Abstract
Objective:
To assess nuclear distribution element-like 1 (Ndel1) enzyme activity following acute administration of sodium nitroprusside (sNP) in a rodent model of schizophrenia (SCZ) and in a cohort of chronic SCZ patients.
Methods:
Ndel1 activity was measured following sNP infusions in spontaneously hypertensive rats (SHR) (2.5 or 5.0 mg/kg) and in a double-blind randomized trial with 15 SCZ patients (0.5 μg/kg/min). Patients were randomized into two groups (group I: n=7; group II: n=8), with one group receiving placebo and the other sNP in phase A. In phase B, the groups switched treatments. sNP was administered as an infusion of 0.5 μg/kg/min, for 4 h, while placebo was a 5% glucose solution infused under the same conditions. The infusions were administered once weekly over 4 weeks. Psychopathology was assessed using the 18-item figure 5 (BPRS-18 – Bech’s version) and the negative subscale of the Positive and Negative Syndrome Scale.
Results:
Ndel1 activity was significantly reduced after sNP infusion in SHR and in patients receiving sNP (t = 7.756, degrees of freedom [df] = 97, p < 0.0001, dcohen=1.44) compared to placebo. Reduced Ndel1 activity from baseline to the end of infusion was only seen in patients after treatment with sNP.
Conclusion:
SCZ patients may benefit from adjunctive therapy with sNP and that the Ndel1 enzyme is a candidate biomarker of psychopathology in the disorder. Future research should look into the role of Ndel1 in SCZ and the potential effects of sNP and drugs with similar profiles of action in both animals and patients.
Keywords: Schizophrenia, sodium nitroprusside, Ndel1, animal model, biomarker
Introduction
Schizophrenia (SCZ), a chronic, devastating, and multi-factorial brain disorder, is the 8th leading cause of disability-adjusted life years (DALYs) worldwide.1 Despite its burden, SCZ is not fully understood, and its etiology and pathophysiology remain elusive. While antipsychotic drugs are still the mainstay of SCZ treatment, a significant proportion of patients exhibit poor responses.2 Alarmingly, approximately one in three patients have treatment resistant SCZ (TRS), a condition defined by the persistence of symptoms after two or more sequential trials with antipsychotic medications at appropriate dosage and duration.3
SCZ has been linked to deficits in nitric oxide (NO), which suggests that enhancement of nitrergic activity might be beneficial to patients. NO is generated within the central nervous system (CNS) through an intricate process that involves activation of the N-methyl-D-aspartate receptor (NMDAR) by glutamate, causing calcium (Ca2+) influx into the cell. This intracellular Ca2+ binds to calmodulin and stimulates neuronal nitric oxide synthase (nNOS) to produce NO in the CNS. This subsequently influences the release of neurotransmitters, including glutamate and dopamine, with direct effects on learning and memory, as well as neurodevelopment.4,5 Specifically, both SCZ and bipolar disorder patients have exhibited lower NO levels compared to control groups.6 In addition, a subtle negative correlation of NO levels with disease duration has been reported in bipolar patients, as well as a subtle positive correlation of NO levels with disease severity in SCZ patients.7
Novel molecules with the ability to increase NO production (such as NO donors) have thus emerged as promising candidates for the treatment of SCZ.8 In a translational clinical trial investigating the effects of the NO donor sodium nitroprusside (sNP) in acute SCZ, compelling evidence shows that sNP infusion led to a rapid amelioration of symptoms in SCZ patients who were concurrently receiving antipsychotic treatment, with noticeable improvements occurring within hours.9 This reduction in psychiatric symptom severity and cognitive deficits in SCZ by sNP was also demonstrated by others in both clinical studies and using animal models.10-13 Furthermore, it has also been proposed that sNP may have the ability to modulate the dopaminergic system.14 This suggests that NO-based therapies may offer substantial value for patients who have a poor response to treatment with antipsychotics or for clinical treatment failure, particularly when employed in combination with other therapeutic approaches.15,16
The activity of nuclear distribution element-like 1 (Ndel1), an oligopeptidase and neurodevelopmental protein, has been observed to be reduced in SCZ generally compared to healthy controls, and reduced to an even greater extent in patients with TRS compared to non-resistant SCZ patients.17 This implies that Ndel1 activity holds promise as a potential biomarker for patients with a SCZ diagnosis and as a support for treatment decisions.18 Furthermore, Ndel1 activity was positively associated with symptom amelioration in a 1-year follow-up study of first episode psychosis (FEP) patients who were treated mainly with the atypical antipsychotic risperidone, suggesting that Ndel1 activity may also have potential as a biomarker of clinical improvement, at least in FEP patients.18
We have demonstrated that Ndel1 enzyme activity in peripheral blood reflects changes in the CNS of a rodent animal model (the SHR strain),19 as assessed before and after the acute administration of psychostimulants or following a long-term treatment mainly with atypical antipsychotics, under conditions in which the SCZ-like animal behaviors were reverted.20 This supports the idea that Ndel1 activity, as measured in peripheral blood samples, could act as a surrogate to monitor changes in the CNS.20,21 Interestingly, Ndel1 has been implicated in serotonin signaling pathways based on nematode worm studies that used strains lacking Ndel1 orthologs.22,23 We believe this to be in line with evidence of a significantly lower Ndel1 activity in TRS,17 as the only Food and Drug Administration (FDA)-approved antipsychotic for TRS (the atypical antipsychotic clozapine) acts through blockade of 5-HT2A/5-HT2C serotonin receptors and the D1-4 dopamine receptor.24
The potential of sNP has been demonstrated for both preventing and reversing the development of SCZ-like behaviors in SHR rats.25 Furthermore, genetic deletion of nNOS appears to increase the binding of Ndel1 to the disrupted-in-schizophrenia 1 (DISC1) protein.26 DISC1 is a scaffold protein and known inhibitor of Ndel1,27 and nNOS deletion was seen to regulate neurite outgrowth, implicating nNOS signaling in cortical development and prefrontal cortex functioning.26 This latter effect may result from Ndel1-DISC1 interactions, given that decreases in Ndel1 activity due to the binding with DISC1 have previously been observed to correlate with decreased neurite outgrowth and neuronal migration.28-31 It is noteworthy that S-nitrosylation at cysteine 203 of Ndel1 accelerates dendritic arborization and enhances NMDAR-mediated neuronal activity, which is the main regulator of dendritic formation.32
Given the potentially convergent findings regarding the role of Ndel1 and sNP in SCZ symptom amelioration as well as the underlying biology of this disorder, we assessed for the first time the Ndel1 enzyme activity following acute administration of sNP in a rodent model of SCZ and in a cohort of chronic SCZ patients.
