public Health. 2020;17:2144. doi: 10.3390/ijerph17062144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Das SK, Mannan M, Faruque ASG, et al. Effect of birth weight on adulthood renal function: a bias-adjusted meta-analytic approach. Nephrology (Carlton) 2016;21:547–65. doi: 10.1111/nep.12732. [DOI] [PubMed] [Google Scholar]
- 107.White SL, Perkovic V, Cass A, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–61. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 108.Li S, Zhang M, Tian H, et al. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta-analysis. Obes Rev. 2014;15:804–11. doi: 10.1111/obr.12214. [DOI] [PubMed] [Google Scholar]
- 109.Harder T, Roepke K, Diller N, et al. Birth weight, early weight gain, and subsequent risk of type 1 diabetes: systematic review and meta-analysis. Am J Epidemiol. 2009;169:1428–36. doi: 10.1093/aje/kwp065. [DOI] [PubMed] [Google Scholar]
- 110.Wang H, Zhang Z, Liu Y, et al. Pre-pregnancy body mass index in mothers, birth weight and the risk of type I diabetes in their offspring: a dose-response meta-analysis of cohort studies. J Gynecol Obstet Hum Reprod. 2021;50:101921. doi: 10.1016/j.jogoh.2020.101921. [DOI] [PubMed] [Google Scholar]
- 111.Cardwell CR, Stene LC, Joner G, et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia. 2010;53:641–51. doi: 10.1007/s00125-009-1648-5. [DOI] [PubMed] [Google Scholar]
- 112.Magnusson Å, Laivuori H, Loft A, et al. The association between high birth weight and long-term outcomes-implications for assisted reproductive Technologies: a systematic review and meta-analysis. Front Pediatr. 2021;9:675775. doi: 10.3389/fped.2021.675775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao H, Song A, Zhang Y, et al. The association between birth weight and the risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Endocr J. 2018;65:923–33. doi: 10.1507/endocrj.EJ18-0072. [DOI] [PubMed] [Google Scholar]
- 114.Harder T, Rodekamp E, Schellong K, et al. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165:849–57. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- 115.Xu Y, Chen S, Yang H, et al. Decreased insulin sensitivity and abnormal glucose metabolism start in preadolescence in low-birth-weight children-meta-analysis and systematic review. Prim Care Diabetes. 2019;13:391–8. doi: 10.1016/j.pcd.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 116.Paquette K, Coltin H, Boivin A, et al. Cancer risk in children and young adults born preterm: a systematic review and meta-analysis. PLoS ONE. 2019;14:e0210366. doi: 10.1371/journal.pone.0210366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harder T, Plagemann A, Harder A. Birth weight and risk of neuroblastoma: a meta-analysis. Int J Epidemiol. 2010;39:746–56. doi: 10.1093/ije/dyq040. [DOI] [PubMed] [Google Scholar]
- 118.Harder T, Plagemann A, Harder A. Birth weight and subsequent risk of childhood primary brain tumors: a meta-analysis. Am J Epidemiol. 2008;168:366–73. doi: 10.1093/aje/kwn144. [DOI] [PubMed] [Google Scholar]
- 119.Dahlhaus A, Prengel P, Spector L, et al. Birth weight and subsequent risk of childhood primary brain tumors: an updated meta-analysis. Pediatr Blood Cancer. 2017;64:e26299. doi: 10.1002/pbc.26299. [DOI] [PubMed] [Google Scholar]
- 120.Georgakis MK, Kalogirou EI, Liaskas A, et al. Anthropometrics at birth and risk of a primary central nervous system tumour: a systematic review and meta-analysis. Eur J Cancer. 2017;75:117–31. doi: 10.1016/j.ejca.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 121.Huang Q, Gao Y, Zhong M, et al. Preterm birth and subsequent risk of acute childhood leukemia: a meta-analysis of observational studies. Cell Physiol Biochem . 2016;39:1229–38. doi: 10.1159/000447828. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y-F, Wu L-Q, Liu Y-N, et al. Gestational age and childhood leukemia: a meta-analysis of epidemiologic studies. Hematology. 2018;23:253–62. doi: 10.1080/10245332.2017.1396056. [DOI] [PubMed] [Google Scholar]
