Abstract
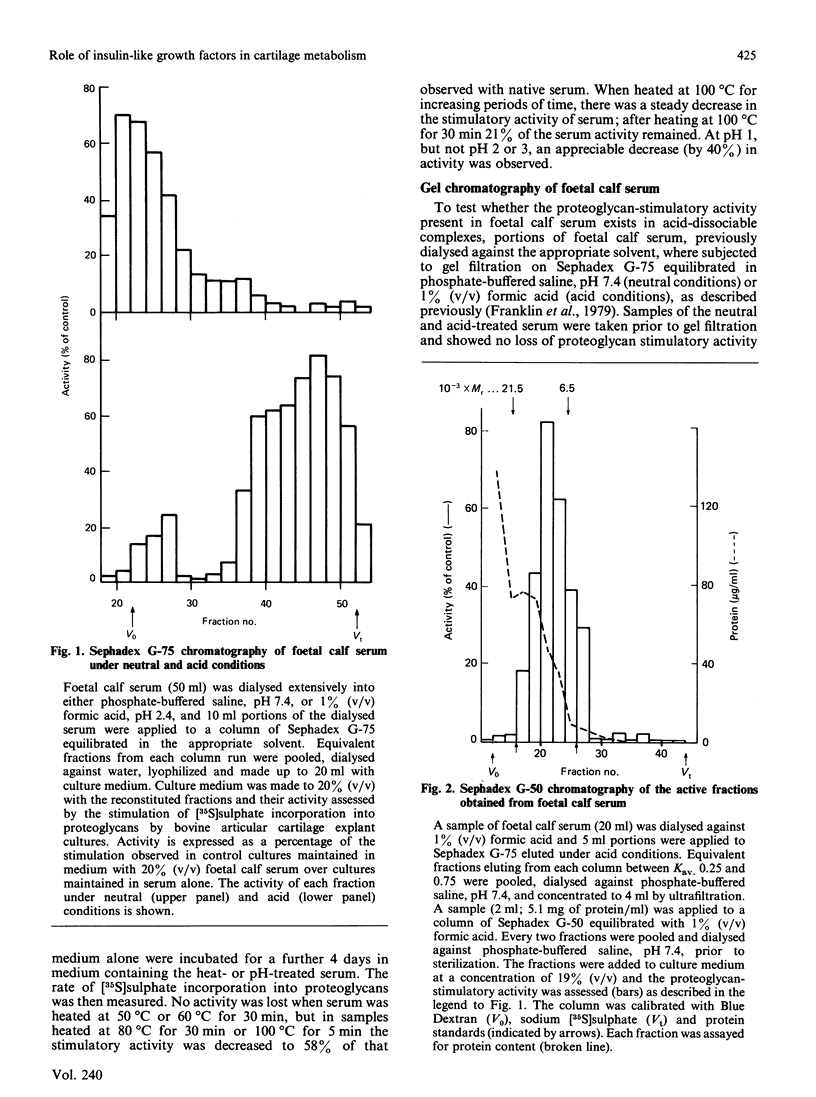
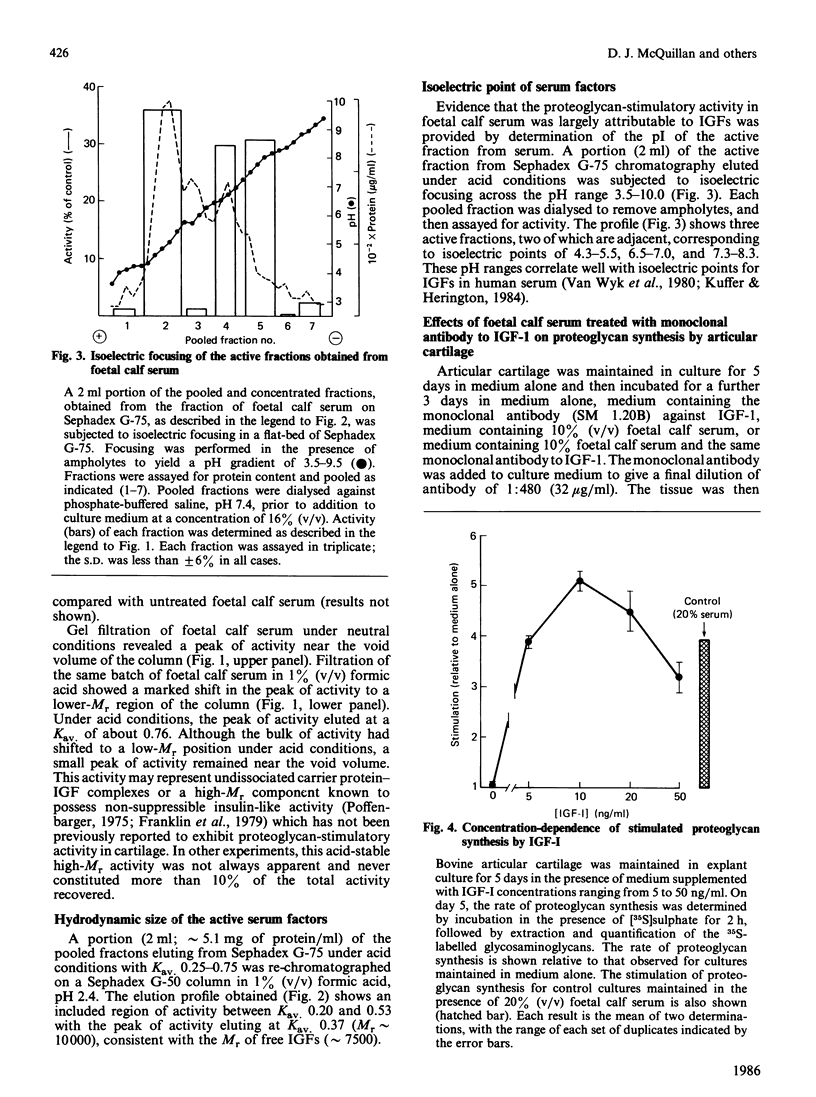
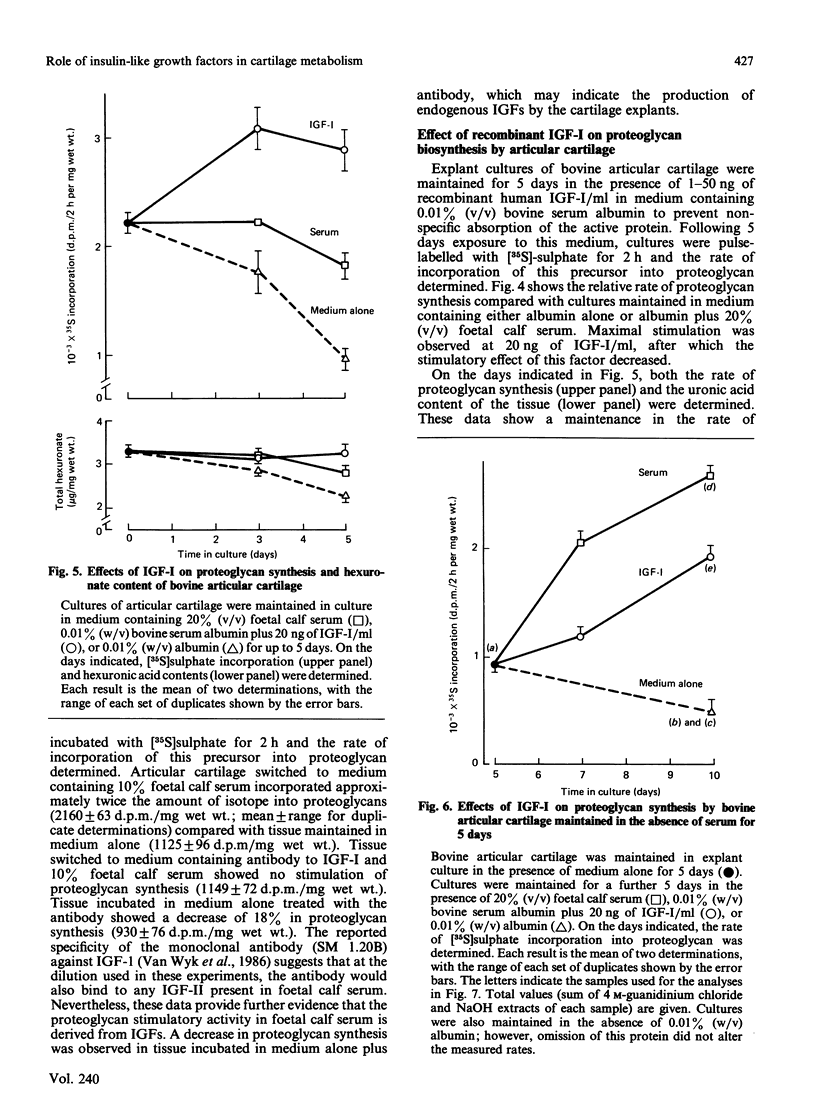
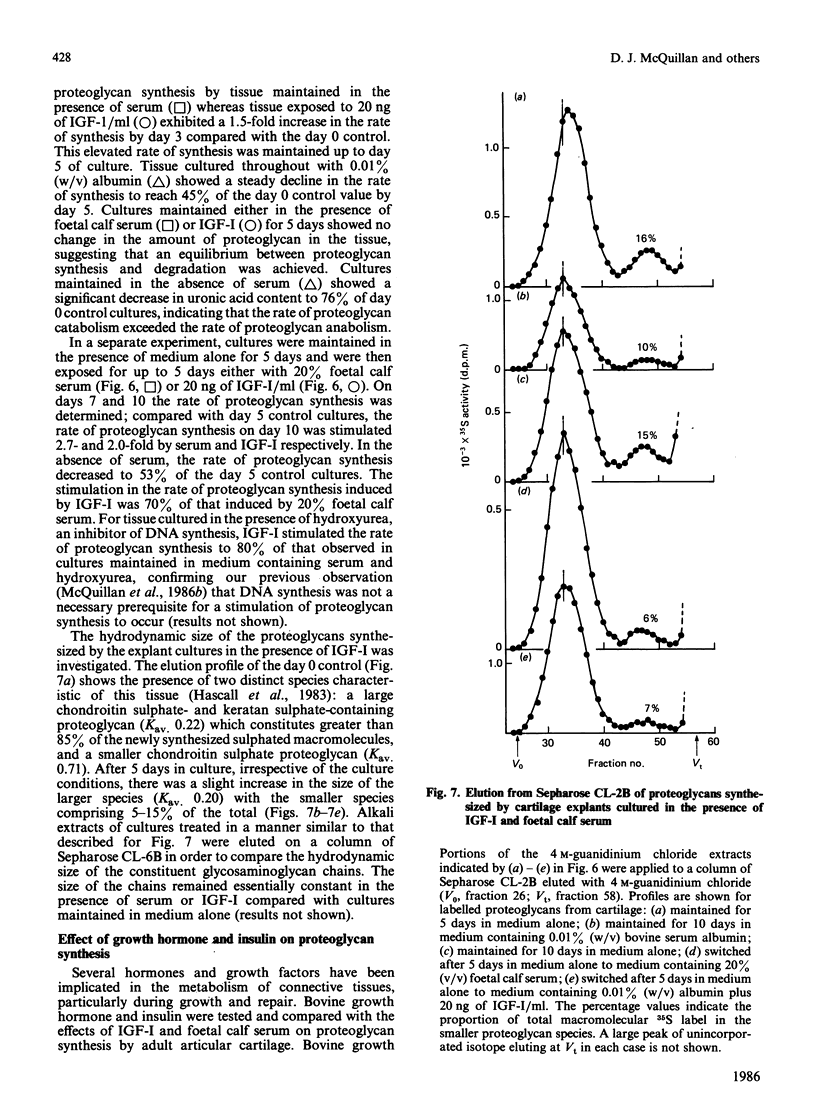
The addition of foetal calf serum to explant cultures of adult bovine articular cartilage is known to stimulate proteoglycan synthesis in a dose-dependent manner. We have now shown the activity in serum responsible for this effect to be heat- and acid-stable, to be associated with a high-Mr complex in normal serum but converted to a low-Mr form under acid conditions. The activity has an apparent Mr approximately 10,000 and isoelectric points similar to those reported for insulin-like growth factors (IGFs). Addition of a monoclonal antibody against insulin-like growth factor-I (IGF-I) prevented foetal calf serum from stimulating proteoglycan synthesis. Physiological concentrations of recombinant IGF-I or pharmacological levels of insulin when added to cartilage cultures mimicked the proteoglycan-stimulatory activity of serum. IGF-I appeared to act by increasing the rate of proteoglycan synthesis and did not change the nature of the proteoglycan synthesized nor the rate of proteoglycan catabolism by the tissue, suggesting that IGF-I may be important in the regulation of proteoglycan metabolism in adult articular cartilage. Furthermore, IGF-I can replace foetal calf serum in the culture medium, thereby allowing the use of a fully-defined medium which will maintain the synthesis and tissue levels of proteoglycan in adult articular cartilage explants for up to 5 days.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Wong S. S., Knowles S. E., Partridge N. C., Martin T. J., Wood C. M., Gunn J. M. Insulin inhibition of protein degradation in cell monolayers. J Cell Physiol. 