Methods
Animals
Male drug-naïve normotensive Wistar rats (NWRs) and spontaneously hypertensive rats (SHRs) aged 3-5 months and weighing 250-300 g were obtained from our own colony at Escola Paulista de Medicina (EPM). The animals were housed in groups of four in Plexiglas cages (41 × 34 × 16.5 cm) under controlled temperature (22-23 °C) and lighting conditions (12/12 h light/dark cycle, lights on at 07:00 a.m.), with free access to food and water. The animals were maintained in accordance with the guidelines of the Committee on Care and Use of Laboratory Animal Resources (National Research Council, USA). This study was approved by the ethics committee of Universidade Federal de São Paulo (UNIFESP/EPM), certificate CEUA no. 7290170315.
Treatment of animals and plasma preparation
Treatment of animals with sNP was performed essentially as previously described.19 Briefly, sNP (NITROPRUS – Cristália, São Paulo, Brazil) diluted in vehicle (0.9% NaCl saline solution) was administered by intraperitoneal (ip) injection (1.0 mL/kg) to adult (4‐months old) NWR or SHR animals, n=5 per group. In addition to sNP diluted in vehicle, NWR or SHR animals also received a vehicle injection (0.9% NaCl saline solution) as a control, n=5 per group.
Blood was collected from animals by caudal puncture in tubes containing heparin both before (baseline) and after (4, 24, and 48 h) ip administration of vehicle or sNP (2.5 or 5.0 mg/kg). Plasma samples were then recovered after centrifugation at 1,000-2,000 g for 10 min at 4 °C, and aliquots were stored at -20 °C until used, as described previously.18,33
Patients
This double-blind randomized trial is part of a larger project that investigated symptom improvement in patients with SCZ following the intravenous administration of sNP. The study protocol was registered in REBEC:RBR-2zhgjw clinical trials database (UTN: U1111-1213-9511).12
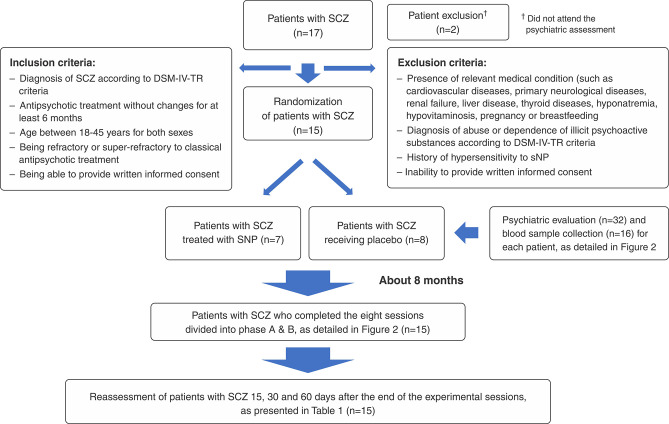
Participants were recruited from community mental health facilities in Ribeirão Preto (Brazil) and surrounding towns. Patients who met the inclusion criteria (Figure 1) underwent a psychiatric assessment before being considered for inclusion in this study.
Figure 1. Flow chart of the sample recruitment process. SCZ = schizophrenia; sNP = sodium nitroprusside.
All patients were invited to participate in the presence of a family member. The patient and the patient’s relatives received comprehensive information on the study procedures and were informed about the characteristics and implications of the study. Participation was conditional on the signing of a consent form, the design of which was approved by the ethics committee of Hospital das Clínicas, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo (HCFMRP-USP) (Resolution 466/2012 of the Conselho Nacional de Saúde, Comitê de Ética em Pesquisa, of the Brazilian Ministry of Health).
All participants had undergone previous assessments to identify relevant general medical conditions, including physical examination and a detailed clinical anamnesis collected from both the patient and the accompanying relative.
Clinical study procedure
According to the recruitment procedures detailed in Figure 1, 15 SCZ outpatients (15 men and two women; mean age, 32.94±6.49 years) in treatment with typical or atypical antipsychotics at the time of the trial were randomly assigned to receive either sNP or placebo according to a pseudo-randomization process (allocation ratio 1:1). Usual treatment was maintained. All patients and front-line study staff were blinded to the experimental treatment received by each patient. A fully trained anesthetist was present during each infusion to ensure safety.
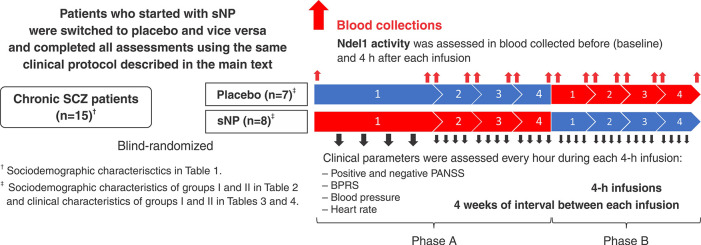
sNP was administered as an infusion of 0.5 μg/kg/min, for 4 h, while placebo was a 5% glucose solution infused under the same conditions. Patients received two rounds of treatment (phases A and B) and the infusions were administered on a weekly basis for 4 weeks (Figure 2). Each participant was interviewed by the same psychiatrist, using the 18-item Brief Psychiatric Rating Scale (BPRS-18 – Bech’s version) and the negative subscale of the Positive and Negative Syndrome Scale (PANSS – negative subscale) (Table S1 (307.2KB, pdf) , available as supplementary material).
Figure 2. Flow chart of clinical procedures. Patients with chronic SCZ were blind-randomized to a group I (n=8) or group II (n=7) to receive four infusions of placebo (in blue) or sNP (in red), respectively, in phase A of treatment. In the second phase (phase B), those receiving four infusions of PLACEBO in phase A were switched to sNP, while the group receiving sNP in phase A was switched to placebo in phase B of treatment. Blood was collected before (at baseline) and 4 h after each infusion of placebo or sNP. Clinical assessments occurred hourly during the 4-h infusion (i.e., total of four assessments for each patient per infusion). The interval between each infusion was of 1 month (i.e., 4 weeks each). BPRS = Brief Psychiatric Rating Scale; Ndel1 = nuclear distribution element-like 1; PANSS = Positive and Negative Syndrome Scale; SCZ = schizophrenia; sNP = sodium nitroprusside.
