Summary
Background
Patients with small cell lung cancer (SCLC) have historically been characterised by poor overall survival (OS) and high risk for brain metastasis (BM), but large-scale real-world evidence on clinical presentation and treatment in this population is lacking. Our aim was to describe the clinical characteristics and outcomes of patients with SCLC and BM in Ontario, Canada.
Methods
This population-based, retrospective cohort study included all patients in Ontario, Canada, who were diagnosed with SCLC between April 1, 2010, and March 31, 2018. Data were analysed between June 2, 2022, and December 20, 2023. Patients with second cancer diagnosis were excluded. Patients were identified and data retrieved from the Institute for Clinical Evaluative Sciences (ICES) databases. Kaplan–Meier and multivariable Cox regression analyses were performed to compare OS between patient cohorts stratified by disease stage, BM diagnosis, and intracranial treatment modality. Propensity score-matching based on age, disease stage, time to BM, and receipt of chemotherapy was performed to compare OS between intracranial treatment modalities.
Findings
8705 patients were included (male: 4433, female: 4272). Median age at diagnosis was 68 years (interquartile range, IQR, 61–75). Median OS of all patients was 7.46 months (95% confidence interval, CI, 7.23–7.69). 32% (n = 2686) of patients developed BM (synchronous, 43.7%; asynchronous, 56.3%) with median OS of 9.76 months (95% CI, 9.36–10.22). 102 (4%), 1654 (62%), and 930 (35%) patients received stereotactic radiosurgery (SRS), whole brain radiation therapy (WBRT), or no treatment, respectively, for their BM in the first-line setting or after prophylactic cranial irradiation (PCI). In propensity score-matched analyses, OS from time of BM diagnosis was non-inferior between SRS- and WBRT-treated cohorts among patients who did not receive PCI (hazard ratio, HR, 0.68, 95% CI, 0.44–1.06, p = 0.091, n = 86) and in favour of SRS for those who received PCI prior to BM development (HR, 0.47, 95% CI, 0.31–0.72, p = 0.0042, n = 112).
Interpretation
OS for patients with SCLC remains poor, and many patients present with BM. With careful selection, patients with SCLC and BM may benefit from SRS treatment. Future research should incorporate information on burden of intracranial disease and novel immunotherapies.
Funding
None.
Keywords: Small cell lung cancer, Brain metastasis, Stereotactic radiosurgery, Whole brain radiation therapy
Research in context.
Evidence before this study
We searched PubMed for peer-reviewed studies from database inception until June 2, 2022 using search terms related to “small cell lung cancer”, “brain metastasis”, and “management”. A total of 826 studies were retrieved of which 39 focused on brain metastasis (BM) in small cell lung cancer (SCLC). Eleven were cohort studies examining the epidemiology of BM, but examination of treatment for BM were limited to only four studies, none of which directly compared stereotactic radiosurgery (SRS) with whole brain radiation therapy (WBRT).
Added value of this study
To our knowledge, this the largest study exploring the epidemiology and management of BM in SCLC, providing insight into the current presentation and management of BM in patients with SCLC. In propensity score-matched analyses, OS from time of BM diagnosis was non-inferior between SRS- and WBRT-treated cohorts among patients who did not receive PCI and in favour of SRS for those who received PCI prior to BM development.
Implications of all the available evidence
OS in patients with SCLC remains poor. Few patients receive SRS for treatment of their BM either in the first-line or salvage setting. Our findings indicate that there may be a subcohort of patients with SCLC and BM who can achieve equitable survival outcomes following SRS compared with WBRT when used as first-line therapy or in the salvage setting following PCI. Given the retrospective nature of this study, prospective trials are needed to evaluate the effect of BM burden and systemic disease status on survival following treatment with SRS or WBRT.
Introduction
Small cell lung cancer (SCLC) accounts for approximately 15% of lung cancers. The historically poor prognosis of patients with SCLC can be partly attributed to its propensity to metastasize, with 60–70% of patients manifesting metastatic disease through the course of their disease.1 Further, patients with SCLC are at high risk for the development of brain metastasis (BM). Retrospective clinical and autopsy studies have shown that among patients with SCLC, as many as 40–60% develop BM during the course of their disease.2,3
SCLC has traditionally been classified into limited and extensive stage, based on the area of disease that can be encompassed within a single radiation field at the time of diagnosis. The majority of patients present with extensive stage disease.1 Survival for these patients has been improving but remains poor.4 Only 21% and 7% of patients with limited and extensive stage, respectively, are expected to survive beyond two years.5 Development of BM has also been found to significantly reduce survival in patients with SCLC to a median of 4–6 months.6, 7, 8, 9 In response to this finding, patients with limited and sometimes extensive stage disease and good response to systemic treatment typically receive prophylactic cranial irradiation (PCI) to reduce the incidence of BM and improve survival.10,11 Management of BM in SCLC further diverges from other primary solid cancers through the use of whole brain radiation therapy (WBRT) as first-line treatment rather than stereotactic radiosurgery (SRS), given the concern of rapid intracranial progression, limited access to routine MRI for follow-up, and lack of prospective data.12 The necessity of PCI and WBRT in patients with SCLC has recently been called into question.12, 13, 14, 15
Despite the development of BM being a common endpoint in patients with SCLC, limited large-scale studies exist describing the epidemiology and treatment of BM in SCLC in the first-line and salvage setting. We therefore performed a retrospective study to assess the current state of disease burden and management of patients with SCLC and BM in Ontario, Canada, and to compare survival outcomes associated with brain-directed treatment modalities.
Methods
Study design and population
This retrospective cohort study included adult patients (age ≥18 years) in Ontario, Canada, with biopsy-confirmed diagnosis of SCLC, who were diagnosed between April 1, 2010, and March 31, 2018, with follow-up through to March 31, 2020. Patients with a second cancer diagnosis or those with missing date of cancer diagnosis were excluded. There were no restrictions on disease stage.
Data sources
Eligible patients were identified through the Institute for Clinical Evaluative Sciences (ICES) database. ICES is an independent, non-profit research institute whose legal status under Ontario's health information privacy law allows it to collect and analyse health care and demographic data, without consent, for health system evaluation and improvement. Ten datasets were used in this study: Cancer Activity Level Reporting (ALR), Yearly Health Services Contact (CONTACT), Discharge Abstract Database (DAD), Ontario Dementia Database (DEMENTIA), Symptom Management Database (ESAS), National Ambulatory Care Reporting System (NACRS), New Drug Funding Program (NDFP), Ontario Cancer Registry (OCR), Ontario Drug Benefits Claims (ODB), and Ontario Health Insurance Plan Claims Benefits (OHIP), Vital Statistics-Death (ORGD), Registered Persons Database (RPDB), and Same Day Surgery Database (SDS). The data was stored securely and confidentially as per ICES protocol. Data were linked using unique encoded identifiers and analysed at ICES. No members of the research team had access to the re-identifying key. Upon study completion, access to data was terminated. The use of data in this cohort study was permitted without individual consent as per section 45 of Ontario's Personal Health Information Protection Act (PHIPA), which does not require review by a research ethics board. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies.
