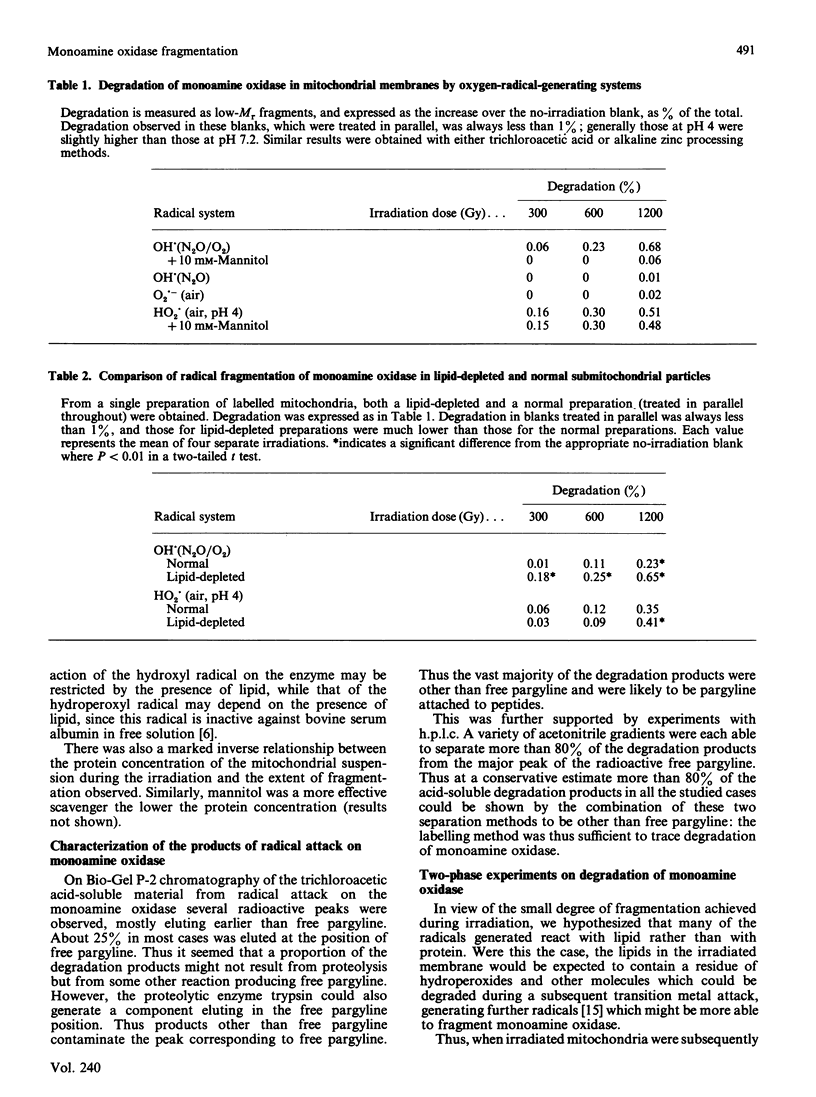
Abstract
A flux of hydroxyl radicals generated by gamma-irradiation can fragment monoamine oxidase in the membrane of submitochondrial particles. This fragmentation can be inhibited by mannitol and in addition is more extensive in monoamine oxidase preparations that have been depleted of lipid. This latter observation is consistent with the higher yields of fragmentation induced by hydroxyl radicals in soluble proteins in the absence of added lipids. In the absence of oxygen, gamma-irradiation of submitochondrial particles leads to cross-linking reactions. A flux of hydroperoxyl radicals also causes fragmentation, whereas one of superoxide is virtually inactive in this respect. The irradiation of submitochondrial particles leads in addition to the accumulation of products of lipid peroxidation. When these irradiated preparations are exposed to ferrous or cupric salts a further fragmentation of monoamine oxidase ensues, especially at acid pH. These transition-metal-catalysed reactions do not occur with irradiated preparations depleted of lipid, and the post-irradiation protein modifications are concomitant with further lipid peroxidation. The data indicate roles for lipid radicals in both fragmentation and cross-linking reactions of proteins in biological membranes. These reactions may have an important bearing on control of protein activity and of protein turnover in membranes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dean R. T., Barrett A. J. Lysosomes. Essays Biochem. 1976;12:1–40. [PubMed] [Google Scholar]
- Dean R. T., Pollak J. K. Endogenous free radical generation may influence proteolysis in mitochondria. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1082–1089. doi: 10.1016/0006-291x(85)90296-7. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Roberts C. R., Forni L. G. Oxygen-centred free radicals can efficiently degrade the polypeptide of proteoglycans in whole cartilage. Biosci Rep. 1984 Dec;4(12):1017–1026. doi: 10.1007/BF01116694. [DOI] [PubMed] [Google Scholar]
- Ekstedt B., Oreland L. Effect of lipid-depletion on the different forms of monoamine oxidase in rat liver mitochondria. Biochem Pharmacol. 1976 Jan 15;25(2):119–124. doi: 10.1016/0006-2952(76)90277-x. [DOI] [PubMed] [Google Scholar]
- Etherington D. J., Pugh D., Silver I. A. Collagen degradation in an experimental inflammatory lesion: studies on the role of the macrophage. Acta Biol Med Ger. 1981;40(10-11):1625–1636. [PubMed] [Google Scholar]
- Evans P. J., Mayer R. J. Comparison of the degradative fate of monoamine oxidase in endogenous and transplanted mitochondrial outer membrane in rat hepatocytes. Implications for the cytomorphological basis of protein catabolism. Biochem J. 1984 Apr 1;219(1):61–72. doi: 10.1042/bj2190061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karel M., Schaich K., Roy R. B. Interaction of peroxidizing methyl linoleate with some proteins and amino acids. J Agric Food Chem. 1975 Mar-Apr;23(2):159–163. doi: 10.1021/jf60198a046. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Brien P. J. Intracellular mechanisms for the decomposition of a lipid peroxide. I. Decomposition of a lipid peroxide by metal ions, heme compounds, and nucleophiles. Can J Biochem. 1969 May;47(5):485–492. doi: 10.1139/o69-076. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Garner A. Radiation-induced generation and properties of lipid hydroperoxide in liposomes. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Dec;44(6):615–625. doi: 10.1080/09553008314551681. [DOI] [PubMed] [Google Scholar]
- Schaich K. M. Free radical initiation in proteins and amino acids by ionizing and ultraviolet radiations and lipid oxidation--part III: free radical transfer from oxidizing lipids. Crit Rev Food Sci Nutr. 1980;13(3):189–244. doi: 10.1080/10408398009527290. [DOI] [PubMed] [Google Scholar]
- Schuessler H., Schilling K. Oxygen effect in the radiolysis of proteins. Part 2. Bovine serum albumin. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Mar;45(3):267–281. doi: 10.1080/09553008414550381. [DOI] [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The stimulatory effects of carbon tetrachloride on peroxidative reactions in rat liver fractions in vitro. Inhibitory effects of free-radical scavengers and other agents. Biochem J. 1971 Aug;123(5):823–828. doi: 10.1042/bj1230823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappel A. L. Lipid peroxidation damage to cell components. Fed Proc. 1973 Aug;32(8):1870–1874. [PubMed] [Google Scholar]
- Vladimirov Y. A., Olenev V. I., Suslova T. B., Cheremisina Z. P. Lipid peroxidation in mitochondrial membrane. Adv Lipid Res. 1980;17:173–249. doi: 10.1016/b978-0-12-024917-6.50011-2. [DOI] [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J. 1986 Mar 1;234(2):399–403. doi: 10.1042/bj2340399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]


