TO THE EDITOR: A paper demonstrating the utility of optogenetic sensors to control interstitial cells of Cajal (ICC) in colonic contractions has been published in the Journal of Neurogastroenterology and Motility.1 This study investigates the potential of using optogenetic stimulation to control contractions generated in the colon by selectively targeting light sensitive ion channels in ICC. The authors employed an inducible Cre-loxP recombination system to generate mice expressing channelrhodopsin-2 (ChR2) in ICC and confirmed expression through genotyping and immunofluorescence analysis. Using such a model, the authors investigated the effects of light stimulation on colonic muscle strips specifically in ICC and demonstrated that 470 nm blue light could induce premature low-frequency and high-amplitude (LFHA) contractions while isometric force was present. Furthermore, this LFHA activity was inhibited by T16Ainh-A01, a specific antagonist of the anoctamin 1 channel in ICC.
Overall, the study suggests that optogenetic stimulation could regulate ICC activity and influence colonic motor patterns, particularly LFHA contractions. It seems that optogenetic stimulation can be used as a tool for gaining insights into the mechanisms underlying gastrointestinal motility and potential therapeutic applications for gastrointestinal motility disorders. As illustrated by a comparison with both optogenetic and electrical stimulations, the similar waveforms evoked in muscle strips are shared with the same unique property of ICC, indicating light-inducible LFHA generated from ICC (Fig. 1).2,3 Furthermore, the suppression of LFHA upon anoctamin 1 antagonist was also evident, as seen in Figure 2.3
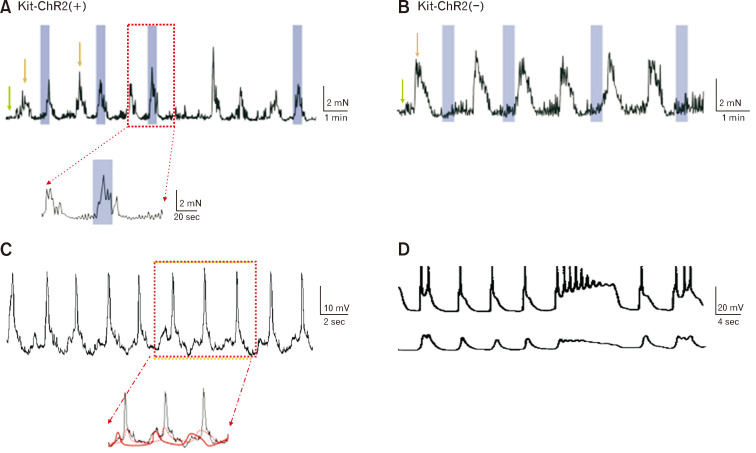
Figure 1.
The generations of low-frequency and high-amplitude (LFHA) contraction respectively from Kit-ChR2 (+) vs (–) mice and their correspondent electrical components recorded from patch clamp and intracellular electrode. (A, B) Light stimulation induced premature LFHA contractions in muscle strips in Kit-ChR2(+) mice, but not in Kit-ChR2(–) mice. The green arrow represented the high-frequency and low-amplitude (HFLA) contractions and the golden arrow represented LFHA contractions. The blue rectangles represented the light stimuli. (C) In situ patch clamp recordings from interstitial cells of Cajal (ICC) associated with the myenteric plexus, a spontaneous rhythmic slow wave was recorded, in which there are magnified as ensuite and the small premature slow wave (solid red) ie, unitary potential displayed before large slow wave (dot red). They are respectively reflected from ICC networks but different properties. Premature slow wave can be generated from ICC alone, slow wave superimposed with other components such as small spikes or action potentials can be composed with myogenic or neuronal sources, ie, ICC-smooth muscle-nerve compound activity. (D) In intracellular electrode recording, the muscle tone at 4 g slow wave was obtained with action potential (upper panel), whilst the muscle tone at 0.4 g slow wave was obtained with no action potential (lower panel). Note: High-amplitude contractions are underlined with compound activity of slow wave, whilst premature contractions are underlined with the unitary potential of slow wave.2,3
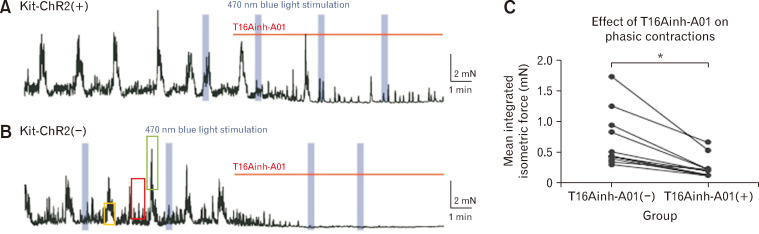
Figure 2.