Outcomes
Overall survival (OS) was measured from the time of SCLC diagnosis and time of BM diagnosis until death.
Classification of variables
Disease stage was identified using OCR records matching the pathological or clinical stage group of patients. Records of AJCC stage I, II, or III were classified as corresponding to limited disease whereas AJCC stage IV was classified as extensive disease. BM were identified in the ALR and DAD datasets with the International Classification of Diseases (ICD)-10 code C79.3 (“secondary malignant neoplasm of brain and cerebral meninges”) or through diagnosis of BM at the time of primary cancer diagnosis in OCR. Synchronous BM were defined as diagnosis of BM within six weeks of primary cancer diagnosis or BM at the time of primary cancer diagnosis in OCR. Data on receipt of brain imaging were merged from OHIP, DAD, and NACRS and identified based on CCI and OHIP fee codes. Brain imaging at the time of diagnosis was defined as imaging received within 6 weeks of SCLC diagnosis date. Given the retrospective nature of this study, we had insufficient information on ECOG scoring. As noted in Table 1, the majority of patients (n = 7469) had missing ECOG category, which would prohibit us from including these patients in multivariable analysis models. Information on ACG comorbidity index, however, was available in the ICES databases and we therefore elected to use the ACG comorbidity index in this study. The Johns Hopkins ACG® System Version 10 was used to collapse patient diagnoses from the preceding 2 years of health service utilization into appropriate ACG® System Aggregated Diagnosis Groups (ADGs) as a measure of comorbidity and categorized as follows: low comorbidity (0-5 ADGs), moderate comorbidity (6-9 ADGs), and high comorbidity (>10 ADGs).16
Table 1.
Baseline characteristics of the overall cohort at the time of primary cancer diagnosis stratified by disease stage.
| Characteristic | Total, n = 8705 | ED, n = 5664 | LD, n = 2572 | Missing, n = 469 | p-value |
|---|---|---|---|---|---|
| Sex | 0.0024 | ||||
| F | 4272 (49%) | 2633 (46%) | 1419 (55%) | 220 (47%) | |
| M | 4433 (51%) | 3031 (54%) | 1153 (45%) | 249 (53%) | |
| Age at SCLC diagnosis (years) | 68.00 (61.00, 75.00) | 68.00 (61.00, 75.00) | 68.00 (61.00, 75.00) | 70.00 (63.00, 77.00) | 0.0080 |
| Diagnosis year | 0.0001 | ||||
| Before 2011 | 2919 (34%) | 1737 (31%) | 894 (35%) | 288 (61%) | |
| After 2011 | 5786 (66%) | 3927 (69%) | 1678 (65%) | 181 (39%) | |
| TNM stage | 0.0001 | ||||
| I | 319 (3.9%) | 0 (0%) | 319 (12%) | 0 (0%) | |
| II | 248 (3.0%) | 0 (0%) | 248 (9.5%) | 0 (0%) | |
| III | 2044 (25%) | 0 (0%) | 2044 (78%) | 0 (0%) | |
| IV | 5625 (68%) | 5625 (100%) | 0 (0%) | 0 (0%) | |
| Unknown | 469 | 0 | 0 | 469 | |
| ACG comorbidity categorization | 0.0045 | ||||
| Low | 2010 (23%) | 1413 (25%) | 507 (20%) | 90 (19%) | |
| Moderate | 3419 (39%) | 2239 (40%) | 1004 (39%) | 176 (38%) | |
| High | 3276 (38%) | 2012 (36%) | 1061 (41%) | 203 (43%) | |
| ECOG PS | |||||
| 0 | 198 (16%) | SC | SC | SC | |
| 1 | 564 (46%) | SC | SC | SC | |
| 2 | 299 (24%) | SC | SC | SC | |
| 3 | 159 (13%) | SC | SC | SC | |
| ≥4 | 16 (1%) | SC | SC | SC | |
| Unknown | 7469 | 4903 | 2119 | 447 | |
| Time to first brain imaging (days) | 12.00 (3.00, 28.00) | 9.00 (2.00, 24.00) | 17.00 (6.00, 34.50) | 19.00 (5.50, 41.50) | 0.0078 |
| Unknown | 766 | 555 | 113 | 98 | |
| Imaging modality at first brain imaging | 0.0067 | ||||
| CT | 6116 (77%) | 4020 (79%) | 1782 (72%) | 314 (85%) | |
| MRI | 1823 (23%) | 1089 (21%) | 677 (28%) | 57 (15%) | |
| Unknown | 766 | 555 | 113 | 98 | |
| Brain imaging at diagnosis | <0.0001 | ||||
| No | 1277 (16%) | 686 (13%) | 498 (20%) | 93 (25%) | |
| Yes | 6662 (84%) | 4423 (87%) | 1961 (80%) | 278 (75%) | |
| Unknown | 766 | 555 | 113 | 98 | |
| Median time between follow-up brain imaging (days) | 100.00 (64.00, 157.50) | 90.50 (57.00, 137.00) | 122.00 (79.00, 195.00) | 110.00 (70.00, 199.00) | <0.0001 |
| Unknown | 3512 | 2630 | 631 | 251 | |
| Number of follow-up brain images | 2.00 (1.00, 4.00) | 2.00 (1.00, 3.00) | 3.00 (2.00, 5.00) | 2.00 (1.00, 3.50) | 0.0049 |
| Unknown | 766 | 555 | 113 | 98 | |
| Receipt of chemotherapy | <0.0001 | ||||
| No | 3153 (36%) | 2262 (40%) | 602 (23%) | 289 (62%) | |
| Yes | 5552 (64%) | 3402 (60%) | 1970 (77%) | 180 (38%) | |
| Receipt of PCI | <0.0001 | ||||
| No | 6947 (80%) | 4909 (87%) | 1622 (63%) | 416 (89%) | |
| Yes | 1758 (20%) | 755 (13%) | 950 (37%) | 53 (11%) | |
| BM diagnosis | <0.0001 | ||||
| Asynchronous | 1511 (17%) | 869 (15%) | 578 (22%) | 64 (14%) | |
| Synchronous | 1175 (13%) | 1152 (20%) | 0 (0%) | 23 (4.9%) | |
| No record of BM diagnosis | 6019 (69%) | 3643 (64%) | 1994 (78%) | 382 (81%) | |
| Age at BM diagnosis (years) | 66.00 (60.00, 73.00) | 66.00 (60.00, 73.00) | 66.00 (59.00, 73.00) | 68.00 (62.00, 72.00) | 0.70 |
| NA | 6019 | 3643 | 1994 | 382 | |
| Time to BM diagnosis (months) | 5.62 (1.08, 12.78) | 2.76 (0.72, 8.51) | 13.27 (6.59, 30.03) | 4.80 (0.82, 14.19) | <0.0001 |
Values are displayed as median (IQR) or n (%). Between-group comparisons were made using the chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. SC: values between 1 and 5, or complementary values that can be used to back-calculate small cells, redacted according to administrative privacy regulations due to re-identification risk.
