Abstract
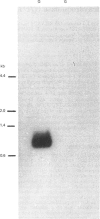
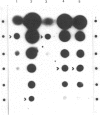
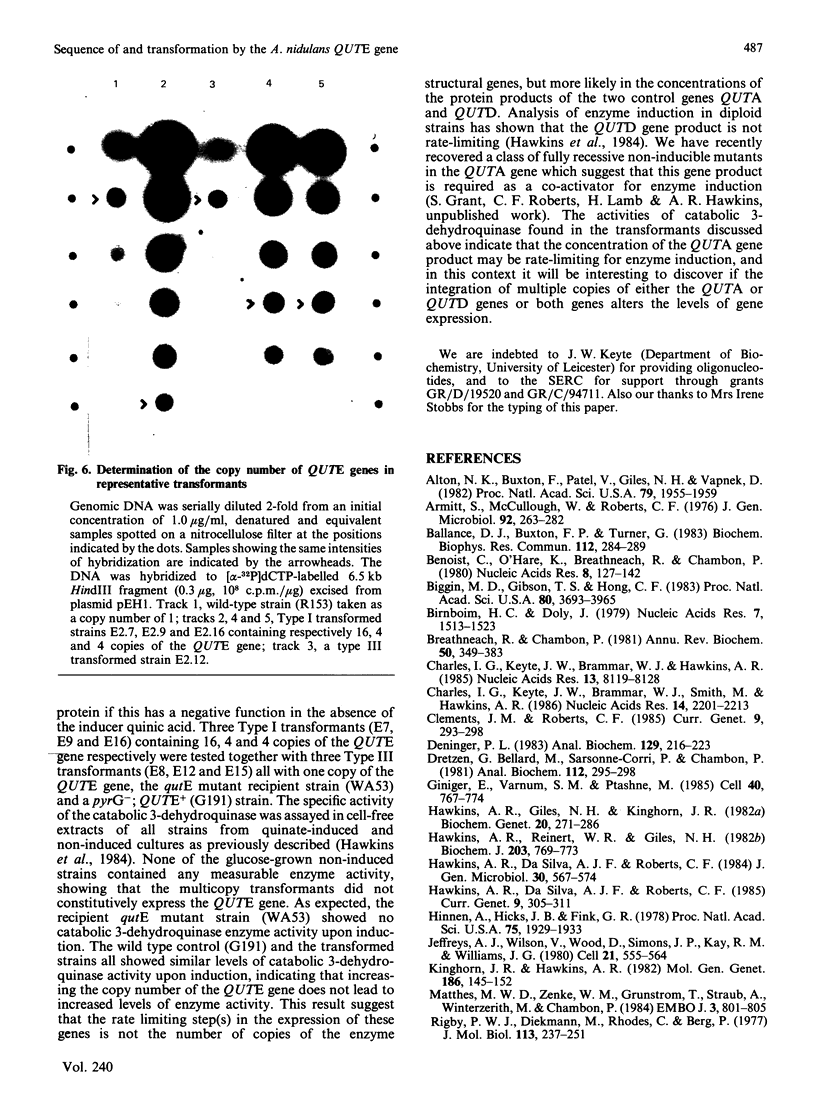
The induction of catabolic 3-dehydroquinase by quinic acid in Aspergillus nidulans has been shown to involve transcriptional control and yields a single major 0.8 kb mRNA. The nucleotide sequence of the catabolic 3-dehydroquinase QUTE gene has been determined and contains a single uninterrupted open reading frame of 462 bases encoding a 16,505 Da protein of 153 residues. Comparison with the corresponding QA2 gene of Neurospora crassa reveals the absence of 75 nucleotides encoding 25 amino acids from the centre of the QUTE gene of A. nidulans and the presence of 21 additional nucleotides at its 3' end. There is no nucleotide or amino acid homology between these two elements. A 16 bp inverted repeat (5' GGCAGAGCGTTCTGCC) shows similarity to such repeats found in other fungal promoters. The functional integrity of the QUTE gene was demonstrated by the transformation of a qutE mutant strain which regains growth on quinic acid as sole carbon source. Four of the twelve transformed strains examined contained vector sequences integrated at the qutE locus, and these strains all exhibited normal regulation of 3-dehydroquinase even when 16 copies of the QUTE gene were present.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Buxton F., Patel V., Giles N. H., Vapnek D. 5'-Untranslated sequences of two structural genes in the qa gene cluster of Neurospora crassa. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1955–1959. doi: 10.1073/pnas.79.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitt S., McCullough W., Roberts C. F. Analysis of acetate non-utilizing (acu) mutants in Aspergillus nidulans. J Gen Microbiol. 1976 Feb;92(2):263–282. doi: 10.1099/00221287-92-2-263. [DOI] [PubMed] [Google Scholar]
- Ballance D. J., Buxton F. P., Turner G. Transformation of Aspergillus nidulans by the orotidine-5'-phosphate decarboxylase gene of Neurospora crassa. Biochem Biophys Res Commun. 1983 Apr 15;112(1):284–289. doi: 10.1016/0006-291x(83)91828-4. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Charles I. G., Keyte J. W., Brammar W. J., Hawkins A. R. Nucleotide sequence encoding the biosynthetic dehydroquinase function of the penta-functional arom locus of Aspergillus nidulans. Nucleic Acids Res. 1985 Nov 25;13(22):8119–8128. doi: 10.1093/nar/13.22.8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles I. G., Keyte J. W., Brammar W. J., Smith M., Hawkins A. R. The isolation and nucleotide sequence of the complex AROM locus of Aspergillus nidulans. Nucleic Acids Res. 1986 Mar 11;14(5):2201–2213. doi: 10.1093/nar/14.5.