- 123.Paltiel O, Tikellis G, Linet M, et al. Birthweight and childhood cancer: preliminary findings from the International childhood cancer cohort consortium (I4C) Paediatr Perinat Epidemiol. 2015;29:335–45. doi: 10.1111/ppe.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009;124:2658–70. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 125.Hjalgrim LL, Westergaard T, Rostgaard K, et al. Birth weight as a risk factor for childhood leukemia: a meta-analysis of 18 epidemiologic studies. Am J Epidemiol. 2003;158:724–35. doi: 10.1093/aje/kwg210. [DOI] [PubMed] [Google Scholar]
- 126.Milne E, Greenop KR, Metayer C, et al. Fetal growth and childhood acute Lymphoblastic leukemia: findings from the childhood leukemia international consortium. Int J Cancer. 2013;133:n. doi: 10.1002/ijc.28314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Panagopoulou P, Skalkidou A, Marcotte E, et al. Parental age and the risk of childhood acute myeloid leukemia: results from the childhood leukemia international consortium. Cancer Epidemiol. 2019;59:158–65. doi: 10.1016/j.canep.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Papadopoulou C, Antonopoulos CN, Sergentanis TN, et al. Is birth weight associated with childhood lymphoma? A meta-analysis. Int J Cancer. 2012;130:179–89. doi: 10.1002/ijc.26001. [DOI] [PubMed] [Google Scholar]
- 129.Chu A, Heck JE, Ribeiro KB, et al. Wilms' tumour: a systematic review of risk factors and meta-analysis. Paediatr Perinat Epidemiol. 2010;24:449–69. doi: 10.1111/j.1365-3016.2010.01133.x. [DOI] [PubMed] [Google Scholar]
- 130.Michos A, Xue F, Michels KB. Birth weight and the risk of testicular cancer: a meta-analysis. Int J Cancer. 2007;121:1123–31. doi: 10.1002/ijc.22771. [DOI] [PubMed] [Google Scholar]
- 131.Chen S, Yang L, Pu F, et al. High birth weight increases the risk for bone tumor: a systematic review and meta-analysis. Int J Environ Res Public Health. 2015;12:11178–95. doi: 10.3390/ijerph120911178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liao L, Deng Y, Zhao D. Association of low birth weight and premature birth with the risk of metabolic syndrome: a meta-analysis. Front Pediatr. 2020;8:405. doi: 10.3389/fped.2020.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sømhovd MJ, Hansen BM, Brok J, et al. Anxiety in adolescents born preterm or with very low birthweight: a meta-analysis of case-control studies. Dev Med Child Neurol. 2012;54:988–94. doi: 10.1111/j.1469-8749.2012.04407.x. [DOI] [PubMed] [Google Scholar]
- 134.Fitzallen GC, Sagar YK, Taylor HG, et al. Anxiety and depressive disorders in children born preterm: a meta-analysis. J Dev Behav Pediatr. 2021;42:154–62. doi: 10.1097/DBP.0000000000000898. [DOI] [PubMed] [Google Scholar]
- 135.Burnett AC, Anderson PJ, Cheong J, et al. Prevalence of psychiatric diagnoses in preterm and full-term children, adolescents and young adults: a meta-analysis. Psychol Med. 2011;41:2463–74. doi: 10.1017/S003329171100081X. [DOI] [PubMed] [Google Scholar]
- 136.Su Y, D’Arcy C, Meng X. Research review: developmental origins of depression - a systematic review and meta-analysis. J Child Psychol Psychiatry . 2021;62:1050–66. doi: 10.1111/jcpp.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wojcik W, Lee W, Colman I, et al. Foetal origins of depression? A systematic review and meta-analysis of low birth weight and later depression. Psychol Med. 2013;43:1–12. doi: 10.1017/S0033291712000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, et al. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–28. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 139.Cassiano RGM, Provenzi L, Linhares MBM, et al. Does Preterm birth affect child temperament? A meta-analytic study. Infant Behav Dev. 2020;58:101417. doi: 10.1016/j.infbeh.2019.101417. [DOI] [PubMed] [Google Scholar]