Patient recruitment and all clinical procedures occurred between 2016 and 2018. For this trial, 15 subjects with chronic SCZ were randomized into two groups (group I: n=7; group II: n=8), with one group receiving placebo and the other sNP in phase A. Clinical interviews were made every hour during the 4-h infusion, and this procedure was repeated four times at intervals of 1 month in both phases A and B. Physiological cardiovascular and pulmonary measures were also recorded every hour to assess the safety of sNP throughout the infusion. After infusion, patients were asked how they felt during the infusion, and if they had experienced any unexpected bodily or psychological sensation or discomfort. Participants were also allowed to rest for at least 1 h after this debriefing with the researcher.
The second round of treatment (phase B) was carried out in exactly the same manner as phase A, except that the group who received placebo in phase A was switched to sNP in phase B and vice versa (flow chart in Figure 2).
Outcome measures
The Structured Clinical Interview for DSM-IV (SCID) was used to confirm the diagnoses of SCZ. The BPRS-18 (Bech’s version) and the positive and negative PANSS subscales were used to measure changes in symptoms. Although the BPRS-18 and the PANSS were not originally intended to track short-term changes in psychopathology, we opted to follow the same method used in the trial by Hallak et al.9 that first described the effects of sNP in SCZ patients.
Safety and tolerability parameters were recorded throughout the infusions. Cardiac function was monitored using an automated monitor (model 2020, Dixtal Medical, São Paulo, Brazil) with electrocardiography, blood pressure, and blood oxygen saturation levels recorded hourly throughout the experimental sessions. The structured Udvalg for Kliniske Undersøgelser (UKU) rating scale was used to prospectively assess adverse treatment effects during this study.
Human blood collection for plasma preparation
Blood was collected into heparin tubes both before and 4 h after the infusion of sNP (or placebo). Plasma samples were then recovered following centrifugation at 1,000-2,000 g, for 10 min, at 4 °C. Aliquots of plasma were stored in microcentrifuge tubes at -20 °C until use, as previously described.18,33
Ndel1 enzyme activity measurements
Ndel1 activity was measured in blood plasma collected from all patients both before and 4 h after each infusion of placebo or sNP, for a total of eight samples per patient per round of treatment (phase A or B). Over both phases, a total of 240 measurements were therefore obtained for the 15 randomized SCZ patients (120 in placebo infusions and 120 in sNP infusions.
Ndel1 enzyme activity was measured as described elsewhere.17 Briefly, hydrolysis of a Ndel1 peptide substrate (Abz-GFSPFRQ-EDDnp) was monitored at 37 °C by measuring fluorescence (λEx = 320 nm and λEm = 420 nm) in a F-7000 spectrofluorometer (Hitachi Ltd., Ibaraki, Japan). Samples for these measurements consisted of 10 μL of plasma and 10 μM of substrate in 200 μL of buffer (NaCl 100 mM, 50 mM Tris-HCl pH 7.4). Ndel1-specific activity was determined through use of 10 μL of a heat-inactivated Ndel1 polyclonal antibody (NOAB inhibitor), which has specific inhibitory activity against Ndel1.27 Ndel1-specific activity was therefore defined as the rate of hydrolysis of the substrate peptide in the absence of NOAB inhibitor minus the rate when NOAB was present.34,35
Statistical analysis
The Gaussian distribution of variables was verified using the Kolmogorov-Smirnoff tests for the total sample and in each comparison group. Data were also tested for homogeneity and sphericity with Welch’s or Greenhouse-Geisser test respectively, and correction was applied when necessary. Chi-square was used to analyze categorical variables such as sex and ethnic background, represented here in sociodemographic tables. Standard parametric tests (paired and unpaired Student’s t test, two-way ANOVA for repeated measures and Pearson correlation) were applied according to variable type and distribution, and Bonferroni’s multiple comparison test was adopted for post-hoc analysis. Ndel1 activity values were log-transformed to suit normality. Additionally, we calculated the difference in Ndel1 activity (▵Ndel1), in nM/min, between post sNP or placebo measurements and their respective baseline values, aiming to enhance the clarity of the observed effects. Effect size analysis was conducted using Cohen’s d method. All results are expressed as mean ± SD. Outlier analysis was performed by the ROUT method (Q = 1%), identifying outliers from nonlinear regression based on the false discovery rate (FDR), with Q being the maximum desired FDR. Both graphs and tables were used to represent clinical outcomes, while Ndel1 activity was represented as graphs. Data analyses were performed using SPSS Statistics software version 22.0 and graphs were generated using GraphPad Prism version 7.00 for Windows. In all experiments, p-values ≤ 0.05 were considered to be statistically significant.
Results
Ndel1 activity in plasma from a rat model of schizophrenia
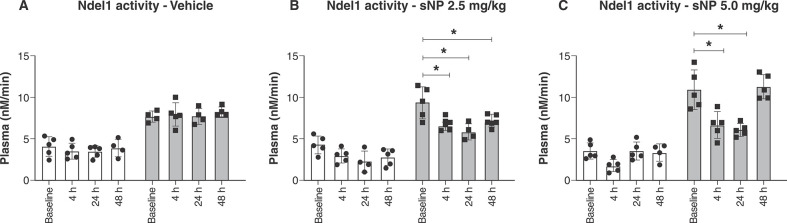
The SHR strain of rodents, a non-pharmacological animal model of SCZ, has been previously used to demonstrate decreases in social interaction and attenuated contextual fear conditioning deficits following acute administration of sNP at various doses.19 Here, we used similar conditions and doses to measure Ndel1 activity in SHR and control NWR, both before and after (4, 24, and 48 h) infusion with vehicle (Figure 3A) or sNP (2.5 or 5.0 mg/kg) (Figures 3B and 3C, respectively). When compared to baseline (9.35±1.89 nM/min), a significant decrease in Ndel1 oligopeptidase activity was seen in the plasma of SHR 4 h after the infusion of sNP (at both a 2.5 and 5.0 mg/kg concentration) (6.51±0.53 nM/min and 6.66±1.59 nM/min respectively). No significant changes in Ndel1 activity were observed in the control NWR after the infusion of sNP at either concentration (Figure 3).
