Abstract
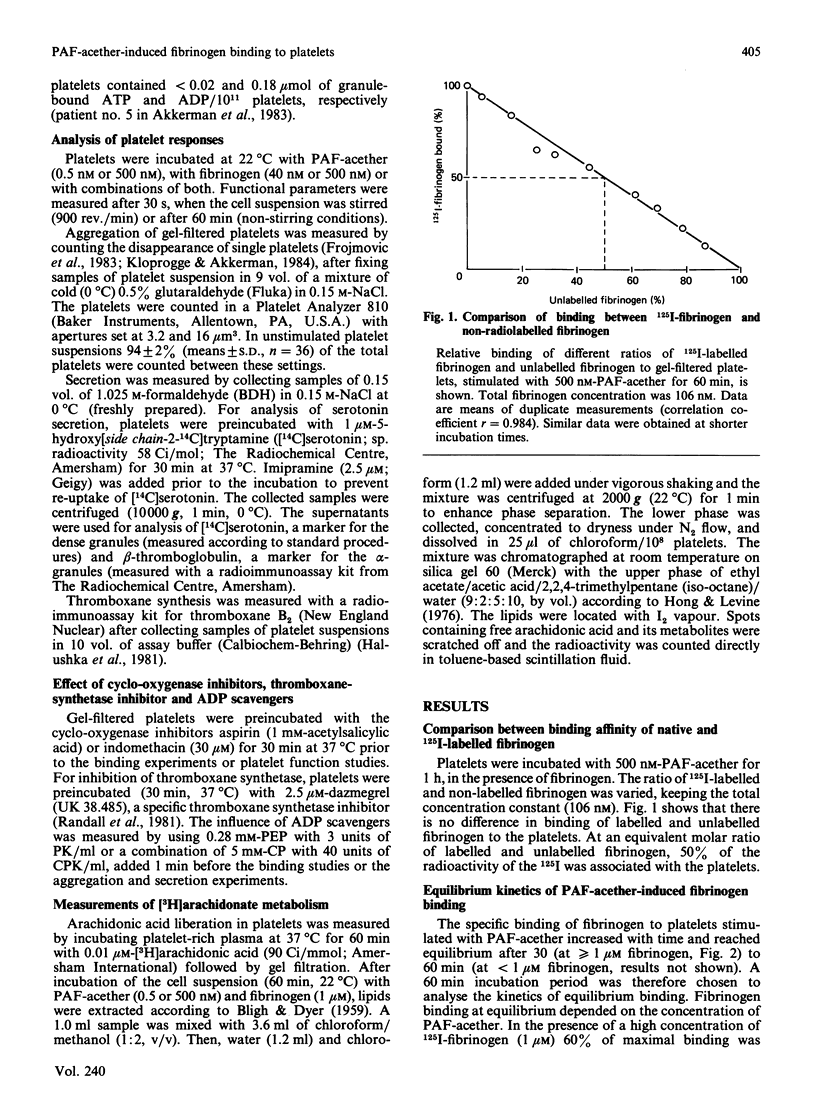
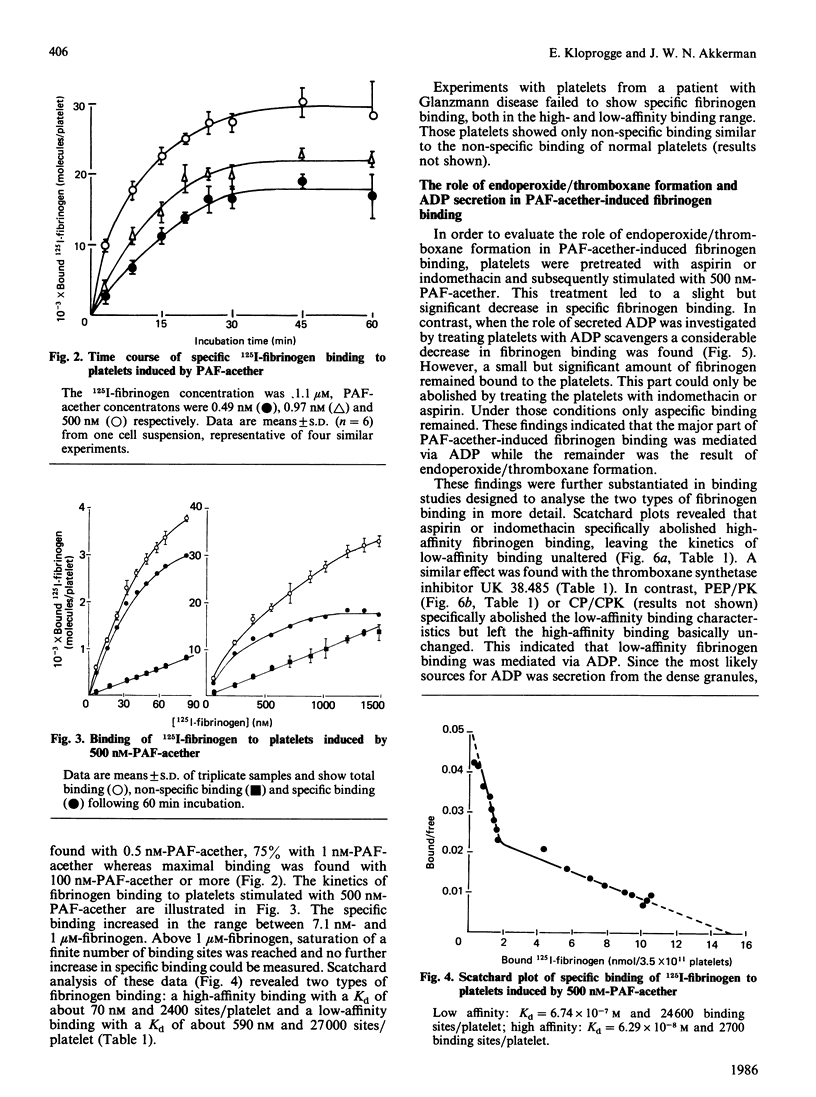
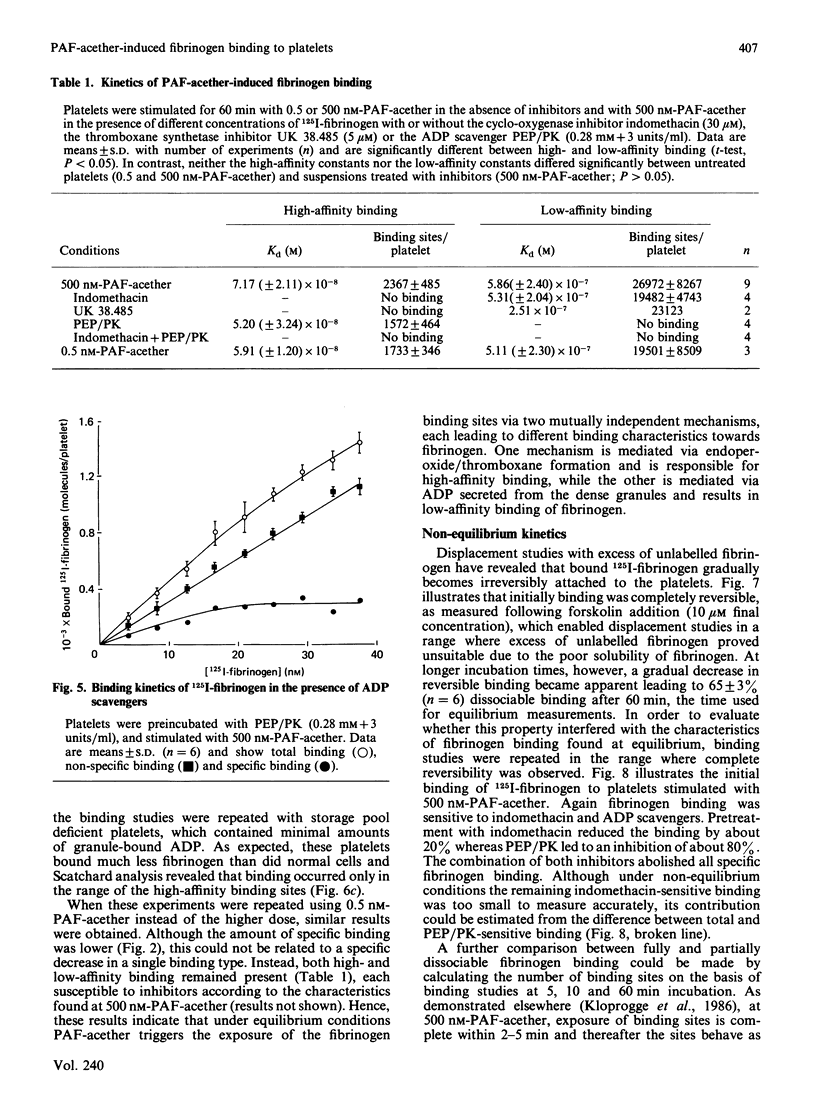
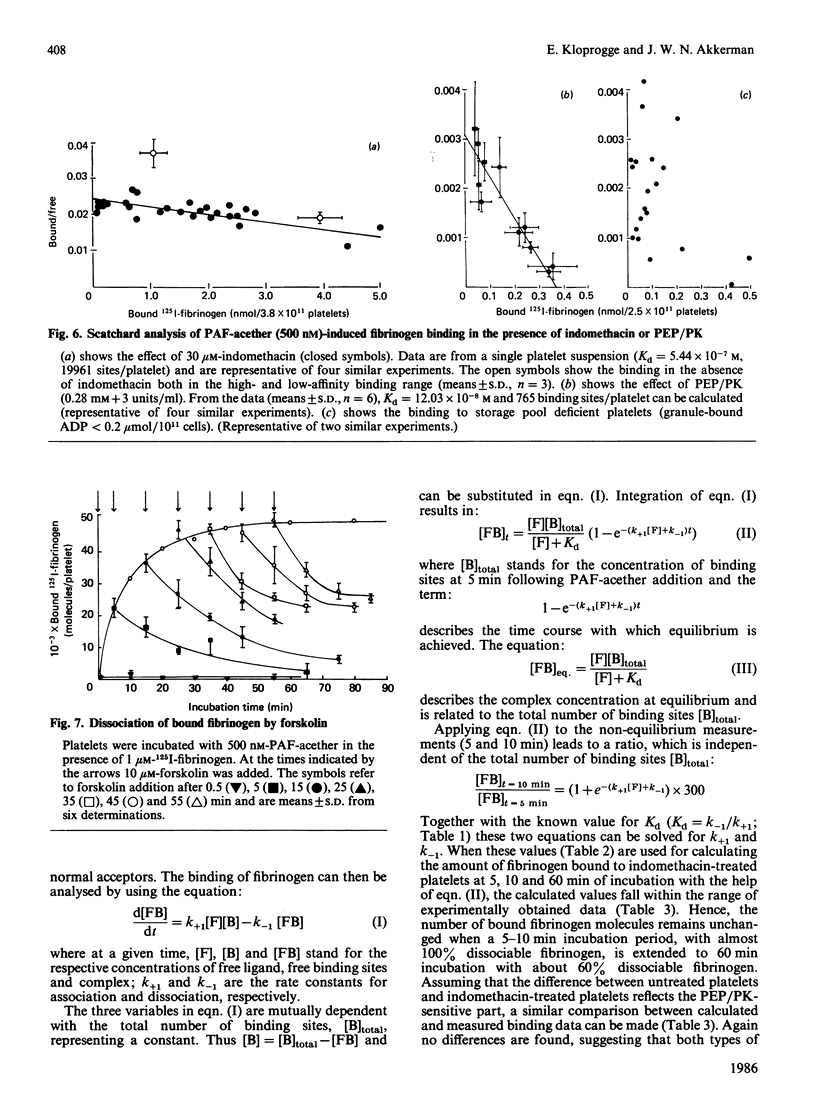
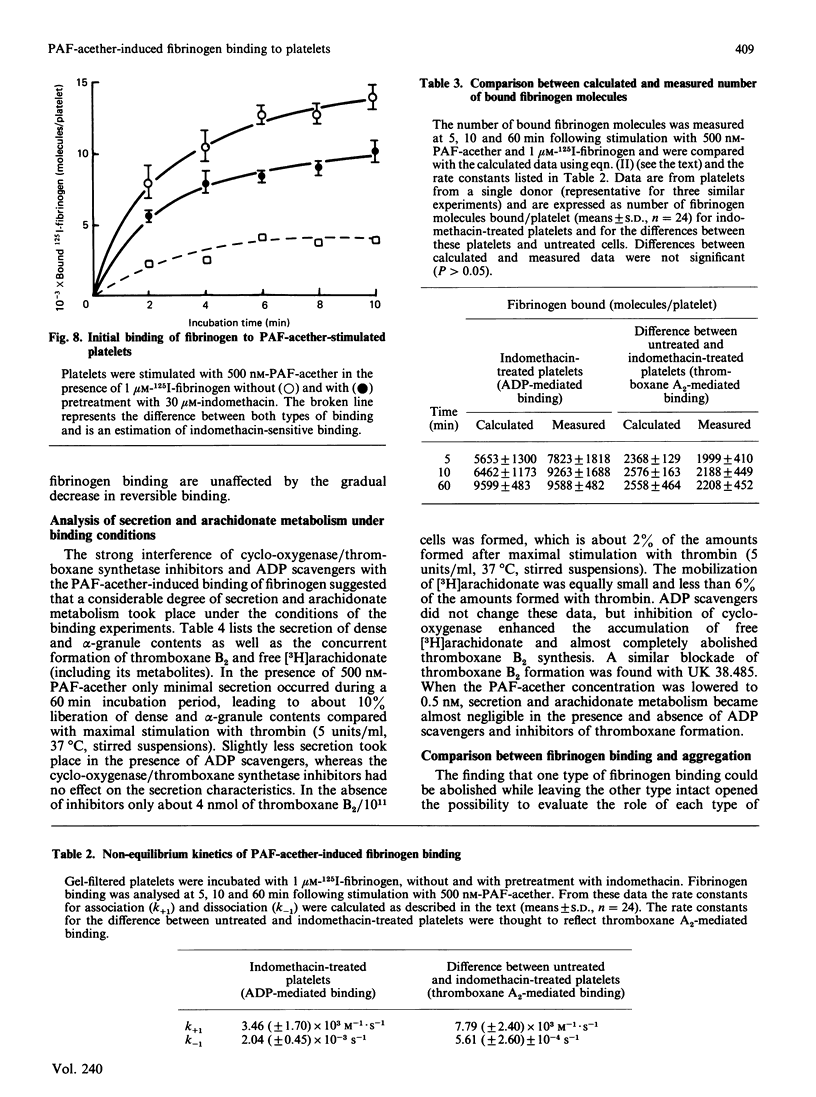
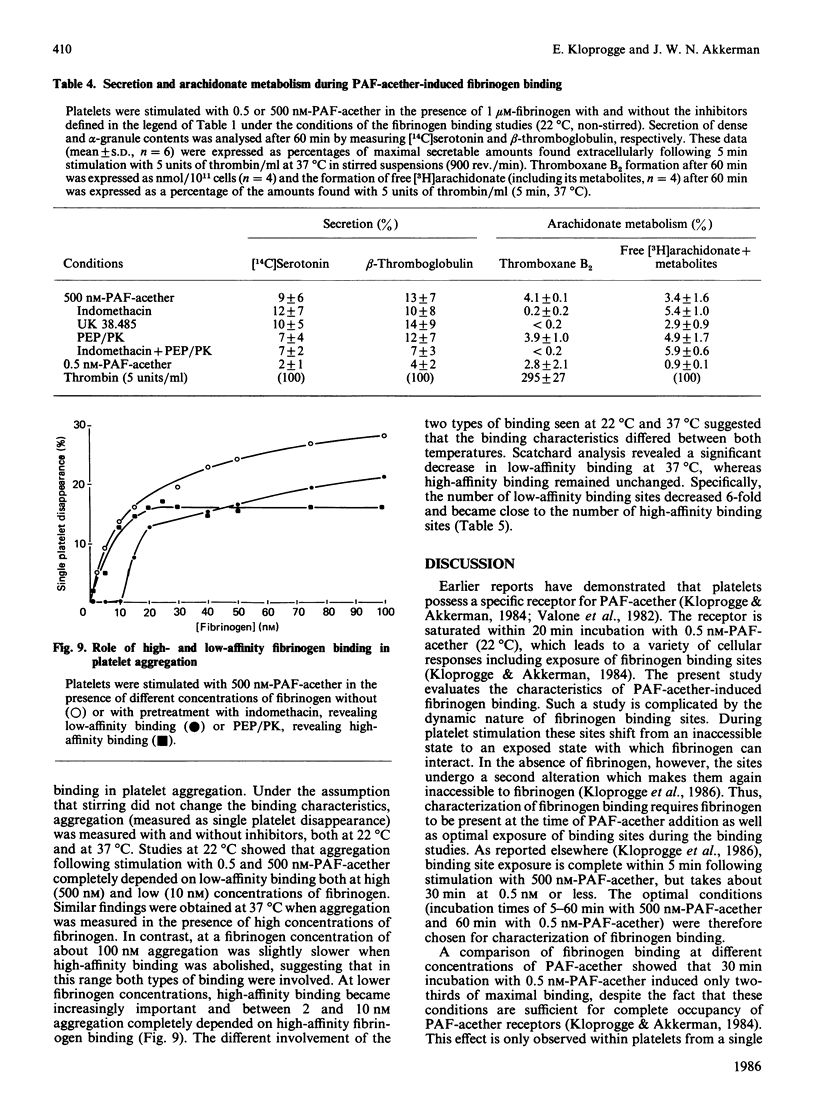
When human platelets are incubated with 500 nM-PAF-acether (platelet-activating factor. 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) under equilibrium conditions (60 min, 22 degrees C, non-stirred suspensions), two classes of fibrinogen binding sites are exposed: one class with a high affinity [Kd (7.2 +/- 2.1) X 10(-8) M, 2367 +/- 485 sites/platelet, n = 9] and one class with a low affinity [Kd (5.9 +/- 2.4) X 10(-7) M, 26972 +/- 8267 sites/platelet]. Preincubation with inhibitors of cyclo-oxygenase (acetylsalicylic acid, indomethacin) or thromboxane synthetase (UK 38.485) completely abolishes high-affinity binding, leaving low-affinity binding unchanged. In contrast, ADP scavengers (phosphocreatine/creatine kinase or phosphoenol pyruvate/pyruvate kinase) completely prevent low-affinity binding, leaving high-affinity binding unaltered. Initial binding studies (2-10 min incubation) confirm these findings with a major part of the binding being sensitive to ADP scavengers, a minor part sensitive to indomethacin and complete blockade with both inhibitors. Increasing the temperature to 37 