Abstract
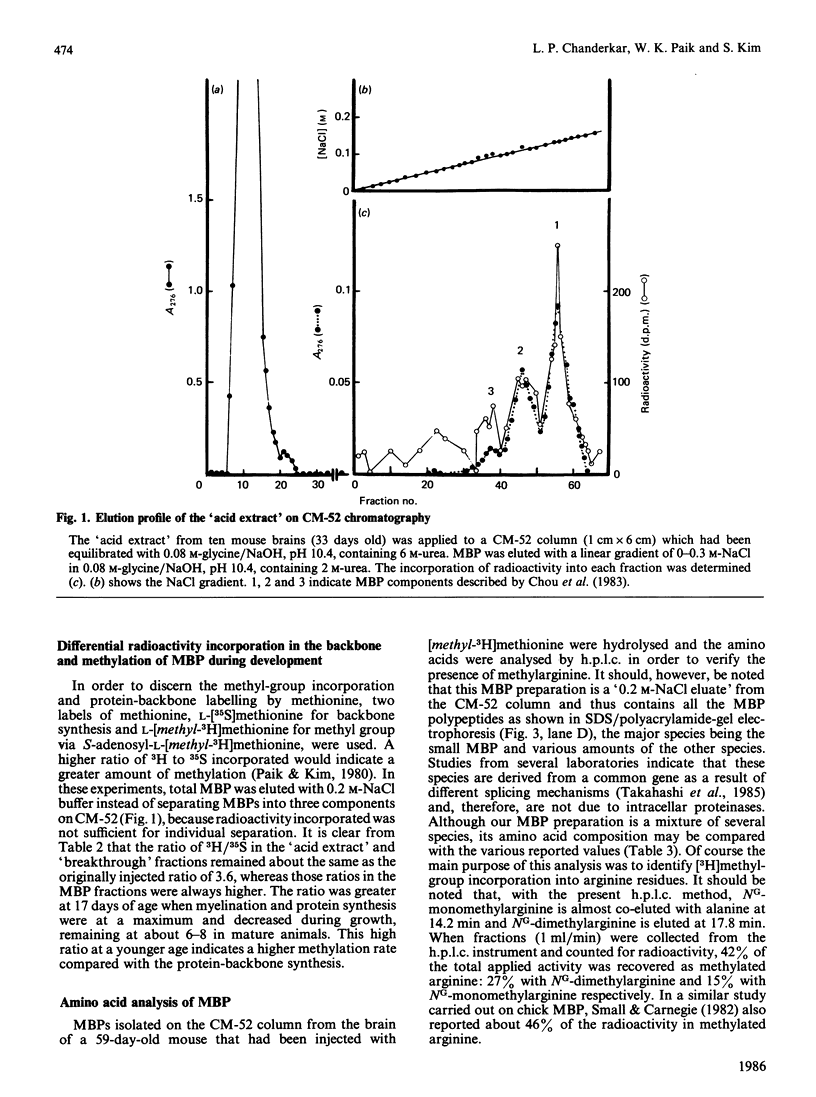
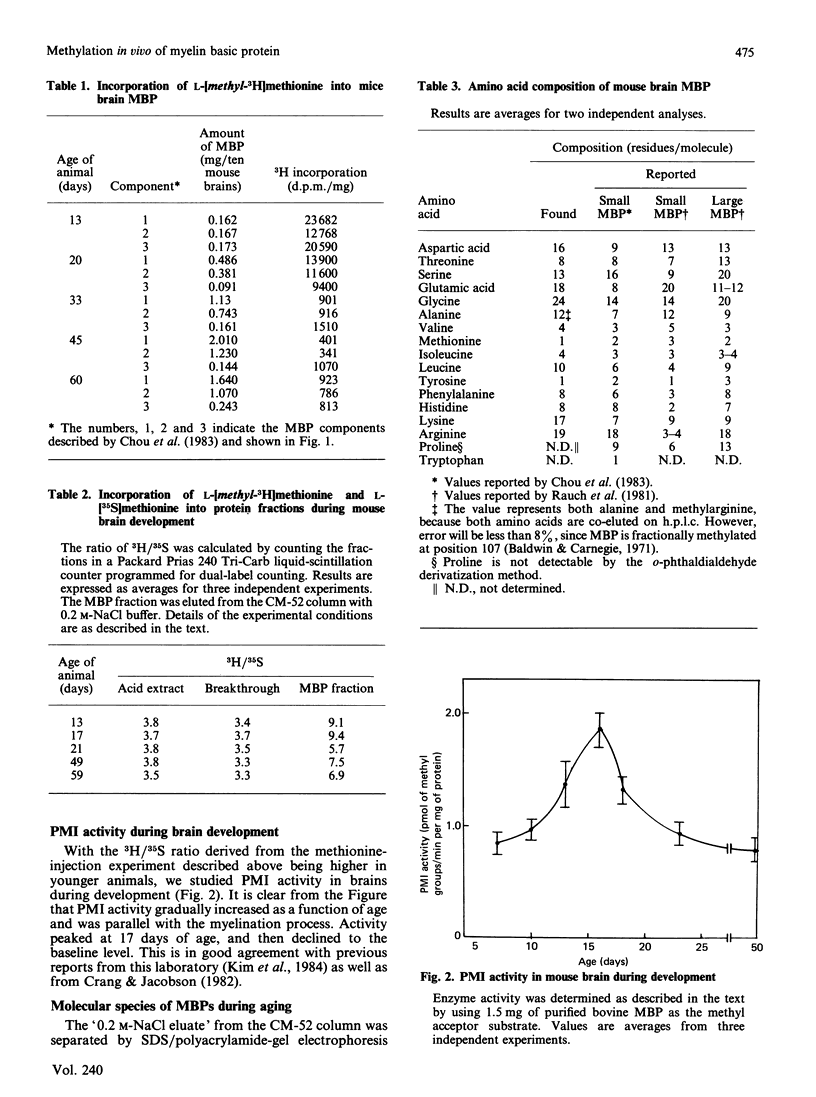
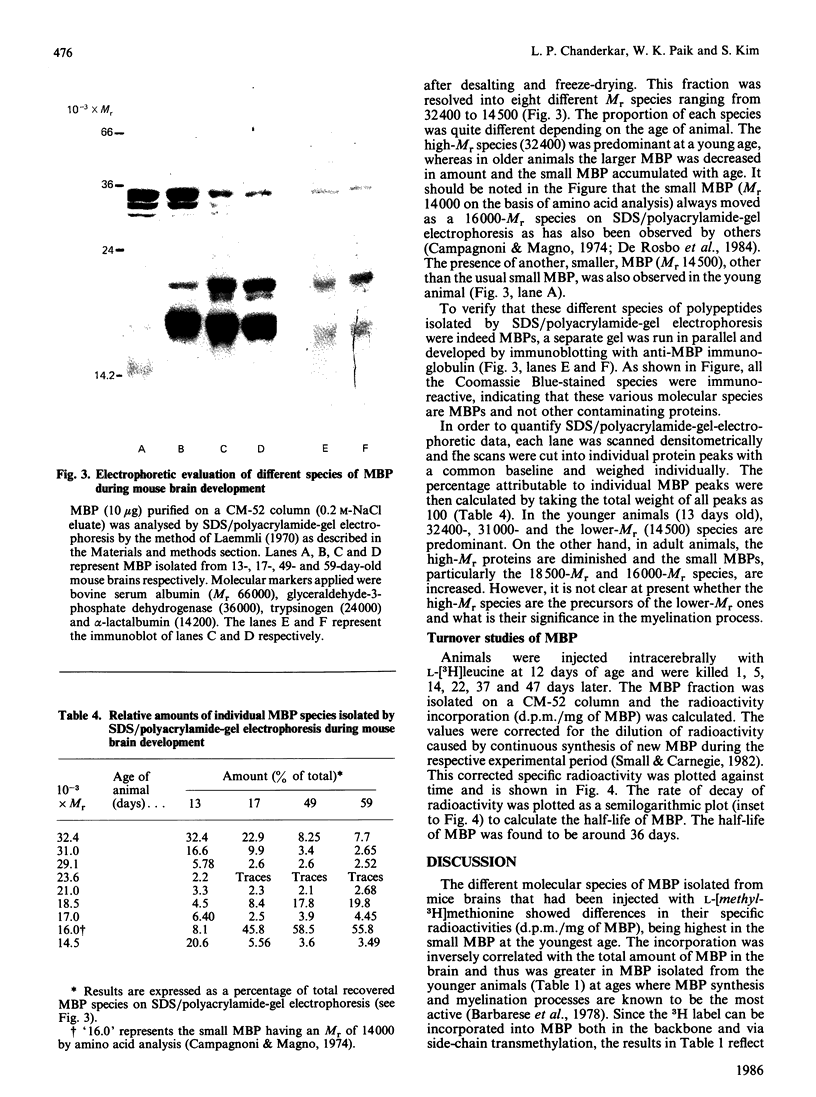
The synthesis and methylation in vivo of myelin basic protein (MBP) during the mouse brain development has been investigated. When mice ranging in age from 13 to 60 days were injected intracerebrally with L-[methyl-3H]methionine, the incorporation of radioactivity into MBP isolated from youngest brain was found to be the highest and declined progressively in mature brains. This pattern of radioactivity incorporation was inversely correlated with the total amount of MBP in the brains, suggesting a higher ratio of MBP methylation to synthesis in younger brain. To differentiate the relative rate of protein synthesis and methylation, animals were given intracerebral injections of a L-[methyl-3H]methionine and L-[35S]methionine mixture and the ratio of 3H/35S (methylation index) was determined. The ratios in the isolated MBP fractions were higher than those of 'acid extracts' and 'breakthrough' fractions, with a maximal ratio in the youngest brain. This high ratio was well correlated with the higher protein methylase I (PMI) activity in younger brains. The MBP fractions were further separated on SDS/polyacrylamide-gel electrophoresis into several species with apparent Mr ranging from 32,400 to 14,500. The results indicated that each protein species accumulated at a characteristic rate as a function of age. The high-Mr (32,400) species was predominant in younger brain, whereas the smaller MBP was the major species in older brain tissue. The importance of this developmental pattern of MBP synthesis and methylation is discussed in relation to PMI activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amur S. G., Shanker G., Pieringer R. A. Regulation of myelin basic protein (arginine) methyltransferase by thyroid hormone in myelinogenic cultures of cells dissociated from embryonic mouse brain. J Neurochem. 1984 Aug;43(2):494–498. doi: 10.1111/j.1471-4159.1984.tb00926.x. [DOI] [PubMed] [Google Scholar]
- Aspillaga M. O., McDermott J. R. The NG-methylated arginine content of rat myelin during development. J Neurochem. 1977 May;28(5):1147–1149. doi: 10.1111/j.1471-4159.1977.tb10683.x. [DOI] [PubMed] [Google Scholar]