- 140.Mathewson KJ, Chow CHT, Dobson KG, et al. Mental health of extremely low birth weight survivors: a systematic review and meta-analysis. Psychol Bull. 2017;143:347–83. doi: 10.1037/bul0000091. [DOI] [PubMed] [Google Scholar]
- 141.Allotey J, Zamora J, Cheong-See F, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG. 2018;125:16–25. doi: 10.1111/1471-0528.14832. [DOI] [PubMed] [Google Scholar]
- 142.Chen J, Chen P, Bo T, et al. Cognitive and behavioral outcomes of intrauterine growth restriction school-age children. Pediatrics. 2016;137 doi: 10.1542/peds.2015-3868. [DOI] [PubMed] [Google Scholar]
- 143.Edwards MO, Kotecha SJ, Lowe J, et al. Effect of preterm birth on exercise capacity: a systematic review and meta-analysis. Pediatr Pulmonol. 2015;50:293–301. doi: 10.1002/ppul.23117. [DOI] [PubMed] [Google Scholar]
- 144.Andersen LG, Angquist L, Gamborg M, et al. Birth weight in relation to leisure time physical activity in adolescence and adulthood: meta-analysis of results from 13 Nordic cohorts. PLoS One. 2009;4:e8192. doi: 10.1371/journal.pone.0008192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Øglund GP, Hildebrand M, Ekelund U. Are birth weight, early growth, and motor development determinants of physical activity in children and youth? A systematic review and meta-analysis. Pediatr Exerc Sci. 2015;27:441–53. doi: 10.1123/pes.2015-0041. [DOI] [PubMed] [Google Scholar]
- 146.Arpi E, D’Amico R, Lucaccioni L, et al. Worse global intellectual and worse neuropsychological functioning in preterm-born children at preschool age: a meta-analysis. Acta Paediatr. 2019;108:1567–79. doi: 10.1111/apa.14836. [DOI] [PubMed] [Google Scholar]
- 147.Burstein O, Zevin Z, Geva R. Preterm birth and the development of visual attention during the first 2 years of life: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:e213687. doi: 10.1001/jamanetworkopen.2021.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mulder H, Pitchford NJ, Hagger MS, et al. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34:393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- 149.Momany AM, Kamradt JM, Nikolas MA. A meta-analysis of the association between birth weight and attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2018;46:1409–26. doi: 10.1007/s10802-017-0371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Franz AP, Bolat GU, Bolat H, et al. Attention-deficit/hyperactivity disorder and very preterm/very low birth weight: a meta-analysis. Pediatrics. 2018;141:e20171645. doi: 10.1542/peds.2017-1645. [DOI] [PubMed] [Google Scholar]
- 151.Bhutta AT, Cleves MA, Casey PH, et al. Cognitive and behavioral outcomes of school-aged children who were born Preterm: a meta-analysis. JAMA. 2002;288:728–37. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 152.Wang C, Geng H, Liu W, et al. Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Medicine (Baltimore) 2017;96:e6696. doi: 10.1097/MD.0000000000006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jenabi E, Bashirian S, Asali Z, et al. Association between small for gestational age and risk of autism spectrum disorders: a meta-analysis. Clin Exp Pediatr . 2021;64:538–42. doi: 10.3345/cep.2020.01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Orri M, Gunnell D, Richard-Devantoy S, et al. In-utero and perinatal influences on suicide risk: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:477–92. doi: 10.1016/S2215-0366(19)30077-X. [DOI] [PubMed] [Google Scholar]
- 155.Johnson MJ, Wootton SA, Leaf AA, et al. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics. 2012;130:e640–9. doi: 10.1542/peds.2011-3379. [DOI] [PubMed] [Google Scholar]