Figure 3. Ndel1 activity in rat plasma 4 h after the infusion of sNP (2.5 or 5.0 mg/kg). Normotensive Wistar rat (white bars) and spontaneously hypertensive rats (gray bars) received the sNP infusion and had their blood collected in heparinized tubes 4, 24, and 24 h after the injection of vehicle or sNP. A) vehicle (0.9% NaCl saline solution). B) sNP 2.5 mg/kg; C) sNP 5.0 mg/kg. Ndel1 oligopeptidase activity was measured in 10 μL of plasma of each animal, and the data are presented here as μM/min. Statistical analysis by two-way analysis of variance (ANOVA) for n=5, * p ≤ 0.05. Ndel1 = nuclear distribution element-like 1; sNP = sodium nitroprusside.
Specifically, Ndel1 activity was not affected by administration of the vehicle in either strain (Figure 3A). In line with previous reports of SCZ-like behavior in SHR animals,13,19 SHR showed significant reductions in Ndel1 activity 4, 24, and 48 h (6.51±0.53 nM/min, 5.86±0.98 nM/min and 7.20±0.77 nM/min respectively) after the infusion of the lowest sNP dose studied here (2.5 mg/kg) when compared to baseline, before-infusion levels (9.35±1.89 nM/min) (F = [1.422, 10.43] = 13.62, p = 0.002 for time, F 1,8 = 84.15, p < 0.001 for strain factors, and F [1.42, 10.43] = 13.62, p = 0.002) for the interaction) (Figure 3B). With the higher dose of sNP (5.0 mg/kg), Ndel1 activity also decreased 4 and 24 h (6.66±1.59 nM/min and 6.08±0.75 nM/min respectively) after treatment, but not at 48 h for time (11.27±1.46 nM/min) (F = [2.117, 21.87] = 13.67, p < 0.001) and strain (F = 1,31 = 169.3, p < 0.001) factors, and for the interaction (F [2.11, 21.87] = 13.67, p < 0.001) (Figure 3C). These findings were used to select time points for the clinical phase of this work, with Ndel1 activity being measured in SCZ patients from blood collected 4 h after infusion with sNP or placebo.
Ndel1 activity in plasma of SCZ patients
Ndel1 activity was examined in a blind-randomized group of SCZ patients (group I: n=7 and group II: n=8). Sociodemographic data for the overall sample as well as for each separate study group appear in Table 1. There were no significant differences in sex, educational level, ethnic background, and age between the groups I and II. Clinical characteristics of these groups are described in Tables S2 and S3 (307.2KB, pdf) (available as supplementary material). The sample consisted almost exclusively of clozapine users (92.86%), with some participants using enhancers of conventional treatment such as lamotrigine and/or other antipsychotics.
Table 1. Sociodemographic characteristics of patients with chronic SCZ (n=15).
| Group I (n=7) | Group II (n=8) | Total (n=15) | Statistics | |||
|---|---|---|---|---|---|---|
| Test value | p-value | df | ||||
| Gender | ||||||
| Male | 6 (86) | 7 (87.5) | 13 (87) | 0.010 | 0.919 | 1 |
| Female | 1 (14) | 1 (12.5) | 2 (13) | |||
| Educational level (years of education) | ||||||
| ≤ 11 | 1 (14) | 2 (25) | 3 (20) | 0.142 | 0.707 | 1 |
| >11 | 6 (86) | 6 (75) | 12 (80) | |||
| Ethnic background | ||||||
| Caucasian | 5 (71) | 6 (75) | 11 (73) | 1.356 | 0.508 | 1 |
| Non-caucasian | 2 (29) | 2 (25) | 4 (27) | |||
| Age, M (± SD), years-old | 33 (5) | 30 (5) | 32 (5) | 0.997 | 0.337 | 13 |
| Min | 23 | |||||
| Max | 42 | |||||
Data presented as n (%), unless otherwise specified.
df = degrees of freedom; SCZ = schizophrenia.
Statistical significance was defined as p ≤ 0.05.
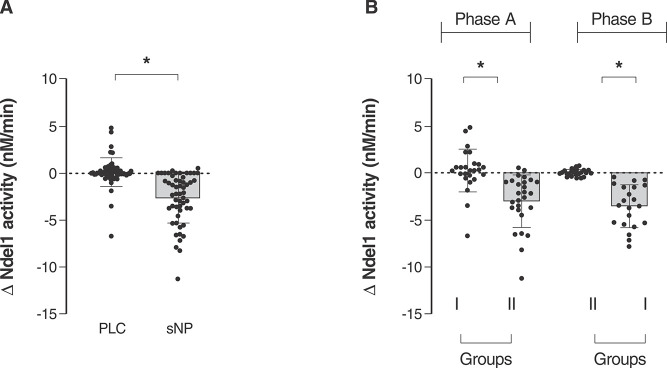
Groups had their Ndel1 activity analyzed before and 4 h after infusion with sNP or placebo, and the change in Ndel1 activity (ΔNdel1) between these two time points was determined. This trial was conducted twice (phases A and B), with patients receiving sNP in phase A and placebo in phase B, or vice versa. There was a significant decrease in Ndel1 activity following sNP infusion compared to placebo across the whole dataset (Figure 4A), and this was also seen when data from each phase (A and B) were analyzed separately, as shown in Figure 4B.
Figure 4. Delta Ndel1 activity in plasma of SCZ patients treated with placebo or sNP. A) Ndel1 activity was measured in the serum of all patients with chronic SCZ (n=15) just before and 4 h after they received placebo or sNP infusion for 4 h. Ndel1 activity (in μM/min) corresponds to Ndel1 activity after each infusion (of placebo or sNP) minus the value at baseline for each SCZ patient. Blood was collected before and 4 h after each infusion (n=4 infusion in each session) of placebo or sNP. Eight samples were collected from each SCZ patient for a total of 120 values of Ndel1 activity, presented for both placebo and sNP infusions. B) Ndel1 activity in blind-randomized group I (n=7) and II (n=8) patients with SCZ. Group I received placebo and group II received sNP in the first round of treatment (phase A), while in the second round of treatment (phase B), group II received placebo and group I received sNP. Each phase (A and B) involved four infusions of 4 h each of placebo or sNP, and the blood was collected just before and 4 h after each infusion. The statistical analysis employed unpaired t test. * p ≤ 0.05 for the comparison of the differences observed after the infusion of placebo or sNP minus their respective baseline values. Ndel1 = nuclear distribution element-like 1; PLC = placebo; SCZ = schizophrenia; sNP = sodium nitroprusside.