- Baldwin G. S., Carnegie P. R. Specific enzymic methylation of an arginine in the experimental allergic encephalomyelitis protein from human myelin. Science. 1971 Feb 12;171(3971):579–581. doi: 10.1126/science.171.3971.579. [DOI] [PubMed] [Google Scholar]
- Barbarese E., Braun P. E., Carson J. H. Identification of prelarge and presmall basic proteins in mouse myelin and their structural relationship to large and small basic proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3360–3364. doi: 10.1073/pnas.74.8.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarese E., Carson J. H., Braun P. E. Accumulation of the four myelin basic proteins in mouse brain during development. J Neurochem. 1978 Oct;31(4):779–782. doi: 10.1111/j.1471-4159.1978.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Brostoff S., Eylar E. H. Localization of methylated arginine in the A1 protein from myelin. Proc Natl Acad Sci U S A. 1971 Apr;68(4):765–769. doi: 10.1073/pnas.68.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnoni A. T., Magno C. S. Molecular weight estimation of mouse and guinea-pig myelin basic proteins by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulphate: influence of ionic strength. J Neurochem. 1974 Oct;23(4):887–890. doi: 10.1111/j.1471-4159.1974.tb04422.x. [DOI] [PubMed] [Google Scholar]
- Campagnoni C. W., Carey G. D., Campagnoni A. T. Synthesis of myelin basic proteins in the developing mouse brain. Arch Biochem Biophys. 1978 Sep;190(1):118–125. doi: 10.1016/0003-9861(78)90258-8. [DOI] [PubMed] [Google Scholar]
- Carson J. H., Nielson M. L., Barbarese E. Developmental regulation of myelin basic protein expression in mouse brain. Dev Biol. 1983 Apr;96(2):485–492. doi: 10.1016/0012-1606(83)90185-9. [DOI] [PubMed] [Google Scholar]
- Chou C. H., Chou F. C., Kowalski T. J., Shapira R., Kibler R. F. The major site of guinea-pig myelin basic protein encephalitogenic in Lewis rats. J Neurochem. 1977 Jan;28(1):115–119. doi: 10.1111/j.1471-4159.1977.tb07716.x. [DOI] [PubMed] [Google Scholar]