BM, brain metastasis; CT, computed tomography; ECOG PS, Eastern Cooperative Oncology Group performance status; ED, extensive stage disease; LD, limited stage disease; MRI, magnetic resonance imaging; NA, not applicable; TNM, tumor, nodes, metastasis; SC, small cell; SCLC, small cell lung cancer.
Receipt of chemotherapy was identified through ALR and NDFP chemotherapy administration dates. A cut-off of 63 days (9 weeks) between consecutive treatment dates was used to define the beginning of a new treatment line rather than breaks between treatment cycles. Intracranial radiation was defined based on ALR body region codes corresponding to intracranial regions and NHPIP codes corresponding to WBRT and SRS. PCI was defined as 1) WBRT administered to patients who received chemotherapy; 2) receipt of WBRT after completion of chemotherapy; 3) absence of synchronous disease; 4) receipt of WBRT at least one month prior to BM diagnosis in those with asynchronous BM; and 5) either WBRT dosage between 2.49 and 2.52 Gy per fraction or receipt of WBRT within 16 weeks of chemotherapy completion. Salvage radiation therapy was defined based on a time difference of more than 31 days between the first day of radiation therapy administration and subsequent administration dates.
Statistical analysis
Between-group comparisons were made using the chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Kaplan Meier methods were used to analyse time-to-event data. Cox-proportional hazard regression models were used to assess differences between Kaplan–Meier survival curves. Post-hoc subgroup analyses were performed on patients who received chemotherapy to consider only patients who were functionally well enough to receive intensive therapy. Multivariable Cox proportional hazards regression analyses were performed adjusting for variables significant in univariable analysis. Patient demographic factors (sex, age at diagnosis, year of diagnosis, ADG comorbidity score), disease stage, and treatment factors (receipt of chemotherapy, receipt of PCI) were assessed. Patients with missing data on any of these variables were omitted from the multivariable regression models. In the overall cohort, PCI was treated as a time-dependent covariate.17 Cumulative incidence curves for incidence of BM were estimated using the competing risk method, and Gray test was used to determine significance between cumulative incidence curves.18,19 PCI was not included in the cumulative incidence model, as cumulative incidence functions cannot be interpreted for time-varying risk factors.20
To prevent confounding of post–index events, analyses in the context of BM development and treatment were re-indexed to time of BM diagnosis. To analyse the effects of intracranial radiation treatment modality, OS analyses were performed separately on cohorts of patients with confirmed BM diagnosis who did or did not receive PCI and stratified by intracranial treatment modality (SRS vs WBRT vs no treatment). Next, differences in OS between SRS and WBRT-treated patients were assessed on a propensity score-matched dataset to reduce bias due to confounding variables (Supplementary Methods). We also performed a second propensity score-matched analysis using only age, disease stage, and receipt of chemotherapy to calculate propensity scores in order to avoid confounding of pre-indexing events (i.e. time to diagnosis of BM). Patients with missing time to diagnosis of BM or disease stage were excluded in this analysis. Small cells, i.e. values between 1 and 5 or complementary values that could be used to back-calculate small cells, were redacted according to administrative privacy regulations due to re-identification risk. Analyses were performed using R versions 3.1.2 and 3.6.1 (R Core Team, Vienna, Austria, 2014 & 2019). All hypothesis tests were 2-sided and an α of 0.05 was considered statistically significant. Analyses were performed between June 2, 2022 and December 20, 2023.
Role of the funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. It is a requirement at ICES that only the individual who is performing the analysis can access the data directly. All outputs were reviewed by KKWC and AWE. The analytic plan was reviewed by four authors (KG, AE, KKWC, and SD).
Results
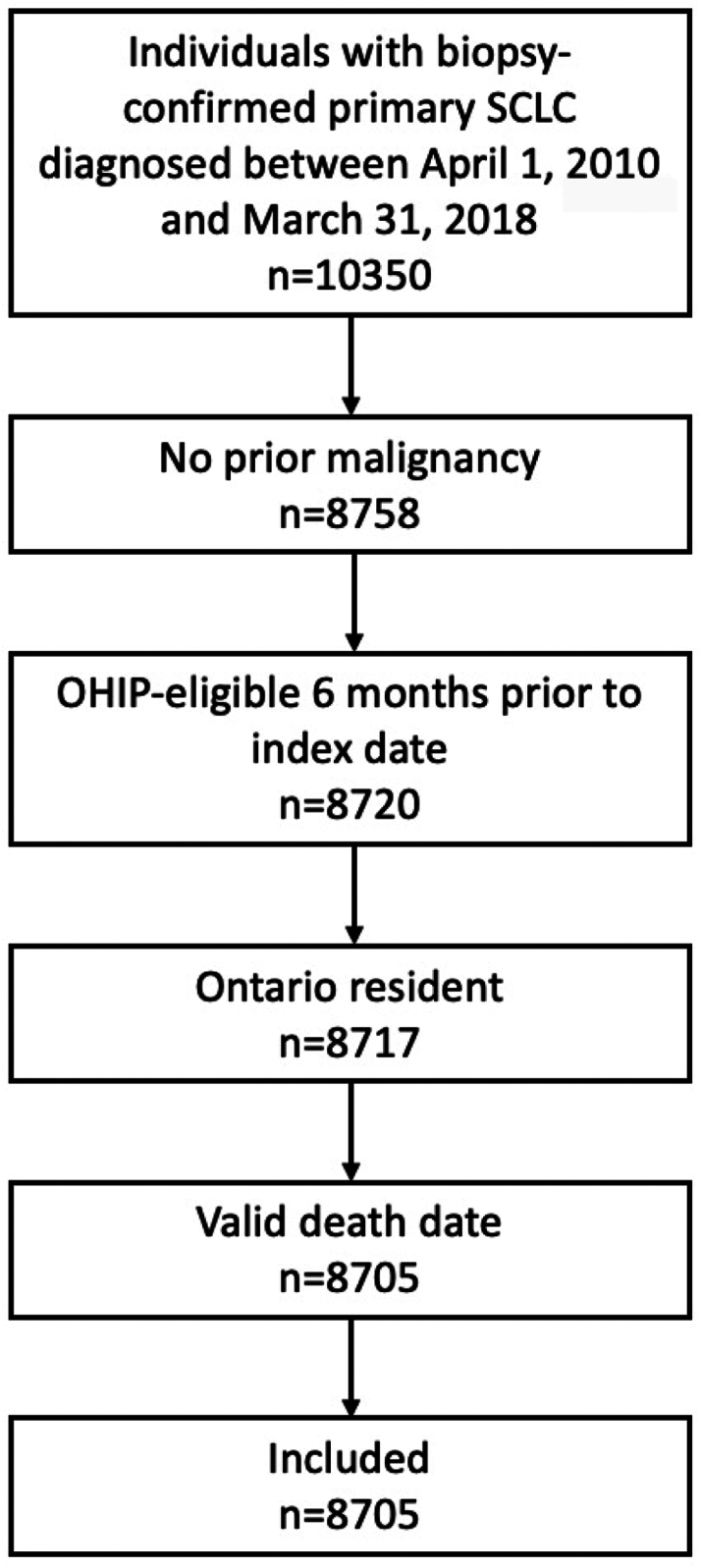
A total of 8705 patients met inclusion criteria (Fig. 1). Median age at the time of SCLC diagnosis was 68 years (interquartile range, IQR, 61–75). Most patients had extensive disease at the time of diagnosis (n = 5664, 64.6%). 63.8% (n = 5552) of patients received chemotherapy and 20.2% (n = 1758) of patients received PCI (Table 1). Median time to intracranial imaging following SCLC diagnosis was 12 days (IQR 3–28). 6662 (76.5%) patients received intracranial imaging at the time of diagnosis. Magnetic resonance imaging (MRI) was more commonly used as first intracranial imaging modality among patients who were diagnosed after 2011, compared with computed tomography (CT) imaging (Table S1). Median follow-up was 28.81 months (IQR 13.77–59.80).