degrees C decreases the number of low affinity-binding sites 6-fold without changing high-affinity binding. Aggregation, measured as the rate of single platelet disappearance, then depends on high-affinity binding at 10 nM-fibrinogen or less, whereas at 100 nM-fibrinogen or more low-affinity binding becomes predominant. These findings point at considerable platelet activation during binding experiments. However, arachidonate metabolism [( 3H]arachidonate mobilization and thromboxane synthesis) and secretion [( 14C]serotonin and beta-thromboglobulin) are about 10% or less of the amounts found under optimal conditions (5 units of thrombin/ml 37 degrees C, stirring). We conclude that PAF-acether induces little platelet activation under binding conditions. The amounts of thromboxane A2 and secreted ADP, however, are sufficient for initiating high- and low-affinity fibrinogen binding via mutually independent mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akkerman J. W., Nieuwenhuis H. K., Mommersteeg-Leautaud M. E., Gorter G., Sixma J. J. ATP-ADP compartmentation in storage pool deficient platelets: correlation between granule-bound ADP and the bleeding time. Br J Haematol. 1983 Sep;55(1):135–143. doi: 10.1111/j.1365-2141.1983.tb01231.x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G., Burch J. W. A role for prostaglandins and thromboxanes in the exposure of platelet fibrinogen receptors. J Clin Invest. 