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. M., Roberts C. F. Molecular cloning of the 3-phosphoglycerate kinase (PGK) gene from Aspergillus nidulans. Curr Genet. 1985;9(4):293–298. doi: 10.1007/BF00419958. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Giniger E., Varnum S. M., Ptashne M. Specific DNA binding of GAL4, a positive regulatory protein of yeast. Cell. 1985 Apr;40(4):767–774. doi: 10.1016/0092-8674(85)90336-8. [DOI] [PubMed] [Google Scholar]
- Hawkins A. R., Francisco Da Silva A. J., Roberts C. F. Cloning and characterization of the three enzyme structural genes QUTB, QUTC and QUTE from the quinic acid utilization gene cluster in Aspergillus nidulans. Curr Genet. 1985;9(4):305–311. doi: 10.1007/BF00419960. [DOI] [PubMed] [Google Scholar]
- Hawkins A. R., Francisco da Silva A. J., Roberts C. F. Evidence for two control genes regulating expression of the quinic acid utilization (qut) gene cluster in Aspergillus nidulans. J Gen Microbiol. 1984 Mar;130(3):567–574. doi: 10.1099/00221287-130-3-567. [DOI] [PubMed] [Google Scholar]
- Hawkins A. R., Giles N. H., Kinghorn J. R. Genetical and biochemical aspects of quinate breakdown in the filamentous fungus Aspergillus nidulans. Biochem Genet. 1982 Apr;20(3-4):271–286. doi: 10.1007/BF00484424. [DOI] [PubMed] [Google Scholar]
- Hawkins A. R., Reinert W. R., Giles N. H. Characterization of Neurospora crassa catabolic dehydroquinase purified from N. crassa and Escherichia coli. Biochem J. 1982 Jun 1;203(3):769–773. doi: 10.1042/bj2030769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Wood D., Simons J. P., Kay R. M., Williams J. G. Linkage of adult alpha- and beta-globin genes in X. laevis and gene duplication by tetraploidization. Cell. 1980 Sep;21(2):555–564. doi: 10.1016/0092-8674(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Kinghorn J. R., Hawkins A. R. Cloning and expression in Escherichia coli K-12 of the biosynthetic dehydroquinase function of the arom cluster gene from the eucaryote, Aspergillus nidulans. Mol Gen Genet. 1982;186(1):145–152. doi: 10.1007/BF00422927. [DOI] [PubMed] [Google Scholar]
- Matthes H. W., Zenke W. M., Grundström T., Staub A., Wintzerith M., Chambon P. Simultaneous rapid chemical synthesis of over one hundred oligonucleotides on a microscale. EMBO J. 1984 Apr;3(4):801–805. doi: 10.1002/j.1460-2075.1984.tb01888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Gait M. J. Chemical synthesis of a gene for somatomedin C. Nucleic Acids Res. 1985 Apr 25;13(8):2959–2977. doi: 10.1093/nar/13.8.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]