Fig. 1.
Study cohort. OHIP, Ontario Health Insurance Plan; SCLC, small cell lung cancer.
Predictors of survival
Median OS from SCLC diagnosis was 7.46 months (95% CI, 7.23–7.69), with 6, 12, and 18-month survival rates of 56.3%, 32.5%, and 20.4%. Survival was longer for females (8.31 vs 6.80 months, p < 0.0001), patients younger than 65 years at the time of diagnosis (9.43 vs 6.14 months, p < 0.0001), patients with limited disease (15.18 vs 5.26 months, p < 0.0001), patients with moderate ACG comorbidity index (8.08 vs 7.00 months, p < 0.0001) and those who received chemotherapy (10.64 vs 1.58 months, p < 0.0001) or PCI (8.94 vs 7.16 months, p < 0.0001). These factors were independently associated with OS in multivariable analysis with the exception of PCI, which was no longer significant. Year of diagnosis and ACG comorbidity index did not influence survival (Table 2). These results were similar in a subgroup analysis of patients who received chemotherapy (Supplementary Materials, page 3; Tables S2 and S3).
Table 2.
Univariable and multivariable analysis of overall survival in all patients with complete data on covariates (n = 8236).
| Variable | No. total | No. events | Median OS | Univariable analysis HR | Multivariable analysis HR |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 4272 | 4011 | 8.31 (95% CI, 7.98–8.74) | 1.19 (95% CI, 1.14–1.25), p < 0.0001 | 1.11 (95% CI, 1.07–1.17), p < 0.0001 |
| Male | 4433 | 4234 | 6.80 (95% CI, 6.47–7.06) | ||
| Age | |||||
| <65 years | 3194 | 2961 | 9.43 (95% CI, 9.07–9.86) | 0.75 (95% CI, 0.72–0.79), p < 0.0001 | 0.82 (95% CI, 0.78–0.86), p < 0.0001 |
| ≥65 years | 5511 | 5284 | 6.14 (95% CI, 5.78–6.54) | ||
| Year of diagnosis | |||||
| Before 2011 | 2919 | 2825 | 7.33 (95% CI, 7–7.75) | 1.04 (95% CI, 0.99–1.08), p = 0.77 | NS |
| After 2011 | 5786 | 5420 | 7.49 (95% CI, 7.23–7.75) | ||
| Disease stage | |||||
| LD | 2572 | 2238 | 15.18 (95% CI, 14.29–15.87) | 0.37 (95% CI, 0.36–0.39), p < 0.0001 | 0.39 (95% CI, 0.36–0.40), p < 0.0001 |
| ED | 5664 | 5558 | 5.26 (95% CI, 4.9–5.55) | ||
| ACG comorbidity index | |||||
| Low | 2010 | 1907 | 7.29 (95% CI, 6.87–7.82) | 0.99 (95% CI, 0.94–1.05), p = 1.2 | NS |
| Moderate | 3419 | 3225 | 8.08 (95% CI, 7.72–8.48) | 0.92 (95% CI, 0.88–0.97), p < 0.0001 | 0.92 (95% CI, 0.87–0.97), p < 0.0001 |
| High | 3276 | 3113 | 7.00 (95% CI, 6.54–7.29) | Ref. | Ref. |
| Receipt of chemotherapy | |||||
| Yes | 5552 | 5151 | 10.64 (95% CI, 10.32–10.91) | 0.36 (95% CI, 0.34–0.38), p < 0.0001 | 0.35 (95% CI, 0.33–0.37), p < 0.0001 |
| No | 3153 | 3094 | 1.58 (95% CI, 1.48–1.74) | ||
| Receipt of PCI | |||||
| Yes | 1758 | 1541 | 8.94 (95% CI, 1.12–22.47) | 0.67 (95% CI, 0.63–0.71), p < 0.0001 | 1.05 (95% CI, 0.98–1.11), p = 0.47 |
| No | 6947 | 6704 | 7.16 (95% CI, 7.00–7.33) | ||
Multivariable Cox proportional hazards regression analyzes were performed adjusting for variables significant in univariable analysis.
95% CI, 95% confidence interval; ACG, Adjusted Clinical Groups; HR, hazard ratio; NS, not significant; OS, overall survival; PCI, prophylactic cranial irradiation.
Development of brain metastasis
2686 (30%) patients developed BM during the course of their disease. 1175 (13%) patients presented with synchronous and 1511 (17%) with asynchronous intracranial disease (Table 3). BM were more common amongst patients with extensive disease (n = 2021, 36%), compared to patients with limited disease (n = 578, 22%, p < 0.0001). Median time to BM diagnosis was 9.82 months (95% CI, 6.34–15.85) in patients who had asynchronous disease. Cumulative incidence of BM at 12 months for the overall cohort was 24.17% (95% CI, 23.27–47.68; Figure S1).
Table 3.
Baseline characteristics of the overall cohort by development of brain metastasis.