There were no significant differences in Ndel1 activity before and after placebo infusion in either phase, with ΔNdel1 activity close to 0 (-0.27±0.45 nM/min, t = 1.113, df = 98, p = 0.268) (Figure 4A). However, mean ΔNdel1 activity decreased after administration of sNP, both when all SCZ patients from both phases were considered together (-3.39±0.43 nM/min, t = 7.756, df = 97, p < 0.0001) (Figure 4A) and when each phase was studied individually (phase A: -3.29±0.71 nM/min, t = 4.604, df = 50, p < 0.0001; phase B: -3.56±0,45 nM/min, t = 7.889, df = 48, p < 0.0001, Figure 4B).
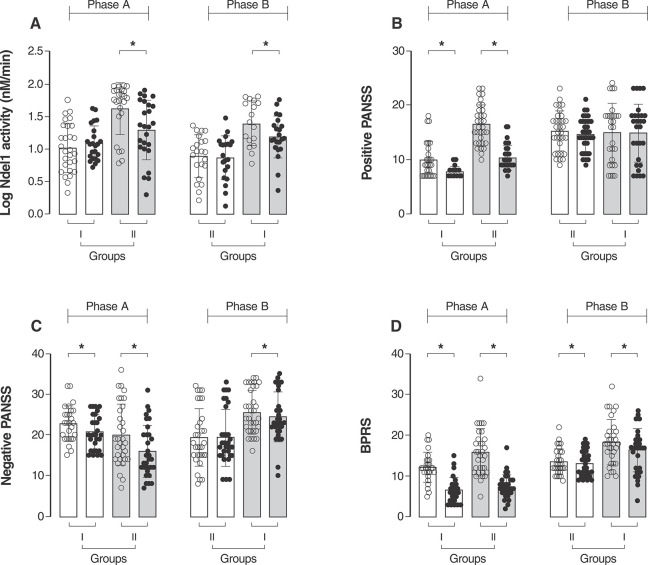
Ndel1 activity was significantly reduced in all subgroups of SCZ patients following the infusion of sNP (F [1, 107] = 25.86, p < 0.001) regardless of the first treatment received, placebo or sNP (mean difference of -2.77 nM/min, 95%CI [-1.95-3.59]) (Figure 5A). Furthermore, in both phases of the trial, patients receiving the sNP infusion showed improvement in symptoms, as assayed by positive and negative PANSS values and the BPRS score (Figure 5B-D, statistical values in Tables S2 and S3 (307.2KB, pdf) ). The only exception was the PANSS positive score after sNP infusions in the group receiving sNP in phase B, after receiving placebo in phase A (Figure 5B, statistical significance values provided in Tables S2 and S3 (307.2KB, pdf) ). We additionally conducted a comparative analysis of Ndel1 activity and psychiatric symptom scores after each session of sNP, confirming the observed effect (Figure S1 (307.2KB, pdf) ).
Figure 5. Values of log Ndel1 activity (A), positive (B) and negative PANSS (C) and BPRS (D) of patients in group I and group II after the first round (phase A) and second round of treatment (phase B). Values represent four infusion sessions with placebo or sNP for each SCZ patient. Ndel1 activity was assessed before and 4 h after infusion. Clinical assessments were performed hourly during the 4 h of placebo or sNP infusion. Paired t test, * p ≤ 0.05. BPRS = Brief Psychiatric Rating Scale; Ndel1 = nuclear distribution element‐like 1; Ndel1 = nuclear distribution element‐like 1; PANSS = Positive and Negative Syndrome Scale; SCZ = schizophrenia; sNP = sodium nitroprusside.
Ndel1 activity was also analyzed within each group of patients (I and II) to compare ▵Ndel1 in each phase — that is, to see whether sNP and placebo administered at different time points, in phase A or B, produced different effects on each specific group of patients. Both groups showed a significant decrease in Nde1l activity following sNP compared to placebo infusion (Figure S2 (307.2KB, pdf) ), despite higher r Ndel1 activity values observed for patients receiving sNP infusions (Figure 5A). This was seen for both the overall cohort (Cohen’s d effect size = 1.44), and the individual groups that received either placebo first (group I, Cohen’s d effect size = 1.68) or sNP first (group II, Cohen’s d effect size = 1.13).
It is also important to note that the clozapine doses used by patients in each group were not significantly different (group I: 550.0±342.3 mg, group II: 471.4±138.0 mg, t = 0.566, df = 13, p = 0.581). In addition, during the course of these experiments, no significant changes were recorded in cardiovascular conditions and/or blood pressure of patients, as shown by systolic blood pressure, diastolic blood pressure, and heart rate measures. The only exception was the increased heart rate seen in group II following placebo infusions, but not sNP infusions (Table S4 (307.2KB, pdf) , available as supplementary material). Crucially, the UKU rating scale used to prospectively assess the secondary outcomes of this study (including safety and tolerability) showed no observable adverse effects arising from the treatments used in this study (data not shown).
Discussion
Treatment of SHR animals with sNP under conditions previously shown to decrease social interaction and attenuate contextual fear conditioning deficits was associated with a significant decrease in plasma Ndel1 activity in SHR, whereas this effect was not observed in control NWR animals. Intriguingly, after infusion of sNP (at doses of 2.5 or 5.0 mg/kg), Ndel1 activity in the plasma of SHR animals was similar to that observed in NWR. This suggests that 4 or 24 h after sNP infusion, a condition more closely aligned with a physiological balance was evident in SHR animals, which is in close agreement with the improvements in animal behavior previously described by others.19 Moreover, it was determined that the interval of 4 h after the infusion of sNP was sufficient to observe a significant decrease in Ndel1 activity in the blood of animal models. Consequently, all clinical samples collected to monitor changes in Ndel1 enzyme activity in human patients were obtained 4 h after placebo or sNP infusions.