1981 Oct;68(4):981–987. doi: 10.1172/JCI110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka J. Enzyme immunoassay for factor VIII-related antigen. Clin Chem. 1982 Jun;28(6):1356–1358. [PubMed] [Google Scholar]
- Chesney C. M., Pifer D. D., Byers L. W., Muirhead E. E. Effect of platelet-activating factor (PAF) on human platelets. Blood. 1982 Mar;59(3):582–585. [PubMed] [Google Scholar]
- Cox C. P., Wardlow M. L., Jorgensen R., Farr R. S. The presence of platelet-activating factor (PAF) in normal human mixed saliva. J Immunol. 1981 Jul;127(1):46–50. [PubMed] [Google Scholar]
- Di Minno G., Thiagarajan P., Perussia B., Martinez J., Shapiro S., Trinchieri G., Murphy S. Exposure of platelet fibrinogen-binding sites by collagen, arachidonic acid, and ADP: inhibition by a monoclonal antibody to the glycoprotein IIb-IIIa complex. Blood. 1983 Jan;61(1):140–148. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G., Duchastel A. Microscopic measurements of platelet aggregation reveal a low ADP-dependent process distinct from turbidometrically measured aggregation. J Lab Clin Med. 1983 Jun;101(6):964–976. [PubMed] [Google Scholar]
- Fujimura K., Phillips D. R. Calcium cation regulation of glycoprotein IIb-IIIa complex formation in platelet plasma membranes. J Biol Chem. 1983 Sep 10;258(17):10247–10252. [PubMed] [Google Scholar]