1980 Nov;105(2):335–346. doi: 10.1002/jcp.1041050216. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell M. A., Handley C. J., Hascall V. C., Campbell R. A., Lowther D. A. Turnover of proteoglycans in cultures of bovine articular cartilage. Arch Biochem Biophys. 1984 Oct;234(1):275–289. doi: 10.1016/0003-9861(84)90350-3. [DOI] [PubMed] [Google Scholar]
- Franklin R. C., Cameron D. P., Burger H. G., Herington A. C. The occurrence of a distinct high molecular weight form of serum non-suppressible insulin-like activity. Mol Cell Endocrinol. 1979 Nov;16(2):81–89. doi: 10.1016/0303-7207(79)90106-0. [DOI] [PubMed] [Google Scholar]
- Hajek A. S., Solursh M. Stimulation of growth and mucopolysaccharide synthesis by insulin treatment of chick embryo chondrocytes in cell culture. Gen Comp Endocrinol. 1975 Apr;25(4):432–446. doi: 10.1016/0016-6480(75)90154-9. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A. Extracellular matrix metabolism by chondrocytes. III. Modulation of proteoglycan synthesis by extracellular levels of proteoglycan in cartilage cells in culture. Biochim Biophys Acta. 1977 Nov 7;500(1):132–139. doi: 10.1016/0304-4165(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A., McQuillan D. J. The structure and synthesis of proteoglycans of articular cartilage. Cell Biol Int Rep. 1985 Sep;9(9):753–782. doi: 10.1016/0309-1651(85)90095-5. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Handley C. J., McQuillan D. J., Hascall G. K., Robinson H. C., Lowther D. A. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983 Jul 1;224(1):206–223. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Kimura J. H. Proteoglycans: isolation and characterization. Methods Enzymol. 1982;82(Pt A):769–800. doi: 10.1016/0076-6879(82)82102-2. [DOI] [PubMed] [Google Scholar]