A significant decrease in Ndel1 activity was consistently observed in all patients with chronic SCZ following sNP infusion. This was evident when comparing the post-sNP infusion values to the baseline levels, measured at different times, in the same SCZ patients, as well as when comparing post-sNP infusion values to post-placebo infusion values, measured at the same time. Interestingly, this decrease in Ndel1 activity was even more pronounced in the first round of treatment with sNP (phase A) compared with the second round of treatment (phase B). These effects seemingly did not arise due to differences in the patients’ main pharmacological treatments, since there was no significant difference in the mean amount of clozapine that each group was receiving.
The use of adjunct sNP treatment for patients with SCZ has been suggested as a strategy to allow lower doses of antipsychotics while reducing the risk of side effects, based on behavioral and biochemical analysis of an animal model receiving low doses of sNP (1 and 1.5 mg/kg) aiming to improve the efficacy of the atypical antipsychotic risperidone.36 The data presented here show that sNP not only improves the symptoms of patients with TRS, but also leads to a significant decrease in Ndel1 activity in their plasma. Previous studies have suggested a correlation between Ndel1 activity in the plasma and the brain.20 In light of the various previous studies that implicate Ndel1 activity in SCZ and symptom severity,17,18 we hypothesize that these two effects of sNP are linked, with Ndel1 being part of the molecular mechanism(s) underlying the effects of sNP infusions on both SCZ-like animal behavior improvement19 and SCZ symptoms amelioration.9
Previous studies by our group have demonstrated a significant decrease in Ndel1 oligopeptidase activity in an SHR animal model, but not in NWR after long-term treatment with the typical antipsychotic haloperidol or the atypical antipsychotic clozapine,20 suggesting that the specific and selective effects of sNP seen in pathological conditions may not be observed in physiological healthy conditions (represented by control drug-naïve NWR animals).20 Decreases in Ndel1 activity were also previously shown to be associated with the amelioration of symptoms in FEP patients.18 Additionally, significantly lower Ndel1 activity was reported in medicated patients with chronic SCZ relative to healthy controls, with even lower Ndel1 activity in treatment-resistant compared with treatment non-resistant SCZ patients.17 Although we could also confirm the significant decrease in Ndel1 activity following sNP infusion in both SCZ animal model and patients with chronic SCZ, it was not possible to demonstrate a clear association of these changes with the improvements in the animal behavior deficits (including the reported decreases in social interaction and attenuated contextual fear conditioning deficits) or with amelioration of clinical symptoms in the patients (increases in several clinical scores, including PANSS).
The interaction between Ndel1 and other cytoskeletal proteins, particularly DISC1, has been proposed as a significant component of the neurodevelopmental hypothesis of SCZ. DISC1 binds to Ndel1 and inhibits its oligopeptidase activity.28,30,33 One potential mechanism through which Ndel1 activity could be decreased following sNP treatment would be an interference with Ndel1 nitrosylation, a post-translational event previously reported by others,32 affecting Ndel1 interaction with DISC1. Further studies are needed to demonstrate this as a potential mechanism.
In statistics, an effect size is a value that expresses the strength of the relationship between two variables in a population, or a sample-based estimate of that quantity. If the difference between the means obtained for two groups is less than 0.2 SDs, the difference is considered negligible, even if statistically significant; in turn, Cohen suggested that d = 0.8 or higher could be expressing a “large” effect size.37 Remarkably, in our study, a significant difference in Ndel1 plasma activity was observed between the placebo controls and sNP treated groups, showing a strong effect of the sNP infusion. This effect was evident both for SCZ patients who received placebo in the initial phase of treatment (phase A), namely group I (effect size Cohen’s d = 1.68), and for those who did not receive placebo in the first phase, namely group II (effect size Cohen’s d = 1.13). Therefore, a “large” effect size for the Ndel1 activity reduction following the administration of sNP was demonstrated here.
We have also noticed a decrease in psychiatric symptom scores following placebo infusion, but only in the first round of treatment (phase A), which also led to significant alterations in the cardiovascular parameters of all patients receiving placebo or sNP (belonging to groups I or II respectively). These observations were not repeated in the second round of treatment (phase B) (Table S4 (307.2KB, pdf) ). However, our study clearly establishes that this placebo-induced effect did not impact Ndel1 activity. Instead, Ndel1 activity was exclusively modulated by sNP infusions and remained unaffected by placebo infusions in both treatment phases. Moreover, we believe that the lack of correlation between the reductions in Ndel1 activity and symptom improvement in this study may be attributed to both the limited sample size and the distinct effects observed for placebo and sNP treatments.
It should be noted that not all the evidence available on the effects of sNP in SCZ points in the same direction, and that some controlled trials failed to find beneficial effects of the drug on both symptoms and cognitive measures.38,39 The divergent findings of these trials might reflect methodological differences, which include important aspects such as patient populations (early/late phase, treatment resistant), symptom severity (higher PANSS scores), and treatment contexts (monotherapy or polytherapy), leading to varying outcomes and highlighting the complexity of addressing SCZ. Our study aims to shed light on specific facets, including the significance of monotherapy, for example the prevalent use of clozapine in our cohort, for the investigation of sNP adjunctive pharmacotherapy. An additional limitation of our study is the relatively small size of the clinical sample enrolled, as well as the impossibility to demonstrate the direct correlation of possible post-translational modifications or complex formation of Ndel1 with decreased activity and/or with symptom improvement. These points will be the focus of future studies aiming to clarify the actual molecular contribution(s) of Ndel1 to the adjunctive effects of NO donors such as sNP on antipsychotic therapies as evaluated herein. Furthermore, we intend to expand our investigations by analyzing larger patient cohorts, as we hypothesize that these findings will bolster the implications of Ndel1 enzyme activity in clinical practice. This will aid in monitoring drug treatment for patients with SCZ and advancing our understanding of the mechanisms underpinning the therapeutic efficacy of sNP.
In conclusion, our results support the hypothesis that Ndel1 activity is linked to the severity of SCZ symptoms, with changes in Ndel1 activity correlating with symptom/behavioral amelioration following treatment with antipsychotics and/or an NO donor in rats and patients. The findings presented suggest that SCZ patients being treated with clozapine may benefit from adjunctive therapy with sNP, and that the Ndel1 enzyme is a candidate biomarker of psychopathology levels in SCZ. Future research should look into the role of Ndel1 in SCZ pathophysiology and study the potential effects of sNP and drugs with similar action profiles in both animals and humans.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grants 2022/00527-8; 2020/01107-7; 2019/13112-8; 2019/08287-3; 2017/02413-1; 2014/50891-1 [INCT 2014 - Translational Medicine]), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). JVN is the recipient of a Fellowship from FAPESP (grants 2022/03297-3 and 2019/09207-3). MAFH is the recipient of a fellowship from CNPq (39337/2016-0).
