Abstract
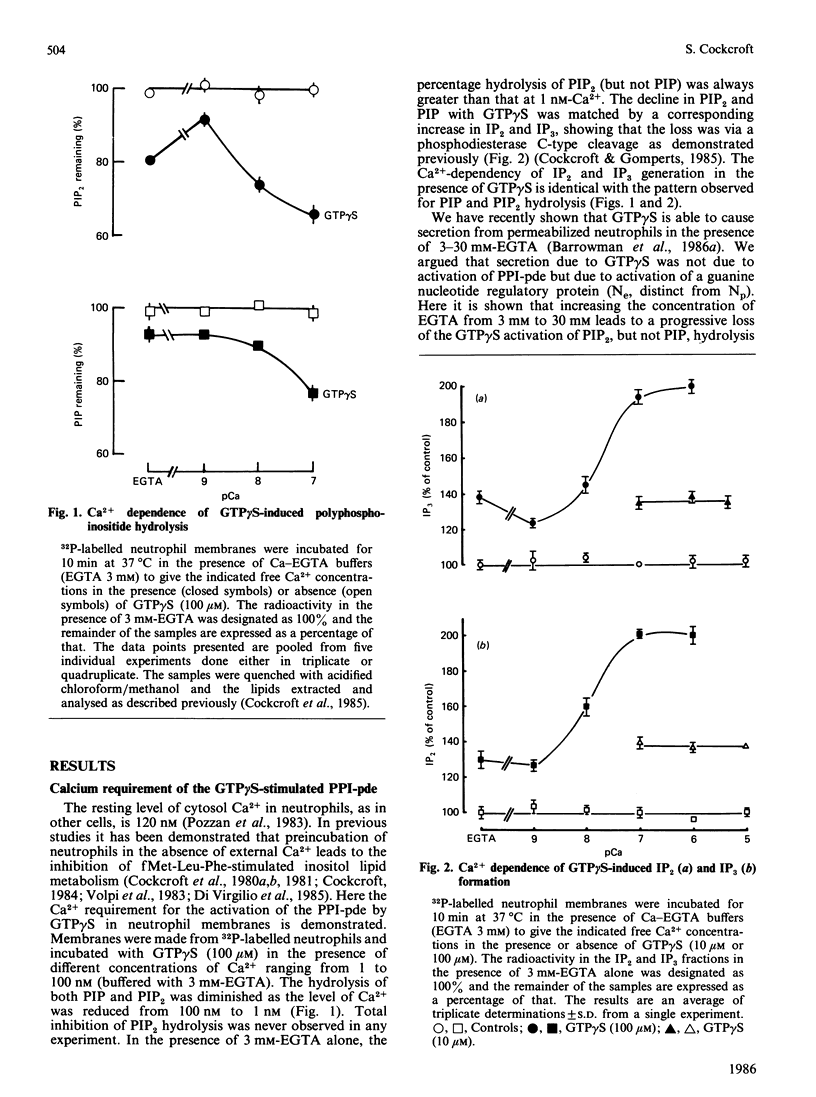
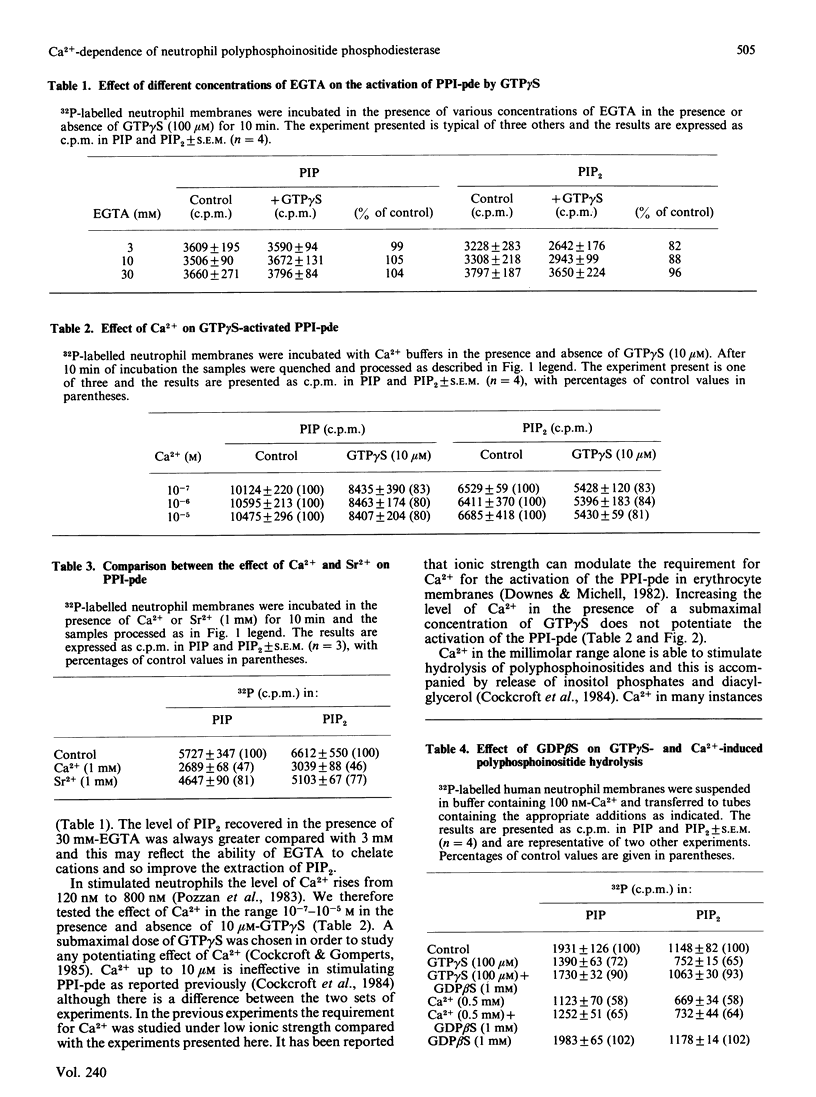
The requirement for Ca2+ for the activation of polyphosphoinositide phosphodiesterase was studied with the guanine nucleotide analogue guanosine 5'-[gamma-thio]triphosphate (GTP gamma S). Levels of Ca2+ that pertain in unstimulated neutrophils (100 nM) are obligatory for the full expression of enzyme activity stimulated with GTP gamma S. Reduction of Ca2+ to 1 nM leads to inhibition. Increasing the level of Ca2+ from 100 nM to 1000 nM does not alter enzyme activity. Guanosine 5'-[beta-thio]diphosphate (GDP beta S) does not stimulate the phosphodiesterase but is an effective inhibitor of activation by GTP gamma S. Ca2+ in the millimolar range can also activate the phosphodiesterase alone and this is not inhibited by GDP beta S. It is also shown that Sr2+ in the millimolar range can stimulate enzyme activity similarly to Ca2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Thomas P. The effects of Ca2+ and Sr2+ on Ca2+-sensitive biochemical changes in human erythrocytes and their membranes. Biochem J. 1981 Sep 15;198(3):441–445. doi: 10.1042/bj1980441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Kermode J. C., Naccache P. H., Yassin R., Marsh M. L., Munoz J. J., Sha'afi R. I. The inhibition of neutrophil granule enzyme secretion and chemotaxis by pertussis toxin. J Cell Biol. 1985 May;100(5):1641–1646. doi: 10.1083/jcb.100.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Caswell A. H., Gomperts B. D. Plasma-membrane location of phosphatidylinositol hydrolysis in rabbit neutrophils stimulated with formylmethionyl-leucylphenylalanine. Biochem J. 1982 Dec 15;208(3):801–808. doi: 10.1042/bj2080801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Use of cytochalasin B to distinguish between early and late events in neutrophil activation. Biochim Biophys Acta. 1980 Oct 2;601(3):584–591. doi: 10.1016/0005-2736(80)90560-x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Characterization of formylmethionyl-leucyl-phenylalanine stimulation of inositol trisphosphate accumulation in rabbit neutrophils. Mol Pharmacol. 1985 Jan;27(1):74–78. [PubMed] [Google Scholar]