- Herington A. C., Cornell H. J., Kuffer A. D. Recent advances in the biochemistry and physiology of the insulin-like growth factor/somatomedin family. Int J Biochem. 1983;15(10):1201–1210. doi: 10.1016/0020-711x(83)90208-2. [DOI] [PubMed] [Google Scholar]
- Honegger A., Humbel R. E. Insulin-like growth factors I and II in fetal and adult bovine serum. Purification, primary structures, and immunological cross-reactivities. J Biol Chem. 1986 Jan 15;261(2):569–575. [PubMed] [Google Scholar]
- Kemp S. F., Mutchnick M., Hintz R. L. Hormonal control of protein synthesis in chick chondrocytes: a comparison of effects of insulin, somatomedin C and triiodothyronine. Acta Endocrinol (Copenh) 1984 Oct;107(2):179–184. doi: 10.1530/acta.0.1070179. [DOI] [PubMed] [Google Scholar]
- Kuffer A. D., Herington A. C. Proteolytic conversion of insulin-like growth factors to an acidic form(s). Biochem J. 1984 Oct 1;223(1):97–103. doi: 10.1042/bj2230097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Robinson H. C. Control of proteoglycan biosynthesis. Further studies on the effect of serum on cultured bovine articular cartilage. Biochem J. 1986 Aug 1;237(3):741–747. doi: 10.1042/bj2370741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Robinson H. C., Ng K., Tzaicos C., Brooks P. R., Lowther D. A. The relation of protein synthesis to chondroitin sulphate biosynthesis in cultured bovine cartilage. Biochem J. 1984 Dec 15;224(3):977–988. doi: 10.1042/bj2240977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Robinson H. C., Ng K., Tzaicos C. The relation of RNA