- Chou C. H., Shapira R., Fritz R. B. Encephalitogenic activity of the small form of mouse myelin basic protein in the SJL/J mouse. J Immunol. 1983 May;130(5):2183–2186. [PubMed] [Google Scholar]
- Crang A. J., Jacobson W. The relationship of myelin basic protein (arginine) methyltransferase to myelination in mouse spinal cord. J Neurochem. 1982 Jul;39(1):244–247. doi: 10.1111/j.1471-4159.1982.tb04726.x. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Boyd L. F., Kies M. W. Enzymatic and nonenzymatic degradation of myelin basic protein. Neurochem Res. 1984 Oct;9(10):1371–1385. doi: 10.1007/BF00964664. [DOI] [PubMed] [Google Scholar]
- DesJardins K. C., Morell P. Phosphate groups modifying myelin basic proteins are metabolically labile; methyl groups are stable. J Cell Biol. 1983 Aug;97(2):438–446. doi: 10.1083/jcb.97.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinn J. J., Weir D. G., McCann S., Reed B., Wilson P., Scott J. M. Methyl group deficiency in nerve tissue: a hypothesis to explain the lesion of subacute combined degeneration. Ir J Med Sci. 1980 Jan;149(1):1–4. doi: 10.1007/BF02939099. [DOI] [PubMed] [Google Scholar]
- Eylar E. H., Thompson M. Allergic encephalomyelitis: the physico-chemical properities of the basic protein encephalitogen from bovine spinal cord. Arch Biochem Biophys. 1969 Feb;129(2):468–479. doi: 10.1016/0003-9861(69)90204-5. [DOI] [PubMed] [Google Scholar]
- Fischer C. A., Morell P. Turnover of proteins in myelin and myelin-like material of mouse brain. Brain Res. 1974 Jul 5;74(1):51–65. doi: 10.1016/0006-8993(74)90111-5. [DOI] [PubMed] [Google Scholar]
- GURNANI S. U., KUMTA U. S., SAHASRABUDHE M. B. Influence of formic acid on the hydrolysis of tissue proteins; a new and rapid method of hydrolysis of proteins. Biochim Biophys Acta. 1955 Apr;16(4):553–557. doi: 10.1016/0006-3002(55)90277-8. [DOI] [PubMed] [Google Scholar]
- Gharib A., Rey C., Fonlupt P., Sarda N., Pacheco H. Phospholipid methylase activity, [3H]S-adenosyl-L-homocysteine binding, and S-adenosyl-L-methionine and S-adenosyl-L-homocysteine levels in rat brain during maturation. J Neurochem. 1985 Jul;45(1):32–36. doi: 10.1111/j.1471-4159.1985.tb05470.x. [DOI] [PubMed] [Google Scholar]