- Buckley J. T., Hawthorne J. N. Erythrocyte membrane polyphosphoinositide metabolism and the regulation of calcium binding. J Biol Chem. 1972 Nov 25;247(22):7218–7223. [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Vallotton M. B. Cytosolic free calcium levels in monolayers of cultured rat aortic smooth muscle cells. Effects of angiotensin II and vasopressin. J Biol Chem. 1985 Jul 5;260(13):7836–7842. [PubMed] [Google Scholar]
- Cockcroft S., Baldwin J. M., Allan D. The Ca2+-activated polyphosphoinositide phosphodiesterase of human and rabbit neutrophil membranes. Biochem J. 1984 Jul 15;221(2):477–482. doi: 10.1042/bj2210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Barrowman M. M., Gomperts B. D. Breakdown and synthesis of polyphosphoinositides in fMetLeuPhe-stimulated neutrophils. FEBS Lett. 1985 Feb 25;181(2):259–263. doi: 10.1016/0014-5793(85)80271-4. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. Stimulus-secretion coupling in rabbit neutrophils is not mediated by phosphatidylinositol breakdown. Nature. 1980 Nov 20;288(5788):275–277. doi: 10.1038/288275a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. The dependence on Ca2+ of phosphatidylinositol breakdown and enzyme secretion in rabbit neutrophils stimulated by formylmethionyl-leucylphenylalanine or ionomycin. Biochem J. 1981 Dec 15;200(3):501–508. doi: 10.1042/bj2000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. f-MetLeuPhe-induced phosphatidylinositol turnover in rabbit neutrophils is dependent on extracellular calcium. FEBS Lett. 1980 Jan 28;110(1):115–118. doi: 10.1016/0014-5793(80)80036-6. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Ca2+-dependent conversion of phosphatidylinositol to phosphatidate in neutrophils stimulated with fMet-Leu-Phe or ionophore A23187. Biochim Biophys Acta. 1984 Aug 15;795(1):37–46. doi: 10.1016/0005-2760(84)90102-4. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Vicentini L. M., Treves S., Riz G., Pozzan T. Inositol phosphate formation in fMet-Leu-Phe-stimulated human neutrophils does not require an increase in the cytosolic free Ca2+ concentration. Biochem J. 1985 Jul 15;229(2):361–367. doi: 10.1042/bj2290361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The control by Ca2+ of the polyphosphoinositide phosphodiesterase and the Ca2+-pump ATPase in human erythrocytes. Biochem J. 1982 Jan 15;202(1):53–58. doi: 10.1042/bj2020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The action of lanthanum and manganese on anaphylactic histamine secretion. Br J Pharmacol. 