- 156.Ou-Yang M-C, Sun Y, Liebowitz M, et al. Accelerated weight gain, Prematurity, and the risk of childhood obesity: a meta-analysis and systematic review. PLoS One. 2020;15:e0232238. doi: 10.1371/journal.pone.0232238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Goto E. Maternal and cord blood adiponectin concentrations in small for gestational age: a meta-analysis. Ann Nutr Metab . 2018;72:57–64. doi: 10.1159/000485748. [DOI] [PubMed] [Google Scholar]
- 158.Young A, Brown LK, Ennis S, et al. Total body water in full-term and Preterm newborns: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2021;106:542–8. doi: 10.1136/archdischild-2020-321112. [DOI] [PubMed] [Google Scholar]
- 159.Martínez-Mesa J, Restrepo-Méndez MC, González DA, et al. Life-course evidence of birth weight effects on bone mass: systematic review and meta-analysis. Osteoporos Int. 2013;24:7–18. doi: 10.1007/s00198-012-2114-7. [DOI] [PubMed] [Google Scholar]
- 160.Christian P, Lee SE, Donahue Angel M, et al. Risk of childhood Undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol. 2013;42:1340–55. doi: 10.1093/ije/dyt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schellong K, Schulz S, Harder T, et al. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS One. 2012;7:e47776. doi: 10.1371/journal.pone.0047776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu ZB, Han SP, Zhu GZ, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev . 2011;12:525–42. doi: 10.1111/j.1467-789X.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 163.Druet C, Stettler N, Sharp S, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26:19–26. doi: 10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 164.de Kieviet JF, Zoetebier L, van Elburg RM, et al. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 2012;54:313–23. doi: 10.1111/j.1469-8749.2011.04216.x. [DOI] [PubMed] [Google Scholar]
- 165.Li K, Sun Z, Han Y, et al. Fractional anisotropy alterations in individuals born preterm: a diffusion tensor imaging meta-analysis. Dev Med Child Neurol. 2015;57:328–38. doi: 10.1111/dmcn.12618. [DOI] [PubMed] [Google Scholar]
- 166.Stipdonk LW, Weisglas-Kuperus N, Franken M-CJ, et al. Auditory brainstem maturation in normal-hearing infants born Preterm: a meta-analysis. Dev Med Child Neurol. 2016;58:1009–15. doi: 10.1111/dmcn.13151. [DOI] [PubMed] [Google Scholar]
- 167.Zhou L, Zhao Y, Liu X, et al. Brain gray and white matter abnormalities in Preterm-born adolescents: a meta-analysis of Voxel-based morphometry studies. PLoS ONE. 2018;13:e0203498. doi: 10.1371/journal.pone.0203498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Geldof CJA, van Wassenaer AG, de Kieviet JF, et al. Visual perception and visual-motor integration in very Preterm and/or very low birth weight children: a meta-analysis. Res Dev Disabil . 2012;33:726–36. doi: 10.1016/j.ridd.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 169.Rudnicka AR, Owen CG, Richards M, et al. Effect of breastfeeding and sociodemographic factors on visual outcome in childhood and adolescence. Am J Clin Nutr. 2008;87:1392–9. doi: 10.1093/ajcn/87.5.1392. [DOI] [PubMed] [Google Scholar]
- 170.Himpens E, Van den Broeck C, Oostra A, et al. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev Med Child Neurol. 2008;50:334–40. doi: 10.1111/j.1469-8749.2008.02047.x. [DOI] [PubMed] [Google Scholar]
- 171.Oskoui M, Coutinho F, Dykeman J, et al. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013;55:509–19. doi: 10.1111/dmcn.12080. [DOI] [PubMed] [Google Scholar]
