Abstract
Purpose
To report a case of multifocal vitelliform lesions in a patient affected by metabolic encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) with the m.3243A>G variant.
Observations
A 37-year-old woman affected by MELAS was referred to our center for progressive vision deterioration. On fundus examination, she presented bilateral macular atrophy associated in the left eye with neurosensory detachment, along with several bilateral vitelliform lesions close to the vascular arcades. We describe the dynamic evolution of the vitelliform lesions, which could either enlarge and coalesce or undergo atrophic evolution.
Conclusions
Mitochondrial retinopathy due to the m.3243A>G variant can be associated with multifocal vitelliform lesions.
Keywords: Mitochondrial retinopathy, OCT, Autofluorescence imaging
1. Introduction
Mitochondrial retinopathy secondary to specific mitochondrial variants may present variable phenotypical manifestations, ranging from simple pigmentary abnormalities to extensive granular alterations and central chorioretinal atrophy.1 Recently, a case of bilateral vitelliform lesion in a patient affected by metabolic encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) was reported.2 We describe a case of multifocal vitelliform lesion in a patient affected by MELAS with the m.3243A>G variant in mitochondrial DNA.
2. Case report
A 37-year-old woman complaining of progressive vision deterioration was referred to our unit for a clinical examination. The patient was previously diagnosed with MELAS, confirmed by identification of m.3243A>G variant, with a heteroplasmy level of 20% in blood and 60% in urine. The region of the MT-TL1 gene containing the variant was amplified by PCR and then quantified by Restriction Fragment Length Polymorphism (RFLP). Genomic DNA was extracted from peripheral blood and analyzed via targeted next-generation sequencing (NGS) using the TruSight One Clinical Exome sequencing panel on an Illumina NexSeq500 platform, enriching for 4800 genes including ABCA4, BEST1, IMPG1, IMPG2, and PRPH2. No pathogenic variants associated with inherited retinal diseases were identified. The patient already presented multisystem manifestations of the disease, namely diabetes, deafness, and muscle cramps after physical exercise. Medical history was similar in her mother, who presented severe cardiomyopathy, visual defect and deafness. The m.3243A>G variant was detected in 20% of mitochondrial genomes extracted from urinary sediment, while it was virtually absent in DNA extracted from blood. A maternal aunt was also affected by diabetes, cardiomyopathy and deafness, though she was not examined.
The proband's best corrected visual acuity (BCVA) was 20/32 and 20/50 Snellen in right and left eye, respectively. Anterior segment and intraocular pressure were normal. Biomicroscopic fundus examination disclosed a round central atrophy in the right eye, and a smaller atrophic patch associated with neurosensory detachment in the macula of the left eye, along with several bilateral vitelliform lesions close to the vascular arcades (Fig. 1). Fundus autofluorescence (FAF) revealed hyperautofluorescence of the vitelliform lesions. Optical coherence tomography (OCT) showed hyperreflective signal corresponding to the vitelliform lesions (Fig. 2). Electrooculogram was normal in both eyes.
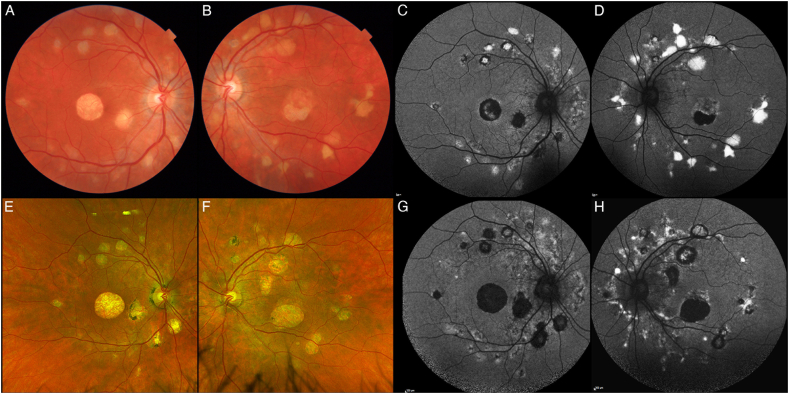
Fig. 1.