1973 Jul;48(3):527–537. doi: 10.1111/j.1476-5381.1973.tb08359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts B. D., Barrowman M. M., Cockcroft S. Dual role for guanine nucleotides in stimulus-secretion coupling. Fed Proc. 1986 Jun;45(7):2156–2161. [PubMed] [Google Scholar]
- Hallam T. J., Thompson N. T., Scrutton M. C., Rink T. J. The role of cytoplasmic free calcium in the responses of quin2-loaded human platelets to vasopressin. Biochem J. 1984 Aug 1;221(3):897–901. doi: 10.1042/bj2210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., O'Flaherty J. T., Orr W., Showell H. J., Ward P. A., Becker E. L. Quantitative comparisons of various biological responses of neutrophils to different active and inactive chemotactic factors. Immunopharmacology. 1978 Dec;1(1):39–47. doi: 10.1016/0162-3109(78)90007-3. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D. O., Bajjalieh S. M., Kowalchyk J. A., Martin T. F. Direct stimulation by thyrotropin-releasing hormone (TRH) of polyphosphoinositide hydrolysis in GH3 cell membranes by a guanine nucleotide-modulated mechanism. Biochem Biophys Res Commun. 1985 Oct 30;132(2):721–728. doi: 10.1016/0006-291x(85)91192-1. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Tatham P. E., O'Flynn K., Linch D. C. The relationship between mitogen-induced membrane potential changes and intracellular free calcium in human T-lymphocytes. Biochim Biophys Acta. 1986 Apr 14;856(2):202–211. doi: 10.1016/0005-2736(86)90029-5. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhing R. J., Jiang H., Prpic V., Exton J. H. Regulation of a liver plasma membrane phosphoinositide phosphodiesterase by guanine nucleotides and calcium. FEBS Lett. 1985 Sep 2;188(2):317–320. doi: 10.1016/0014-5793(85)80394-x. [DOI] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Volpi M., Yassin R., Naccache P. H., Sha'afi R. I. Chemotactic factor causes rapid decreases in phosphatidylinositol,4,5-bisphosphate and phosphatidylinositol 4-monophosphate in rabbit neutrophils. Biochem Biophys Res Commun. 1983 May 16;112(3):957–964. doi: 10.1016/0006-291x(83)91711-4. [DOI] [PubMed] [Google Scholar]