- Glass W. F., 2nd, Briggs R. C., Hnilica L. S. Identification of tissue-specific nuclear antigens transferred to nitrocellulose from polyacrylamide gels. Science. 1981 Jan 2;211(4477):70–72. doi: 10.1126/science.7003713. [DOI] [PubMed] [Google Scholar]
- Hall C., Mahadevan L. C., Whatley S. A., Ling T. S., Lim L. The polyadenylated RNA directing the synthesis of the rat myelin basic proteins is present in both free and membrane-bound forebrain polyribosomes. Biochem J. 1982 Feb 15;202(2):407–417. doi: 10.1042/bj2020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Vidali G., Boffa L. C., Allfrey V. G. Characterization of the non-histone nuclear proteins associated with rapidly labeled heterogeneous nuclear RNA. J Biol Chem. 1977 Oct 25;252(20):7307–7322. [PubMed] [Google Scholar]
- Kerlero De Rosbo N., Carnegie P. R., Bernard C. C., Linthicum D. S. Detection of various forms of brain myelin basic protein in vertebrates by electroimmunoblotting. Neurochem Res. 1984 Oct;9(10):1359–1369. doi: 10.1007/BF00964663. [DOI] [PubMed] [Google Scholar]
- Kim S., Tuck M., Kim M., Campagnoni A. T., Paik W. K. Studies on myelin basic protein-specific protein methylase I in various dysmyelinating mutant mice. Biochem Biophys Res Commun. 1984 Sep 17;123(2):468–474. doi: 10.1016/0006-291x(84)90254-7. [DOI] [PubMed] [Google Scholar]
- Kim S., Wasserman L., Lew B., Paik W. K. Studies on the natural substrate for protein methylase II in mammalian brain and blood. J Neurochem. 1975 Apr;24(4):625–629. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee H. W., Kim S., Paik W. K. S-adenosylmethionine: protein-arginine methyltransferase. Purification and mechanism of the enzyme. Biochemistry. 1977 Jan 11;16(1):78–85. doi: 10.1021/bi00620a013. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Cook R. G., Ahn Y. S., Yeoman L. C., Busch H. Clustering of glycine and NG,NG-dimethylarginine in nucleolar protein C23. Biochemistry. 1985 Oct 22;24(22):6025–6028. doi: 10.1021/bi00343a001. [DOI] [PubMed] [Google Scholar]
- Miyake M. Methylases of myelin basic protein and histone in rat brain. J Neurochem. 1975 May;24(5):909–915. doi: 10.1111/j.1471-4159.1975.tb03655.x. [DOI] [PubMed] [Google Scholar]