| Characteristic | Overall, n = 8705 | Asynchronous, n = 1511 | Synchronous, n = 1175 | No record of BM diagnosis, N = 6019 | p-value |
|---|---|---|---|---|---|
| Sex | 0.13 | ||||
| F | 4272 (49%) | 772 (51%) | 555 (47%) | 2945 (49%) | |
| M | 4433 (51%) | 739 (49%) | 620 (53%) | 3074 (51%) | |
| Age at SCLC diagnosis (years) | 68.00 (61.00, 75.00) | 65.00 (58.00, 71.00) | 66.00 (60.00, 72.00) | 69.00 (62.00, 76.00) | 0.0072 |
| Diagnosis year | 0.0048 | ||||
| Before 2011 | 2919 (34%) | 564 (37%) | 270 (23%) | 2085 (35%) | |
| After 2011 | 5786 (66%) | 947 (63%) | 905 (77%) | 3934 (65%) | |
| ACG comorbidity categorization | <0.0001 | ||||
| Low | 2010 (23%) | 364 (24%) | 381 (32%) | 1265 (21%) | |
| Moderate | 3419 (39%) | 620 (41%) | 453 (39%) | 2346 (39%) | |
| High | 3276 (38%) | 527 (35%) | 341 (29%) | 2408 (40%) | |
| SCLC stage | <0.0001 | ||||
| LD | 2572 (31%) | 578 (40%) | 0 (0%) | 1994 (35%) | |
| ED | 5664 (69%) | 869 (60%) | 1152 (100%) | 3643 (65%) | |
| Unknown | 469 | 64 | 23 | 382 | |
| TNM stage | <0.0001 | ||||
| I | 319 (3.9%) | 55 (4%) | 0 | 264 (5%) | |
| II | 248 (3.0%) | 50 (3%) | 0 | 198 (3%) | |
| III | 2044 (25%) | 512 (35%) | 0 | 1532 (27%) | |
| IV | 5625 (68%) | 830 (58%) | 1152 | 3643 (65%) | |
| Unknown | 469 | 64 | 23 | 382 | |
| ECOG PS | 0.0072 | ||||
| 0 | 198 (16%) | 59 (21%) | 27 (14%) | 112 (15%) | |
| 1 | 564 (46%) | 139 (50%) | 81 (43%) | 344 (45%) | |
| 2 | 299 (24%) | 57 (20%) | 44 (23%) | 198 (26%) | |
| 3 | 159 (13%) | 23 (8.2%) | 33 (18%) | 103 (13%) | |
| ≥4 | 16 (1%) | SC | SC | SC | |
| Unknown | 7469 | 1232 | 987 | 5250 | |
| Imaging modality at first brain imaging | 0.0080 | ||||
| CT | 6116 (77%) | 1078 (72%) | 927 (79%) | 4111 (78%) | |
| MRI | 1823 (23%) | 427 (28%) | 243 (21%) | 1153 (22%) | |
| Unknown | 766 | SC | SC | SC | |
| Brain imaging at diagnosis | 0.0067 | ||||
| No | 1277 (16%) | 324 (22%) | 78 (6.7%) | 875 (17%) | |
| Yes | 6662 (84%) | 1181 (78%) | 1092 (93%) | 4389 (83%) | |
| Unknown | 766 | SC | SC | SC | |
| Receipt of chemotherapy | <0.0001 | ||||
| Yes | 5552 (64%) | 1285 (85%) | 697 (59%) | 3570 (59%) | |
| No | 3153 (36%) | 226 (15%) | 478 (41%) | 2449 (41%) | |
| Receipt of PCI | <0.0001 | ||||
| Yes | 1758 (20%) | 461 (31%) | 0 (0%) | 1297 (22%) | |
| No | 6792 (78%) | 923 (61%) | 1175 (100%) | 4694 (78%) | |
| Age at BM diagnosis (years) | 66.00 (60.00, 73.00) | 66.00 (59.00, 73.00) | 66.00 (60.00, 73.00) | NA | 0.60 |
| Time to BM diagnosis (months) | 5.62 (1.08, 12.78) | 9.82 (6.34, 15.85) | 0.39 (0.00, 1.05) | NA | <0.0001 |
Values are displayed as median (IQR) or n (%). Between-group comparisons were made using the chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. SC: values between 1 and 5, or complementary values that can be used to back-calculate small cells, redacted according to administrative privacy regulations due to re-identification risk.
BM, brain metastasis; CT, computed tomography; ECOG PS, Eastern Cooperative Oncology Group performance status; ED, extensive stage disease; LD, limited stage disease; MRI, magnetic resonance imaging; NA, not applicable; TNM, tumor, nodes, metastasis; SC, small cell; SCLC, small cell lung cancer.
Median OS following development of BM was 95% CI, 2.40 months (95% CI, 2.23–2.60). Median survival from time of BM diagnosis was longer for patients with synchronous disease (3.29 months; 95% CI, 2.89–3.75), compared to those who had asynchronous disease (1.97 months; 95% CI, 1.84–2.17; HR, 0.79; 95% CI, 0.73–0.85; p < 0.0001).
Treatment of brain metastasis among patients who did not receive prophylactic cranial irradiation
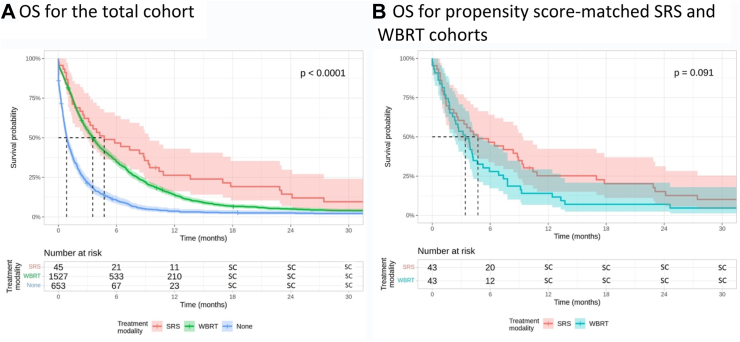
There were 2225 patients who did not receive PCI and had or developed BM. 1175 (53%) of these presented with BM at the time of diagnosis while 1050 (47%) patients developed BM asynchronously at a median time of 8.05 months (IQR 5.20–12.12) following SCLC diagnosis. Median OS following BM diagnosis was 2.50 months (95% CI, 2.33–2.69). In this group, first-line treatment consisted of SRS (n = 45, 2%) or WBRT (n = 1527, 67%); 653 patients (29%) had no record of intracranial radiation. Baseline difference between these cohorts existed in terms of age, cancer stage, diagnosis year, receipt of chemotherapy, presence of synchronous disease, and time to BM diagnosis (Table S4). Median OS from time of SCLC diagnosis was 18.56 months (95% CI, 11.73–24.64), 9.17 months (95% CI, 8.87–9.50), and 5.72 months (95% CI, 4.76–6.47) in patients who received SRS, WBRT, or no treatment for their BM, respectively. In unadjusted analysis, median OS from time of BM diagnosis was comparable between patients who received SRS (4.73 months, 95% CI, 2.66–9.43) or WBRT (3.55 months, 95% CI, 3.29–3.84; HR, 0.74, 95% CI, 0.54–1.01, p = 0.054) and in favour of SRS compared with those who received no treatment (0.85 months, 95% CI, 0.76–1.02; HR, 0.34, 95% CI, 0.25–0.47, p < 0.0001; Fig. 2). These findings remained unchanged after multivariable adjustment (SRS vs WBRT, HR 0.83, 95% CI, 0.61–1.14, p = 0.25, Table S5). In multivariable subgroup analyses of patients with synchronous or asynchronous disease only, survival differences between SRS and WBRT were non-significant (Supplementary Materials, page 3).
Fig. 2.
Median overall survival from time of brain metastasis diagnosis among patients who did not receive prophylactic cranial irradiation stratified by intracranial treatment modality for total (panel A) and propensity score-matched (panel B) cohorts. Kaplan–Meier methods were used to analyse overall survival in months from time of brain metastasis diagnosis. Cox-proportional hazard regression models were used to assess differences between Kaplan–Meier survival curves. SC: values between 1 and 5, or complementary values that can be used to back-calculate small cells, redacted according to administrative privacy regulations due to re-identification risk. OS, overall survival; PCI, prophylactic cranial irradiation; SC, small cell; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy.