Both phasic contractions from Kit-ChR2(+) and (–) mice are blocked by anoctamin 1 specific antagonist. Channelrhodopsin-2 (ChR2) regulated the low-frequency and high-amplitude (LFHA) contractions of colonic muscle strips via interstitial cells of Cajal (ICC). (A) The effect of anoctamin 1 antagonists, T16Ainh-A01 (10-20 µM), on phasic contractions Kit-ChR2(+) mice. The light evoked LFHA contractions could not be reproduced under T16Ainh-A01 (10 µM). (B) The effect of T16Ainh-A01 on phasic contractions Kit ChR2(–) mice. The blue rectangles represented the light stimuli, and the red lines represented the administration of T16Ainh-A01. Note: here we point out the component contributed from ICC (yellow box), smooth muscle (red box), and neuronal (green box). (C) The effect of T16Ainh-A01 (10 µM) on the mean integrated isometric force. *P < 0.001. Note: the phasic contractions either from Kit-ChR2(+) or (–) mice are both blocked by T16Ainh-A01 (10-20 µM), although some activity remained in a population of mice, indicating both of phasic contractions are from ICC.3
However, there is a discrepancy noted between LFHA and high-frequency and low-amplitude (HFLA) contractions, those are terms used to describe different patterns of contractions (or slow waves). Authors identified HFLA activity from the tissue of Kit-ChR2(–), attempting to lead audiences that HFLA comes solely from myogenic sources. Both Figures 1D and 2B indicate that the contraction is orchestrated from both ICC and smooth muscle cells; especially in addition of tetrodotoxin and/or nifedipine the superimposed spikes (information from nerve or muscle) on slow wave can be removed. It is expected a further clarification on frequency from LFHA and HFLA to have a better understanding on whether the different frequency comes from the deviations of optogenetic stimulation, mechanic force or muscle tone.
In conclusion, a potentially feasible novel approach to modulate the activity of ICC by optogenetics was demonstrated by Zhao and Tong.1 When the light-sensitive protein ChR2 is expressed in ICC, a single light stimulus can evoke a premature LFHA contraction, whereas periodic light stimulation enhances the frequency of LFHA contractions. This approach will facilitate a more comprehensive examination of the pacemaker mechanism in ICC and its role in colonic motility. Further investigation may yield fresh perspectives for treating ICC-associated motility disorders, such as constipation, in clinical settings.4,5
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Jing Wang initiated the research topic and both Jing Wang and Jun Xiao conceived and designed research, Jing Wang analyzed experiments and data, and wrote the manuscript. All authors read and approved the manuscript.
References
- 1.Zhao S, Tong W. An optogenetics-based approach to regulate colonic contractions by modulating the activity of the interstitial cells of Cajal in mice. J Neurogastroenterol Motil. 2023;29:388–399. doi: 10.5056/jnm22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckett EA, Bayguinov YR, Sanders KM, Ward SM, Hirst GD. Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus. J Physiol. 2004;559(Pt 1):259–269. doi: 10.1113/jphysiol.2004.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang B, Kunze WA, Zhu Y, Huizinga JD. In situ recording from gut pacemaker cells. Pflugers Arch. 2008;457:243–251. doi: 10.1007/s00424-008-0513-6. [DOI] [PubMed] [Google Scholar]
- 4.Hibberd TJ, Feng J, Luo J, et al. Optogenetic induction of colonic motility in mice. Gastroenterology. 2018;155:514–528. doi: 10.1053/j.gastro.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drumm BT, Cobine CA, Baker SA. Insights on gastrointestinal motility through the use of optogenetic sensors and actuators. J Physiol. 2022;600:3031–3052. doi: 10.1113/JP281930. [DOI] [PMC free article] [PubMed] [Google Scholar]