- Gogstad G. O., Brosstad F., Krutnes M. B., Hagen I., Solum N. O. Fibrinogen-binding properties of the human platelet glycoprotein IIb-=IIIa complex: a study using crossed-radioimmunoelectrophoresis. Blood. 1982 Sep;60(3):663–671. [PubMed] [Google Scholar]
- Goldstein B. M., Gabel R. A., Huggins F. J., Cervoni P., Crandall D. L. Effect of platelet activating factor (PAF) on blood flow distribution in the spontaneously hypertensive rat. Life Sci. 1984 Sep 24;35(13):1373–1378. doi: 10.1016/0024-3205(84)90394-1. [DOI] [PubMed] [Google Scholar]
- Halushka P. V., Rogers R. C., Loadholt C. B., Colwell J. A. Increased platelet thromboxane synthesis in diabetes mellitus. J Lab Clin Med. 1981 Jan;97(1):87–96. [PubMed] [Google Scholar]
- Handley D. A., Van Valen R. G., Melden M. K., Saunders R. N. Evaluation of dose and route effects of platelet activating factor-induced extravasation in the guinea pig. Thromb Haemost. 1984 Aug 31;52(1):34–36. [PubMed] [Google Scholar]
- Harfenist E. J., Guccione M. A., Packham M. A., Kinlough-Rathbone R. L., Mustard J. F. Arachidonate-induced fibrinogen binding to thrombin-degranulated rabbit platelets is independent of released ADP. Blood. 1982 May;59(5):956–962. [PubMed] [Google Scholar]