Propensity score matching in this cohort revealed 43 patients who received SRS and 43 patients who received WBRT and were well-balanced in terms of cancer stage, age, time to BM, and receipt of chemotherapy (Table S6). Considering these matched cohorts, median OS from SCLC diagnosis was comparable between the two treatment modalities (SRS: 18.00 months, 95% CI, 11.73–24.60; WBRT: 12.60 months, 95% CI, 8.81–17.70; HR, 0.66, 95% CI, 0.42–1.03, p = 0.98). Median OS from time of BM diagnosis was also not statistically different (SRS: 4.73 months, 95% CI, 2.53–9.43; WBRT: 3.42 months, 95% CI, 2.10–5.26; HR, 0.68, 95% CI, 0.44–1.06, p = 0.091; Fig. 2). Findings of a sensitivity analysis of propensity scores that did not include time to BM diagnosis can be found in the supplement.
Treatment of brain metastasis among patients who received prophylactic cranial irradiation
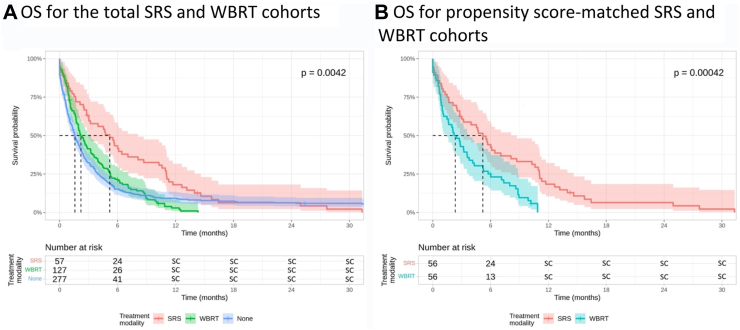
461 patients who received PCI developed BM (Table S8). Salvage therapy consisted of WBRT (n = 127, 28%), SRS (n = 57, 12%), and no treatment (n = 227, 49%), with median OS from time of SCLC diagnosis of 22.40 months (95% CI, 20.50–24.10), 24.90 months (95% CI, 23.20–28.70), and 15.60 months (95% CI, 14.50–17.70), respectively. Median OS from BM diagnosis was 5.19 months (95% CI, 3.02–8.12), 2.20 (95% CI, 1.84–3.22), and 1.58 months (95% CI, 1.31–1.97) for patients who received SRS, WBRT, or no treatment for their BM, respectively. In univariable analysis, survival was in favour of SRS over WBRT (HR, 0.65, 95% CI, 0.47–0.89, p = 0.0070) and no treatment (HR, 0.61, 95% CI, 0.46–0.82, p < 0.0001). This remained unchanged in multivariate analysis (SRS vs WBRT: HR, 0.64, 95% CI, 0.46–0.88, p = 0.0060; SRS vs no treatment: HR, 0.61, 95% CI, 0.45–0.82, p = 0.0010; Table S9; Fig. 3).
Fig. 3.
Median overall survival from time of brain metastasis diagnosis among patients who received prophylactic cranial irradiation stratified by intracranial treatment modality for total (panel A) and propensity score-matched (panel B) cohorts. Kaplan–Meier methods were used to analyse overall survival in months from time of brain metastasis diagnosis. Cox-proportional hazard regression models were used to assess differences between Kaplan–Meier survival curves. SC: values between 1 and 5, or complementary values that can be used to back-calculate small cells, redacted according to administrative privacy regulations due to re-identification risk. OS, overall survival; PCI, prophylactic cranial irradiation; SC, small cell; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy.
Propensity score matching for time to BM, age, and disease stage identified 112 well-balanced patients treated with SRS (n = 56) and WBRT (n = 56, Table S10). Median OS from time of SCLC diagnosis was 25.0 months (95% CI, 23.9–28.80) for patients who received SRS and 23.50 months (95% CI, 22.4–26.6; HR, 0.77, 95% CI, 0.53–1.13, p = 0.19) for patients who received WBRT. Survival from time of BM diagnosis in the matched cohort was longer in patients who received salvage SRS (5.19 months, 95% CI, 3.02–8.71) compared to patients who received salvage WBRT (2.33 months, 95% CI, 1.48–3.78; HR, 0.47, 95% CI, 0.31–0.72, p = 0.0042; Fig. 3). The findings remained unchanged in a sensitivity analysis of propensity scores that did not include time to BM (Supplementary Materials, page 3; Table S11, Figure S2).
Discussion
In this large database study of 8705 patients diagnosed with SCLC within the modern era (2010–2018), survival outcomes continue to be poor. Male sex, increasing age, extensive disease at time of diagnosis, and absence of chemotherapy were associated with poor survival. 30% of patients had detected BM over their disease course, with 13% of all patients presenting with BM at the time of diagnosis. Only a minority of patients received SRS for treatment of BM either in the first-line or salvage setting with encouraging survival comparable to those achieved with WBRT. This finding was unchanged in subgroup analyses of patients who presented with synchronous or asynchronous BM.
Prior studies demonstrating non-inferior survival outcomes following SRS compared with WBRT, even in the light of inferior intracranial control, and superior outcomes for cognition and quality-of-life, have established SRS as the preferred first-line treatment for BM arising from most solid tumors.21 In our cohort, SRS was associated with longer survival compared with patients who received WBRT in propensity score-matched analyses of patients who received first-line treatment for their BM without prior PCI. This finding is in line with other recently published data suggesting that survival outcomes following SRS in patients with SCLC may be analogous to those for patients with BM from other solid cancer types.13 As radiation oncologists consider factors such as performance status, cognitive function, extent of extracranial disease and BM burden (i.e. number of BM) when making treatment decisions, patients with favourable prognosis and limited intracranial disease were likely selected to receive SRS in this cohort.22 Under this assumption, our findings indicate that patients with limited intracranial disease can be treated safely with SRS and may achieve comparable survival. This finding should prompt a careful reassessment of current decision-making algorithms to identify patients who may potentially be spared from the neurocognitive sequelae of WBRT. There is a possibility for change in practice should the prospective trials that are currently underway for evaluating SRS in comparison with WBRT and evolving WBRT techniques (NCT03297788, NCT04804644, NCT04804644) continue to reflect this survival trend.
Development of BM following PCI is a common occurrence.15 However, retrospective data on the use of salvage radiation therapy in this setting are limited.13 Our analysis showed survival outcomes in favour of SRS over WBRT. This finding may be due to the fact that salvage WBRT following PCI is low-dose and may not be as effective in patients previously exposed to PCI. Prior exposure to PCI may also lead to radioresistance to subsequent radiation therapy. SRS as a high-dose focal ablative treatment may overcome these two barriers and induce effective local control.23 Furthermore, compound effects of repeat WBRT may compromise survival compared with SRS, which is associated with better neurocognitive and performance status outcomes.24
The high incidence rate of synchronous BM in our cohort parallels findings from the American Surveillance, Epidemiology, and End Results (SEER) database, according to which 15.5% of patients with SCLC had BM at the time of diagnosis.25 A further 15% of patients in our study developed BM during the course of their disease. Our data also show provincial adherence to screening recommendations for BM, with over 90% of patients receiving either CT or MRI at the time of diagnosis and regular three-monthly follow-up imaging.26,27 These incidence rates exceed those reported in patients with non-small cell lung cancer, where there has been debate around the use of routine screening for BM.28,29 Routine screening can lead to detection of BM at an earlier time point, identifying patients when their intracranial disease may be more amenable to SRS. Surveillance may also impact systemic treatment approaches, particularly if practitioners are considering the use of PCI.