Footnotes
How to cite this article: Nani JV, Ushirohira JM, Bradshaw NJ, Machado-de-Sousa JP, Hallak JEC, Hayashi MAF. Sodium nitroprusside as an adjunctive treatment for schizophrenia reduces Ndel1 oligopeptidase activity. Braz J Psychiatry. 2024;46:e20233315. http://doi.org/10.47626/1516-4446-2023-3315
References
- 1.GBD 2016 Neurology Collaborators Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jauhar S, Lawrie SM. What is the evidence for antipsychotic medication and alternative psychosocial interventions for people with acute, non-affective psychosis? Lancet Psychiatry. 2022;9:253–60. doi: 10.1016/S2215-0366(21)00293-5. [DOI] [PubMed] [Google Scholar]
- 3.Correll CU, Agid O, Crespo-Facorro B, de Bartolomeis A, Fagiolini A, Seppälä N, et al. A Guideline and checklist for initiating and managing clozapine treatment in patients with treatment-resistant schizophrenia. CNS Drugs. 2022;36:659–79. doi: 10.1007/s40263-022-00932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segieth J, Fowler L, Whitton P, Pearce B. Nitric oxide-mediated regulation of dopamine release in the hippocampus in vivo. Neuropharmacology. 2000;39:571–7. doi: 10.1016/s0028-3908(99)00178-1. [DOI] [PubMed] [Google Scholar]
- 5.Richards LA, Schonhoff CM. Nitric oxide and sex differences in dendritic branching and arborization. J Neurosci Res. 2021;99:1390–400. doi: 10.1002/jnr.24789. [DOI] [PubMed] [Google Scholar]
- 6.Cheah SY, Lawford BR, Young RM, Morris CP, Voisey J. Association of NOS1AP variants and depression phenotypes in schizophrenia. J Affect Disord. 2015;188:263–9. doi: 10.1016/j.jad.2015.08.069. [DOI] [PubMed] [Google Scholar]
- 7.Ustundag MF, Ozcan H, Gencer AG, Yilmaz ED, Uğur K, Oral E, et al. Nitric oxide, asymmetric dimethylarginine, symmetric dimethylarginine and L-arginine levels in psychotic exacerbation of schizophrenia and bipolar disorder manic episode. Saudi Med J. 2020;41:38–45. doi: 10.15537/smj.2020.1.24817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitsikas N. The role of nitric oxide donors in schizophrenia: basic studies and clinical applications. Eur J Pharmacol. 2015;766:106–13. doi: 10.1016/j.ejphar.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 9.Hallak JE, Maia-de-Oliveira JP, Abrao J, Evora PR, Zuardi AW, Crippa JAS, et al. Rapid improvement of acute schizophrenia symptoms after intravenous sodium nitroprusside: a randomized, double-blind, placebo-controlled trial. JAMA Psychiatry. 2013;70:668–76. doi: 10.1001/jamapsychiatry.2013.1292. [DOI] [PubMed] [Google Scholar]
- 10.Maia-de-Oliveira JP, Abrao J, Evora PR, Zuardi AW, Crippa JAS, Belmonte-de-Abreu P, et al. The effects of sodium nitroprusside treatment on cognitive deficits in schizophrenia: a pilot study. J Clin Psychopharmacol. 2015;35:83–5. doi: 10.1097/JCP.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 11.Brown HE, Freudenreich O, Fan X, Heard SO, Goff D, Petrides G, et al. Efficacy and tolerability of adjunctive intravenous sodium nitroprusside treatment for outpatients with schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2019;76:691–9. doi: 10.1001/jamapsychiatry.2019.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adelino MPM, Nunes MV, Nunes MFQ, Costa ER, Jr, Ajub E, Mitrovitch MPB, et al. Treatment-resistant schizophrenia – A RCT on the effectiveness of repeated-dose sodium nitroprusside. Schizophr Res. 2021;231:70–2. doi: 10.1016/j.schres.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Zoupa E, Pitsikas N. The nitric oxide (NO) donor sodium nitroprusside (SNP) and its potential for the schizophrenia therapy: lights and shadows. Molecules. 2021;26:3196. doi: 10.3390/molecules26113196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maia-de-Oliveira JP, Lobão-Soares B, Baker GB, Dursun SM, Hallak JEC. Sodium nitroprusside, a nitric oxide donor for novel treatment of schizophrenia, may also modulate dopaminergic systems. Schizophr Res. 2014;159:558–9. doi: 10.1016/j.schres.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Maia-de-Oliveira JP, Kandratavicius L, Nunes EA, Machado-de-Sousa JP, Hallak JE, Murat Dursun SM. Nitric oxide’s involvement in the spectrum of psychotic disorders. Curr Med Chem. 2016;23:2680–91. doi: 10.2174/0929867323666160721144549. [DOI] [PubMed] [Google Scholar]
- 16.MacKay MAB, Paylor JW, Wong JTF, Winship IR, Baker GB, Dursun SM. Multidimensional connectomics and treatment-resistant schizophrenia: linking phenotypic circuits to targeted therapeutics. Front Psychiatry. 2018;9:537. doi: 10.3389/fpsyt.2018.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadelha A, Machado MFM, Yonamine CM, Sato JR, Juliano MA, Oliveira V, et al. Plasma Ndel1 enzyme activity is reduced in patients with schizophrenia--a potential biomarker? J Psychiatr Res. 2013;47:657–63. doi: 10.1016/j.jpsychires.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Dal Mas C, Nani JV, Noto C, Yonamine CM, da Cunha GR, Mansur RB, et al. Ndel1 oligopeptidase activity as a potential biomarker of early stages of schizophrenia. Schizophr Res. 2019;208:202–8. doi: 10.1016/j.schres.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Diana MC, Peres FF, Justi V, Bressan RA, Lacerda ALT, Crippa JA, et al. Sodium nitroprusside is effective in preventing and/or reversing the development of schizophrenia-related behaviors in an animal model: the SHR strain. CNS Neurosci Ther. 2018;24:624–32. doi: 10.1111/cns.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nani JV, Lee RS, Yonamine CM, Sant’Anna OA, Juliano MA, Gadelha A, et al. Evaluation of NDEL1 oligopeptidase activity in blood and brain in an animal model of schizophrenia: effects of psychostimulants and antipsychotics. Sci Rep. 2020;10:18513. doi: 10.1038/s41598-020-75616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nani JV, Fonseca MC, Engi SA, Perillo MG, Dias Cs, Gazarini ML, et al. Decreased nuclear distribution nudE-like 1 enzyme activity in an animal model with dysfunctional disrupted-in-schizophrenia 1 signaling featuring aberrant neurodevelopment and amphetamine-supersensitivity. J Psychopharmacol. 2020;34:467–77. doi: 10.1177/0269881119897562. [DOI] [PubMed] [Google Scholar]