Color fundus photographs (CFP) and short-wavelength fundus autofluorescence (SW-AF). At baseline, our patient presented with bilateral macular atrophy, associated in the left eye to neurosensory detachment (A, B). In both eyes, several vitelliform lesions can be found close to the vascular arcades. Seven years later the macular atrophy grew in size, with reabsorption of the subretinal fluid in the left eye, while the vitelliform lesions either underwent atrophic evolution or enlarged in size (E, F). SW-AF at baseline (C, D) and six years later (G, H) show the transition of the lesions from hyperautofluorescence to hypoautofluorescence. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
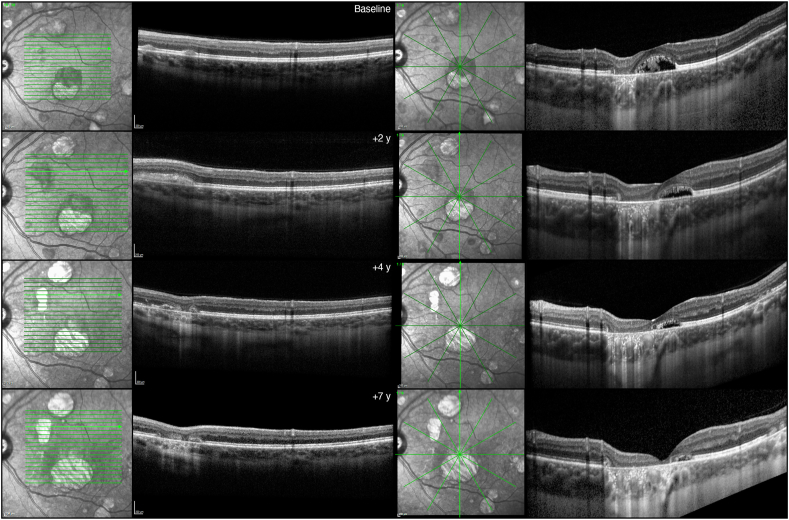
Fig. 2.
Optical coherence tomography (OCT). Left column: at baseline, OCT revealed two separate vitelliform lesions in the superior macular region, identifiable as subretinal hyperreflective material. Over 2 years, these lesions coalesced, before the development of retinal pigment epithelium (RPE) and outer retinal atrophy in the next 5 years. Right column: a vertical OCT scan passing through the fovea demonstrates the concomitant presence of RPE and outer retinal atrophy, along with neurosensory detachment in the foveal region at baseline. Over time, the atrophy enlarges while the subretinal fluid is reabsorbed.
Over the 7-year follow-up, BCVA slowly reduced to 20/50 and 20/80 Snellen in right and left eye, respectively. Macular atrophy expanded from the baseline area of 2.46 mm2 and 1.27 mm2 to the final area of 5.40 mm2 and 4.34 mm2, in the right and left eyes, respectively. The vitelliform lesions showed a dynamic evolution, some of them showing enlargement and coalescence, with progressive fading and evolution towards retinal pigment epithelium and outer retinal atrophy. OCT revealed the gradual reabsorption of the hyperreflective material leaving neurosensory detachment and advancing toward atrophic changes (Fig. 2).
MAIA microperimetry was performed 4 years after the initial presentation and at the last follow-up. The exams revealed an absence of retinal sensitivity in the area of macular atrophy, with fixation close to the border of the atrophy, and reduced retinal sensitivity even in the biomicroscopically uninvolved macular area. Predictably, following the enlargement of the lesions, mean sensitivity slowly decreased over a 3-year timeframe (Fig. 3).
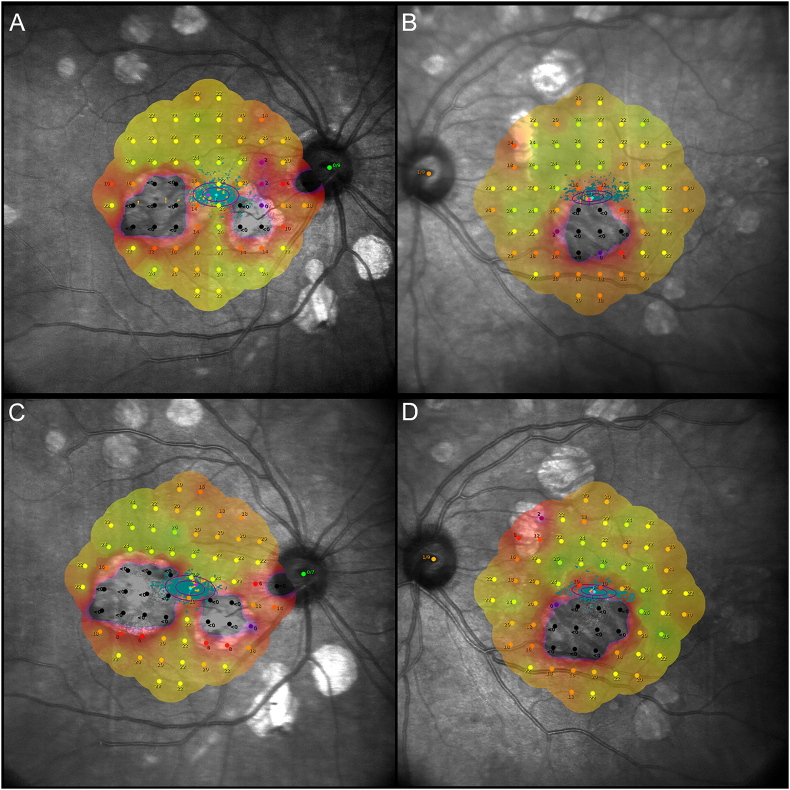
Fig. 3.