Paradoxically, we found that patients with asynchronous BM lived longer than patients who never had a recorded diagnosis of BM. One possibility is that patients who developed intracranial disease later in their disease course may have had better response to chemotherapy or favourable tumor characteristics. In this sub-cohort, later development of BM could be a result of prolonged life expectancy and time for BM development, rather than the aggressive nature of the disease. Development of BM has historically been considered a negative prognostic factor for survival.8,29 Our data suggests that there may be a divergence between patients with BM and stable or progressive extracranial disease.30,31 Characterization of this cohort of patients with stable disease could have clinical implications for monitoring and treatment intensification, which could lead to improved survival outcomes for some patients with SCLC.
Despite advances in systemic treatment, our results mirror findings from studies published over a decade ago, which reported median survival estimates at 12–17 months and 5–10 months for patients with limited and extensive stage disease, respectively.32 First-line treatment for limited stage SCLC, consisting of cisplatin and etoposide with concurrent radiotherapy and followed by PCI, has remained unchanged since its inception in the mid-1980s.33 Recently, the addition of PD-L1 inhibitors to chemotherapy has become standard of care in patients with extensive stage disease after trials showing that addition of PD-L1 inhibitors improved OS in these patients.34,35 An exploratory analysis of the CASPIAN study even suggests that addition of immunotherapies may prolong time to BM and intracranial radiation.36 However, research in this area remains limited, due to the aggressive natural history of SCLC and rapid initiation of treatment following diagnosis. Better understanding of molecular characteristics of SCLC are needed to further advance systemic and intracranial treatment in this patient population.37,38 The recently created Collaborative Canadian SCLC (CASCADE) database may help clarify the impact of treatment approach in the management of patients with SCLC through real-world clinical evidence.39
Our study has several limitations. First, given its retrospective nature, we were unable to collect information on potentially confounding variables, including race, tobacco use, extent of intracranial disease, number of BM, performance status, treatment-related toxicities, treatment response, or receipt of salvage therapy. We also did not investigate the role of WBRT type, systemic radiation therapy, chemotherapy, or surgery on OS or intracranial or extracranial disease progression due to limited data access. Second, there may selection bias contributing influencing the decision making for administration of SRS vs WBRT. we were unable to analyse or predict reasons for administration or forgoing of certain types of therapy. Third, patients with very aggressive disease may not have lived long enough to receive intracranial imaging or treatment for BM, skewing BM incidence estimates and estimation of treatment efficacy. Lastly, addition of newly approved immunotherapies after accrual of our cohort for systemic SCLC treatment may impact the clinical response to intracranial treatment limiting the generalisability of our results.
In summary, our study finds poor survival outcomes in patients with SCLC with little improvement compared to prior estimates. The incidence of BM in this population remains high. Treatment of BM with SRS was associated with longer survival compared with treatment with WBRT. This finding indicates the presence of a sub-cohort of patients with SCLC with BM who can show prolonged survival even in the presence of intracranial disease. Future research efforts should characterize this patient cohort to offer maximally effective therapy for patients with SCLC.
Contributors
KG, AE, KKWC, and SD conceived and designed the study. SD supervised the project. KG carried out the formal data analysis with results checked and verified by AE and KKWC. KG wrote the original manuscript draft with support from SC. All authors critically revised the report for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. KG had the final responsibility to submit the report for publication.
Data sharing statement
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g., healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Declaration of interests
IBM declares honoraria from Bristol-Myers Squibb, Agence Unik, AstraZeneca, and Merck. IBM is on the Advisory board for InSightec and received trainee funding from Takeda, AstraZeneca, and BMS as well as research funding from AstraZeneca.
BHL declares institutional grants from AstraZeneca and Pfizer and honoraria and support for travel/attending meetings from AstraZeneca. AS received a research grant and acted as a consultant for Elekta/Elekta AB and BrainLab. AS received Honoraria for educational seminars from Elekta/Elekta AB, Varian, BrainLab, AstaZeneca, Seagen Inc, Cerapedics, and CarboFIX. AS was reimbursed for travel expenses by Elekta/Elekta AB, Varian, and BrainLab. AS received research grants from Seagen Inc and is Vice President for the International Stereotactic Radiosurgery Society. SD has received grant funding from the Canadian Institutes of Health Research, Gratitude 10, the Canadian Cancer Society, the Calum Macbeth fund and the Keenan Chair in Surgery. SD received royalties from Oxford University Press. SD reports support for travel and accommodation from the Congress of Neurological Surgeons and the American Association of Neurological Surgeons. SD participated on a data safety monitoring board or advisory board for the Subcortical Surgery Group and XPan Medical. SD is the Provincial Lead for CNS Cancers at Ontario Health, Cancer Care Ontario. SD receives research support from the Keenan Chair in Surgery. KG, AWE, SC, and KKWC declare no competing interests.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES, the MOH or MLTC is intended or should be inferred.
Parts of the materials in this paper are based on data and information provided by Ontario Health (OH), CIHI, and the Ontario MOH. The analyses, opinions, results, view, and conclusions reported in this paper are those of the authors and do not reflect those of the data sources. No endorsement is intended or should be inferred. We thank IQVIA Solutions Canada Inc. for use of their Drug Information File.
We thank Lena Nguyen, MSc, for cutting the patient cohorts from the data holdings at IC/ES, where she was a salaried employee at IC/ES, the facility from which data were obtained. Her contributions fell within the remit of that employment. She was not directly compensated by any member of our team.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102871.