- Morell P., Greenfield S., Costantino-Ceccarini E., Wisniewski H. Changes in the protein composition of mouse brain myelin during development. J Neurochem. 1972 Nov;19(11):2545–2554. doi: 10.1111/j.1471-4159.1972.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Protein methylase I. Purification and properties of the enzyme. J Biol Chem. 1968 May 10;243(9):2108–2114. [PubMed] [Google Scholar]
- Rauch H. C., Katar M., Montgomery I. N. Experimental allergic encephalomyelitis in mice: encephalitogenicity of mouse central myelin basic proteins. Eur J Immunol. 1981 Jul;11(7):545–549. doi: 10.1002/eji.1830110705. [DOI] [PubMed] [Google Scholar]
- Reidl L. S., Campagnoni C. W., Campagnoni A. T. Preparation and properties of an immunosorbent column specific for the myelin basic protein. J Neurochem. 1981 Aug;37(2):373–380. doi: 10.1111/j.1471-4159.1981.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Ro J. Y., Jensen D. E., Kim S. Quantitation of S-methylcysteine formed in O6-methylguanine-DNA:methyltransferase. Cancer Lett. 1984 Jun;23(2):213–221. doi: 10.1016/0304-3835(84)90156-3. [DOI] [PubMed] [Google Scholar]
- Sabri M. I., Bone A. H., Davison A. N. Turnover of myelin and other structural proteins in the developing rat brain. Biochem J. 1974 Sep;142(3):499–507. doi: 10.1042/bj1420499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. H., Carnegie P. R. In vivo methylation of an arginine in chicken myelin basic protein. J Neurochem. 1982 Jan;38(1):184–190. doi: 10.1111/j.1471-4159.1982.tb10870.x. [DOI] [PubMed] [Google Scholar]
- Smith M. E. The turnover of myelin proteins. Neurobiology. 1972;2(1):35–40. [PubMed] [Google Scholar]
- Takahashi N., Roach A., Teplow D. B., Prusiner S. B., Hood L. Cloning and characterization of the myelin basic protein gene from mouse: one gene can encode both 14 kd and 18.5 kd MBPs by alternate use of exons. Cell. 1985 Aug;42(1):139–148. doi: 10.1016/s0092-8674(85)80109-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. T., Campagnoni A. T. Vitro synthesis of the four mouse myelin basic proteins: evidence for the lack of a metabolic relationship. J Neurochem. 1982 Dec;39(6):1559–1568. doi: 10.1111/j.1471-4159.1982.tb07988.x. [DOI] [PubMed] [Google Scholar]