MAIA microperimetry. (A, B) Microperimetry taken 4 years after presentation reveals a mean retinal sensitivity of 15.4 dB in the right eye and 18.2 dB in the left eye. As demonstrated by the bivariate contour ellipse area (BCEA), fixation was relatively unstable in the right eye (area: 7.5°2) but stable in the left eye (area: 3.5°2). (C, D) Three years later, mean sensitivity decreased by 0.8 dB in the right eye and 1.5 dB in the left eye. Similarly, fixation stability deteriorated, with an enlargement in BCEA of 1°2 in the right eye and 0.5°2 in the left eye.
Family members who underwent an ophthalmological examination did not show any sign of retinopathy, with the exception of her 58-year-old mother, already diagnosed with MELAS. BCVA was 20/25 in both eyes and showed focal yellowish lesions at the posterior pole, which turned out to be hyperautofluorescent on FAF. Over the 7-year-follow-up the focal lesions showed progressive reabsorption of the yellowish material, whereas other yellowish focal lesions developed at the posterior pole (Fig. 4). No significant functional change was registered over the follow-up.
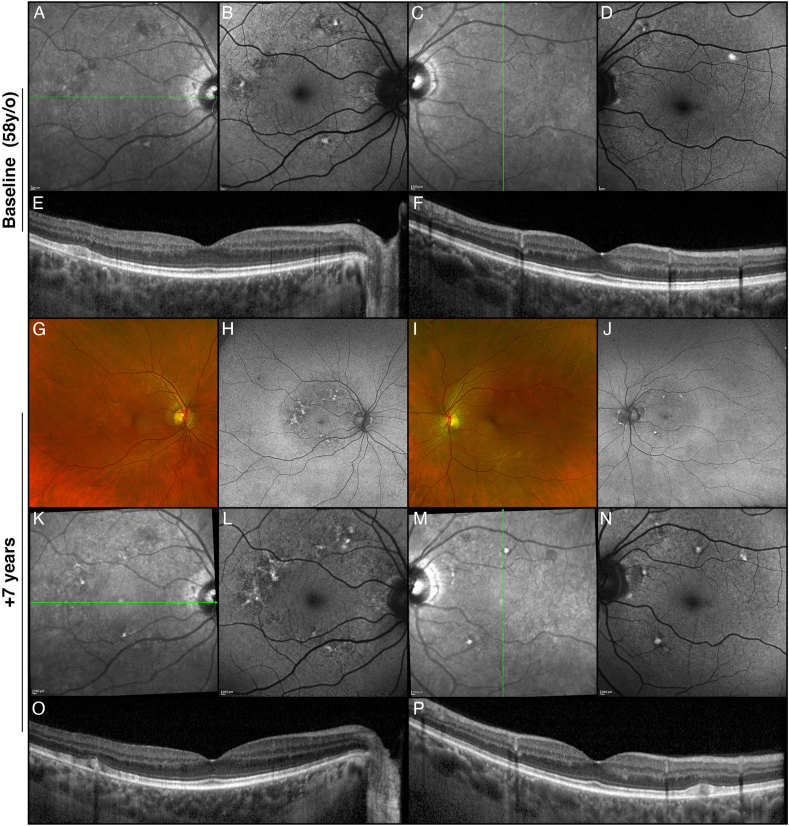
Fig. 4.
Multimodal imaging of the proband's mother. Infrared reflectance (A, C), fundus autofluorescence (B, D), and optical coherence tomography (E, F) of the right and left eyes. The yellowish lesions observed on fundus examination appeared hyperautofluorescent on fundus autofluorescence and corresponded to flat accumulations of subretinal hyperreflective material on OCT. Seven years later (G–P), the fovea remained uninvolved, despite the development of new yellowish lesions at the posterior pole (G, I).