synthesis to chondroitin sulphate biosynthesis in cultured bovine cartilage. Biochem J. 1986 Apr 15;235(2):499–505. doi: 10.1042/bj2350499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poffenbarger P. L. The purification and partial characterization of an insulin-like protein from human serum. J Clin Invest. 1975 Dec;56(6):1455–1463. doi: 10.1172/JCI108226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. II. Preparative isoelectric focusing. Biochim Biophys Acta. 1975 Mar 28;386(1):181–195. doi: 10.1016/0005-2795(75)90258-5. [DOI] [PubMed] [Google Scholar]
- Smith G. L. Somatomedin carrier proteins. Mol Cell Endocrinol. 1984 Feb;34(2):83–89. doi: 10.1016/0303-7207(84)90058-3. [DOI] [PubMed] [Google Scholar]
- Trippel S. B., Van Wyk J. J., Foster M. B., Svoboda M. E. Characterization of a specific somatomedin-c receptor on isolated bovine growth plate chondrocytes. Endocrinology. 1983 Jun;112(6):2128–2136. doi: 10.1210/endo-112-6-2128. [DOI] [PubMed] [Google Scholar]
- Van Wyk J. J., Svoboda M. E., Underwood L. E. Evidence from radioligand assays that somatomedin-C and insulin-like growth factor-I are similar to each other and different from other somatomedins. J Clin Endocrinol Metab. 1980 Jan;50(1):206–208. doi: 10.1210/jcem-50-1-206. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Rosenfeld R. G., Hintz R. L., Dollar L. A., Smith R. L. Characterization of a specific insulin-like growth factor-I/somatomedin-C receptor on high density, primary monolayer cultures of bovine articular chondrocytes: regulation of receptor concentration by somatomedin, insulin and growth hormone. J Endocrinol. 1985 Nov;107(2):275–283. doi: 10.1677/joe.0.1070275. [DOI] [PubMed] [Google Scholar]