- Hong S. L., Levine L. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proc Natl Acad Sci U S A. 1976 May;73(5):1730–1734. doi: 10.1073/pnas.73.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloprogge E., Akkerman J. W. Binding kinetics of PAF-acether (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) to intact human platelets. Biochem J. 1984 Nov 1;223(3):901–909. doi: 10.1042/bj2230901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloprogge E., Mommersteeg M., Akkerman J. W. Kinetics of platelet-activating factor 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine-induced fibrinogen binding to human platelets. J Biol Chem. 1986 Aug 25;261(24):11071–11076. [PubMed] [Google Scholar]
- Kloprogge E., de Haas G. H., Gorter G., Akkerman J. W. Properties of PAF-acether-induced platelet aggregation and secretion. Studies in gel-filtered human platelets. Thromb Res. 1983 Mar 15;29(6):595–608. doi: 10.1016/0049-3848(83)90214-1. [DOI] [PubMed] [Google Scholar]
- Kornecki E., Niewiarowski S., Morinelli T. A., Kloczewiak M. Effects of chymotrypsin and adenosine diphosphate on the exposure of fibrinogen receptors on normal human and Glanzmann's thrombasthenic platelets. J Biol Chem. 1981 Jun 10;256(11):5696–5701. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marguerie G. A., Edgington T. S., Plow E. F. Interaction of fibrinogen with its platelet receptor as part of a multistep reaction in ADP-induced platelet aggregation. J Biol Chem. 1980 Jan 10;255(1):154–161. [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F. The fibrinogen-dependent pathway of platelet aggregation. Ann N Y Acad Sci. 1983 Jun 27;408:556–566. doi: 10.1111/j.1749-6632.1983.tb23272.x. [DOI] [PubMed] [Google Scholar]
- Morinelli T. A., Niewiarowski S., Kornecki E., Figures W. R., Wachtfogel Y., Colman R. W. Platelet aggregation and exposure of fibrinogen receptors by prostaglandin endoperoxide analogues. Blood. 1983 Jan;61(1):41–49. [PubMed] [Google Scholar]
- Nieuwenhuizen W., Emeis J. J., Vermond A., Kurver P., van der Heide D. Studies on the catabolism and distribution of fibrinogen in rats. Application of the Iodogen labelling technique. Biochem Biophys Res Commun. 1980 Nov 17;97(1):49–55. doi: 10.1016/s0006-291x(80)80132-x. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I. Effect of epinephrine on fibrinogen receptor exposure by aspirin-treated platelets and platelets from concentrates in response to ADP and thrombin. Am J Hematol. 1984 May;16(4):335–345. doi: 10.1002/ajh.2830160404. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I. Induction of human platelet fibrinogen receptors by epinephrine in the absence of released ADP. Blood. 1982 Jul;60(1):71–77. [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B., Grant R. A., Egan J. J., Johnson M. M. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood. 1980 May;55(5):841–847. [PubMed] [Google Scholar]