3. Discussion
Vitelliform lesions can be ascribed to several genetic or acquired conditions.3 Biomicroscopically, vitelliform lesions are characterized by yellowish subretinal material that enlarges or, more frequently, decreases in size and progresses toward atrophic evolution over time.
The present case report expands our knowledge about the phenotypic manifestations associated with mitochondrial retinopathies. This term refers to the retinal manifestations of a broad spectrum of disorders linked to pathogenic variants and structural changes in the mitochondrial genome, including those linked to the m.3243G > A variant in the MT-TL1 gene.3 In 2013, de Laat et al. classified the variable phenotypes associated with this variant into 4 different grades.4 These included mild pigmentary abnormalities in the central fundus (grade 1), fleck-like hyperautofluorescent lesions at the posterior pole (grade 2), and profound chorioretinal atrophy with or without foveal involvement (grade 4 and 3, respectively). Even at that time, the authors identified a single case with an atypical vitelliform lesion that progressed to chorioretinal atrophy over a period of five years.
More recently, a case showing single central vitelliform lesion similar to the one typical of BEST1-related dystrophy was reported in an 11-year-old girl.2 Although the patient was genetically confirmed to have MELAS, carrying the same m.3243A>G variant, the genetic assessment disclosed also the c.1840C>T variant in the IMPG1 gene. On the contrary, the present patient was found to be negative for any genes associated with vitelliform lesions. Bearing in mind the clinical picture of the mother, who showed focal yellowish pigmented lesions at the posterior pole, we postulate that the vitelliform lesions in the daughter represent an exaggerated subretinal material accumulation taking the form of multiple vitelliform lesions. The central atrophy may represent the result of the atrophic evolution of a vitelliform lesion. The OCT-documented reabsorption of the vitelliform material along the vascular arcades supports this interpretation. The pathogenesis of the vitelliform lesions in the setting of MELAS remains unclear. Dysfunctions of the mitochondria at the level of the retinal pigment epithelium may lead to a cellular impairment with vitelliform material accumulation leading to final apoptosis and clinically visible atrophy.5, 6, 7
Further studies are warranted to investigate the complex spectrum of phenotypic manifestations of mitochondrial retinopathy.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Patient consent
Each study subject provided signed informed consent.
CRediT authorship contribution statement
Maurizio Battaglia Parodi: Writing – original draft, Conceptualization. Alessio Antropoli: Writing – review & editing, Writing – original draft, Data curation. Lorenzo Bianco: Validation, Data curation. Alessandro Arrigo: Validation, Supervision. Sebastiano Del Fabbro: Investigation, Data curation. Paola Carrera: Supervision. Ivana Spiga: Validation. Alessia Catania: Investigation. Costanza Lamperti: Supervision, Investigation. Francesco Bandello: Supervision. Ahmad Mansour: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Birtel J., von Landenberg C., Gliem M., et al. Mitochondrial retinopathy. Ophthalmol Retina. 2022;6(1):65–79. doi: 10.1016/j.oret.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Jahrig C., Ku C.A., Marra M., et al. Vitelliform maculopathy in MELAS syndrome. Am J Ophthalmol Case Rep. 2023 Apr 6;30 doi: 10.1016/j.ajoc.2023.101842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iovino C., Ramtohul P., Au A., et al. Vitelliform maculopathy: diverse etiologies originating from one common pathway. Surv Ophthalmol. 2023 May-Jun;68(3):361–379. doi: 10.1016/j.survophthal.2023.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Birtel J., von Landenberg C., Gliem M., et al. Mitochondrial retinopathy. Ophthalmol Retina. 2022;6(1):65–79. doi: 10.1016/j.oret.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 5.de Laat P., Smeitink J.A.M., Janssen M.C.H., et al. Mitochondrial retinal dystrophy associated with the m.3243A>G mutation. Ophthalmology. 2013;120(12):2684–2696. doi: 10.1016/j.ophtha.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 6.König J., Ott C., Hugo M., et al. Mitochondrial contribution to lipofuscin formation. Redox Biol. 2017 Apr;11:673–681. doi: 10.1016/j.redox.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sridevi Gurubaran I., Viiri J., Koskela A., et al. Mitophagy in the retinal pigment epithelium of dry age-related macular degeneration investigated in the NFE2L2/PGC-1α-/- mouse model. Int J Mol Sci. 2020 Mar 13;21(6):1976. doi: 10.3390/ijms21061976. [DOI] [PMC free article] [PubMed] [Google Scholar]