- 172.Zhao M, Dai H, Deng Y, et al. SGA as a risk factor for cerebral palsy in moderate to late Preterm infants: a system review and meta-analysis. Sci Rep. 2016;6:38853. doi: 10.1038/srep38853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dong Y, Chen S, Yu J. A systematic review and meta-analysis of long-term development of early term infants. Neonatology. 2012;102:212–21. doi: 10.1159/000338099. [DOI] [PubMed] [Google Scholar]
- 174.Sania A, Sudfeld CR, Danaei G, et al. Early life risk factors of motor, cognitive and language development: a pooled analysis of studies from low/middle-income countries. BMJ Open. 2019;9:e026449. doi: 10.1136/bmjopen-2018-026449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Kieviet JF, Piek JP, Aarnoudse-Moens CS, et al. Motor development in very preterm and very low-birth-weight children from birth to adolescence: a meta-analysis. JAMA. 2009;302:2235–42. doi: 10.1001/jama.2009.1708. [DOI] [PubMed] [Google Scholar]
- 176.FitzGerald TL, Kwong AKL, Cheong JLY, et al. Body structure, function, activity and participation in 3-to 6-year-old children born very Preterm: an ICF-based systematic review and meta-analysis. Phys Ther. 2018;98:691–704. doi: 10.1093/ptj/pzy050. [DOI] [PubMed] [Google Scholar]
- 177.Upadhyay RP, Naik G, Choudhary TS, et al. Cognitive and motor outcomes in children born low birth weight: a systematic review and meta-analysis of studies from South Asia. BMC Pediatr. 2019;19:35. doi: 10.1186/s12887-019-1408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Edwards J, Berube M, Erlandson K, et al. Developmental coordination disorder in school-aged children born very preterm and/or at very low birth weight: a systematic review. J Dev Behav Pediatr. 2011;32:678–87. doi: 10.1097/DBP.0b013e31822a396a. [DOI] [PubMed] [Google Scholar]
- 179.Maitra K, Park HY, Eggenberger J, et al. Difficulty in mental, Neuromusculoskeletal, and movement-related school functions associated with low birthweight or Preterm birth: a meta-analysis. Am J Occup Ther. 2014;68:140–8. doi: 10.5014/ajot.2014.009985. [DOI] [PubMed] [Google Scholar]
- 180.Dodds R, Denison HJ, Ntani G, et al. Birth weight and muscle strength: a systematic review and meta-analysis. J Nutr Health Aging. 2012;16:609–15. doi: 10.1007/s12603-012-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brydges CR, Landes JK, Reid CL, et al. Cognitive outcomes in children and adolescents born very Preterm: a meta-analysis. Dev Med Child Neurol. 2018;60:452–68. doi: 10.1111/dmcn.13685. [DOI] [PubMed] [Google Scholar]
- 182.van Houdt CA, Oosterlaan J, van Wassenaer-Leemhuis AG, et al. Executive function deficits in children born Preterm or at low birthweight: a meta-analysis. Dev Med Child Neurol. 2019;61:1015–24. doi: 10.1111/dmcn.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chan E, Leong P, Malouf R, et al. Long-term cognitive and school outcomes of late-Preterm and early-term births: a systematic review. Child Care Health Dev . 2016;42:297–312. doi: 10.1111/cch.12320. [DOI] [PubMed] [Google Scholar]
- 184.Twilhaar ES, de Kieviet JF, Aarnoudse-Moens CS, et al. Academic performance of children born Preterm: a meta-analysis and meta-regression. Arch Dis Child Fetal Neonatal Ed. 2018;103:F322–30. doi: 10.1136/archdischild-2017-312916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kerr-Wilson CO, Mackay DF, Smith GCS, et al. Meta-analysis of the association between preterm delivery and intelligence. Journal of Public Health. 2012;34:209–16. doi: 10.1093/pubmed/fdr024. [DOI] [PubMed] [Google Scholar]
- 186.Gu H, Wang L, Liu L, et al. A gradient relationship between low birth weight and IQ: a meta-analysis. Sci Rep. 2017;7:18035. doi: 10.1038/s41598-017-18234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aylward GP, Pfeiffer SI, Wright A, et al. Outcome studies of low birth weight infants published in the last decade: a Metaanalysis. J Pediatr. 1989;115:515–20. doi: 10.1016/s0022-3476(89)80273-2. [DOI] [PubMed] [Google Scholar]