Appendix A. Supplementary data
References
- 1.Rudin C.M., Brambilla E., Faivre-Finn C., Sage J. Small-cell lung cancer. Nat Rev Dis Prim. 2021;7(1):3. doi: 10.1038/s41572-020-00235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nugent J.L., Bunn P.A., Jr., Matthews M.J., et al. CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer. 1979;44(5):1885–1893. doi: 10.1002/1097-0142(197911)44:5<1885::aid-cncr2820440550>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch F.R., Paulson O.B., Hansen H.H., Larsen S.O. Intracranial metastases in small cell carcinoma of the lung. Prognostic aspects. Cancer. 1983;51(3):529–533. doi: 10.1002/1097-0142(19830201)51:3<529::aid-cncr2820510327>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Govindan R., Page N., Morgensztern D., et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 5.Amarasena I.U., Chatterjee S., Walters J.A., Wood-Baker R., Fong K.M. Platinum versus non-platinum chemotherapy regimens for small cell lung cancer. Cochrane Database Syst Rev. 2015;2015(8) doi: 10.1002/14651858.CD006849.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postmus P.E., Haaxma-Reiche H., Gregor A., et al. Brain-only metastases of small cell lung cancer; efficacy of whole brain radiotherapy. An EORTC phase II study. Radiother Oncol. 1998;46(1):29–32. doi: 10.1016/s0167-8140(97)00149-7. [DOI] [PubMed] [Google Scholar]
- 7.Videtic G.M., Adelstein D.J., Mekhail T.M., et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for small-cell lung cancer-only brain metastases. Int J Radiat Oncol Biol Phys. 2007;67(1):240–243. doi: 10.1016/j.ijrobp.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Sperduto P.W., Kased N., Roberge D., et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernhardt D., Bozorgmehr F., Adeberg S., et al. Outcome in patients with small cell lung cancer re-irradiated for brain metastases after prior prophylactic cranial irradiation. Lung Cancer. 2016;101:76–81. doi: 10.1016/j.lungcan.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Slotman B., Faivre-Finn C., Kramer G., et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357(7):664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 11.Sun A., Abdulkarim B., Blais N., et al. Use of radiation therapy among patients with Extensive-stage Small-cell lung cancer receiving Immunotherapy: Canadian consensus recommendations. Lung Cancer. 2023;179 doi: 10.1016/j.lungcan.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Rusthoven C.G., Yamamoto M., Bernhardt D., et al. Evaluation of first-line radiosurgery vs whole-brain radiotherapy for small cell lung cancer brain metastases: the FIRE-SCLC cohort study. JAMA Oncol. 2020;6(7):1028–1037. doi: 10.1001/jamaoncol.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaebe K., Li A.Y., Park A., et al. Stereotactic radiosurgery versus whole brain radiotherapy in patients with intracranial metastatic disease and small-cell lung cancer: a systematic review and meta-analysis. Lancet Oncol. 2022;23(7):931–939. doi: 10.1016/S1470-2045(22)00271-6. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T., Yamanaka T., Seto T., et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(5):663–671. doi: 10.1016/S1470-2045(17)30230-9. [DOI] [PubMed] [Google Scholar]
- 15.Gaebe K., Erickson A.W., Li A.Y., et al. Re-examining prophylactic cranial irradiation in small cell lung cancer: a systematic review and meta-analysis. eClinicalMedicine. 2024;67 doi: 10.1016/j.eclinm.2023.102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin P.C., Walraven C. The mortality risk score and the ADG score: two points-based scoring systems for the Johns Hopkins aggregated diagnosis groups to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49(10):940–947. doi: 10.1097/MLR.0b013e318229360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therneau T., Cynthia C., Atkinson E. Using time dependent covariates and time dependent coeffcients in the Cox model. 2024. https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf
- 18.Scrucca L., Santucci A., Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 19.Scrucca L., Santucci A., Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45(9):1388–1395. doi: 10.1038/bmt.2009.359. [DOI] [PubMed] [Google Scholar]
- 20.Poguntke I., Schumacher M., Beyersmann J., Wolkewitz M. Simulation shows undesirable results for competing risks analysis with time-dependent covariates for clinical outcomes. BMC Med Res Methodol. 2018;18(1):79. doi: 10.1186/s12874-018-0535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsao M.N., Xu W., Wong R.K., et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2018;(1) doi: 10.1002/14651858.CD003869.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gjyshi O., Lin S.H., Pezzi T.A., et al. Care patterns for stereotactic radiosurgery in small cell lung cancer brain metastases. Clin Lung Cancer. 2022;23(2):185–190. doi: 10.1016/j.cllc.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Nakazaki K., Yomo S., Kondoh T., et al. Salvage gamma knife radiosurgery for active brain metastases from small-cell lung cancer after whole-brain radiation therapy: a retrospective multi-institutional study (JLGK1701) J Neuro Oncol. 2020;147(1):67–76. doi: 10.1007/s11060-020-03397-9. [DOI] [PubMed] [Google Scholar]
- 24.Chang E.L., Wefel J.S., Hess K.R., et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 25.Li N., Chu Y., Song Q. Brain metastasis in patients with small cell lung cancer. Int J Gen Med. 2021;14:10131–10139. doi: 10.2147/IJGM.S342009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melosky B.L., Leighl N.B., Dawe D., et al. Canadian consensus recommendations on the management of extensive-stage small-cell lung cancer. Curr Oncol. 2023;30(7):6289–6315. doi: 10.3390/curroncol30070465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seute T., Leffers P., ten Velde G.P., Twijnstra A. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI) Cancer. 2008;112(8):1827–1834. doi: 10.1002/cncr.23361. [DOI] [PubMed] [Google Scholar]
- 28.Schoenmaekers J.J., Dingemans A.-M.C., Hendriks L.E. Brain imaging in early stage non-small cell lung cancer: still a controversial topic? J Thorac Dis. 2018;10(Suppl 18):S2168. doi: 10.21037/jtd.2018.06.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habbous S., Forster K., Darling G., et al. Incidence and real-world burden of brain metastases from solid tumors and hematologic malignancies in Ontario: a population-based study. Neurooncol Adv. 2021;3(1) doi: 10.1093/noajnl/vdaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li A.Y., Gaebe K., Zulfiqar A., et al. Association of brain metastases with survival in patients with limited or stable extracranial disease: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(2) doi: 10.1001/jamanetworkopen.2023.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erickson A.W., Habbous S., Wright F., Lofters A.K., Jerzak K.J., Das S. Assessing the association of targeted therapy and intracranial metastatic disease. JAMA Oncol. 2021;7(8):1220–1224. doi: 10.1001/jamaoncol.2021.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sculier J.P., Chansky K., Crowley J.J., Van Meerbeeck J., Goldstraw P. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol. 2008;3(5):457–466. doi: 10.1097/JTO.0b013e31816de2b8. [DOI] [PubMed] [Google Scholar]
- 33.Noronha V., Sekhar A., Patil V.M., et al. Systemic therapy for limited stage small cell lung carcinoma. J Thorac Dis. 2020;12(10):6275–6290. doi: 10.21037/jtd-2019-sclc-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paz-Ares L., Dvorkin M., Chen Y., et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 35.Horn L., Mansfield A.S., Szczęsna A., et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y., Paz-Ares L., Reinmuth N., et al. Impact of brain metastases on treatment patterns and outcomes with first-line durvalumab plus platinum-etoposide in extensive-stage SCLC (CASPIAN): a brief report. JTO Clin Res Rep. 2022;3(6) doi: 10.1016/j.jtocrr.2022.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogart J.A., Waqar S.N., Mix M.D. Radiation and systemic therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2022;40(6):661–670. doi: 10.1200/JCO.21.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li A.Y., Gaebe K., Jerzak K.J., Cheema P.K., Sahgal A., Das S. Intracranial metastatic disease: present challenges, future opportunities. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.855182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore S., Zhan L., Liu G., et al. EP03. 01-016 the Canadian small cell lung cancer database (CASCADE): results from a multi-institutional real-world evidence collaboration. J Thorac Oncol. 2022;17(9):S244. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.