- Pieroni G., Hanahan D. J. Metabolic behavior of acetyl glyceryl ether phosphorylcholine on interaction with rabbit platelets. Arch Biochem Biophys. 1983 Jul 15;224(2):485–493. doi: 10.1016/0003-9861(83)90236-9. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall M. J., Parry M. J., Hawkeswood E., Cross P. E., Dickinson R. P. UK-37, 248, a novel, selective thromboxane synthetase inhibitor with platelet anti-aggregatory and anti-thrombotic activity. Thromb Res. 1981 Jul 1;23(1-2):145–162. doi: 10.1016/0049-3848(81)90247-4. [DOI] [PubMed] [Google Scholar]
- Valone F. H., Coles E., Reinhold V. R., Goetzl E. J. Specific binding of phospholipid platelet-activating factor by human platelets. J Immunol. 1982 Oct;129(4):1637–1641. [PubMed] [Google Scholar]
- Vargaftig B. B., Chignard M., Benveniste J., Lefort J., Wal F. Background and present status of research on platelet-activating factor (PAF-acether). Ann N Y Acad Sci. 1981;370:119–137. doi: 10.1111/j.1749-6632.1981.tb29727.x. [DOI] [PubMed] [Google Scholar]
- Walsh P. N. Albumin density gradient separation and washing of platelets and the study of platelet coagulant activities. Br J Haematol. 1972 Feb;22(2):205–217. doi: 10.1111/j.1365-2141.1972.tb08801.x. [DOI] [PubMed] [Google Scholar]