- 22.Monte GG, Nani JV, de Almeida Campos MR, Dal Mas C, Marins LAN, Martins LG, et al. Impact of nuclear distribution element genes in the typical and atypical antipsychotics effects on nematode Caenorhabditis elegans: putative animal model for studying the pathways correlated to schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:19–30. doi: 10.1016/j.pnpbp.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Campeiro JD, Nani JV, Monte GG, Almeida PGC, Mori MA, Hayashi MAF. Regulation of monoamine levels by typical and atypical antipsychotics in Caenorhabditis elegans mutant for nuclear distribution element genes. Neurochem Int. 2021;147:105047. doi: 10.1016/j.neuint.2021.105047. [DOI] [PubMed] [Google Scholar]
- 24.Haidary HA, Padhy RK. StatPearls [Internet] Treasure Island: StatPearls Publishing; 2023. Clozapine. [PubMed] [Google Scholar]
- 25.Nani JV, Rodríguez B, Cruz FC, Hayashi MAF. Animal models in psychiatric disorder studies. Cap 4. In: Tvrdá E, Yenisetti SC, editors. Animal models in medicine and biology. London: IntechOpen; 2019. pp. 66–74. [Google Scholar]
- 26.Zoubovsky SP, Pogorelov VM, Taniguchi Y, Kim SH, Yoon P, Nwulia E, et al. Working memory deficits in neuronal nitric oxide synthase knockout mice: potential impairments in prefrontal cortex mediated cognitive function. Biochem Biophys Res Commun. 2011;408:707–12. doi: 10.1016/j.bbrc.2011.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi MAF, Portaro FCV, Bastos MF, Guerreiro JR, Oliveira V, Gorrão SS, et al. Inhibition of NUDEL (nuclear distribution element-like)-oligopeptidase activity by disrupted-in-schizophrenia 1. Proc Natl Acad Sci U S A. 2005;102:3828–33. doi: 10.1073/pnas.0500330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi MAF, Felicori LF, Fresqui MAC, Yonamine CM. Protein-protein and peptide-protein interactions of NudE-Like 1 (Ndel1): a protein involved in schizophrenia. Curr Protein Pept Sci. 2015;16:754–67. doi: 10.2174/1389203716666150505225251. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi MAF, Guerreiro JR, Charych E, Kamiya A, Barbosa RL, Machado MF, et al. Assessing the role of endooligopeptidase activity of Ndel1 (nuclear-distribution gene E homolog like-1) in neurite outgrowth. Mol Cell Neurosci. 2010;44:353–61. doi: 10.1016/j.mcn.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Bradshaw NJ, Hayashi MAF. NDE1 and NDEL1 from genes to (mal)functions: parallel but distinct roles impacting on neurodevelopmental disorders and psychiatric illness. Cell Mol Life Sci. 2017;74:1191–210. doi: 10.1007/s00018-016-2395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez B, Nani JV, Almeida PGC, Brietzke E, Lee RS, Hayashi MAF. Neuropeptides and oligopeptidases in schizophrenia. Neurosci Biobehav Rev. 2020;108:679–93. doi: 10.1016/j.neubiorev.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Saito A, Taniguchi Y, Kim SH, Selvakumar B, Perez G, Ballinge MD, et al. Developmental alcohol exposure impairs activity-dependent S-Nitrosylation of NDEL1 for neuronal maturation. Cereb Cortex. 2017;27:3918–29. doi: 10.1093/cercor/bhw201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nani JV, Yonamine CM, Castro Musial D, Dal Mas C, Mari JJ, Hayashi MAF. ACE activity in blood and brain axis in an animal model for schizophrenia: effects of dopaminergic manipulation with antipsychotics and psychostimulants. World J Biol Psychiatry. 2020;21:53–63. doi: 10.1080/15622975.2019.1583372. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi MA, Portaro FC, Tambourgi DV, Sucupira M, Yamane T, Fernandes BL, et al. Molecular and immunochemical evidences demonstrate that endooligopeptidase A is the predominant cytosolic oligopeptidase of rabbit brain. Biochem Biophys Res Commun. 2000;269:7–13. doi: 10.1006/bbrc.2000.2243. [DOI] [PubMed] [Google Scholar]
- 35.Nani JV, Almeida PGC, Hayashi MAF. Neuropeptidases in psychiatric disorders. In: Della Sala S, editor. Encyclopedia of behavioral neuroscience. Vol 1. Amsterdam: Elsevier; 2021. [Google Scholar]
- 36.Titulaer J, Malmerfelt A, Marcus MM, Svensson TH. Enhancement of the antipsychotic effect of risperidone by sodium nitroprusside in rats. Eur Neuropsychopharmacol. 2019;29:1282–7. doi: 10.1016/j.euroneuro.2019.08.302. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. Statistical power analysis for the behavioral sciences. Abingdon: Routledge; 1988. [Google Scholar]
- 38.Stone JM, Morrison PD, Koychev I, Gao F, Reilly TJ, Kolanko M, et al. The effect of sodium nitroprusside on psychotic symptoms and spatial working memory in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. Psychol Med. 2016;46:3443–50. doi: 10.1017/S0033291716002245. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Zhao J, Hu YQ, Jiao z, Lu Y, Ding M, et al. Sodium nitroprusside treatment for psychotic symptoms and cognitive deficits of schizophrenia: a randomized, double-blind, placebo-controlled trial. Psychiatry Res. 2018;269:271–7. doi: 10.1016/j.psychres.2018.08.079. [DOI] [PubMed] [Google Scholar]