- 188.Twilhaar ES, Wade RM, de Kieviet JF, et al. Cognitive outcomes of children born extremely or very Preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 2018;172:361. doi: 10.1001/jamapediatrics.2017.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kormos CE, Wilkinson AJ, Davey CJ, et al. Low birth weight and intelligence in adolescence and early adulthood: a meta-analysis. J Public Health (Oxf) 2014;36:213–24. doi: 10.1093/pubmed/fdt071. [DOI] [PubMed] [Google Scholar]
- 190.Barre N, Morgan A, Doyle LW, et al. Language abilities in children who were very Preterm and/or very low birth weight: a meta-analysis. J Pediatr. 2011;158:766–774. doi: 10.1016/j.jpeds.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 191.Zimmerman E. Do infants born very premature and who have very low birth weight catch up with their full term peers in their language abilities by early school age. J Speech Lang Hear Res. 2018;61:53–65. doi: 10.1044/2017_JSLHR-L-16-0150. [DOI] [PubMed] [Google Scholar]
- 192.van Noort-van der Spek IL, Franken M-CJP, Weisglas-Kuperus N. Language functions in Preterm-born children: a systematic review and meta-analysis. Pediatrics. 2012;129:745–54. doi: 10.1542/peds.2011-1728. [DOI] [PubMed] [Google Scholar]
- 193.McBryde M, Fitzallen GC, Liley HG, et al. Academic outcomes of school-aged children born Preterm: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e202027. doi: 10.1001/jamanetworkopen.2020.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kovachy VN, Adams JN, Tamaresis JS, et al. Reading abilities in school-aged Preterm children: a review and meta-analysis. Dev Med Child Neurol. 2015;57:410–9. doi: 10.1111/dmcn.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Domellöf E, Johansson A-M, Rönnqvist L. Handedness in Preterm born children: a systematic review and a meta-analysis. Neuropsychologia. 2011;49:2299–310. doi: 10.1016/j.neuropsychologia.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 196.Barros FC, Huttly SRA, Victora CC, et al. Comparison of the causes and consequences of Prematurity and Intrauterine growth retardation: a longitudinal study in Southern Brazil. Pediatrics. 1992;90:238–44. doi: 10.1542/peds.90.2.238. [DOI] [PubMed] [Google Scholar]
- 197.Han Z, Lutsiv O, Mulla S, et al. Maternal height and the risk of Preterm birth and low birth weight: a systematic review and meta-analyses. J Obstet Gynaecol Can. 2012;34:721–46. doi: 10.1016/S1701-2163(16)35337-3. [DOI] [PubMed] [Google Scholar]
- 198.Shaw JC, Crombie GK, Palliser HK, et al. Impaired Oligodendrocyte development following Preterm birth: promoting GABAergic action to improve outcomes. Front Pediatr. 2021;9:618052. doi: 10.3389/fped.2021.618052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Goedicke-Fritz S, Härtel C, Krasteva-Christ G, et al. Preterm birth affects the risk of developing immune-mediated diseases. Front Immunol. 2017;8:1266. doi: 10.3389/fimmu.2017.01266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cosmi E, Fanelli T, Visentin S, et al. Consequences in infants that were Intrauterine growth restricted. J Pregnancy. 2011;2011:364381. doi: 10.1155/2011/364381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Armengaud JB, Yzydorczyk C, Siddeek B, et al. Intrauterine growth restriction: clinical consequences on health and disease at adulthood. Reprod Toxicol. 2021;99:168–76. doi: 10.1016/j.reprotox.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 202.Yegros-Yegros A, van de Klippe W, Abad-Garcia MF, et al. Exploring why global health needs are unmet by research efforts: the potential influences of geography, industry and publication incentives. Health Res Policy Syst. 2020;18:47. doi: 10.1186/s12961-020-00560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository.