Abstract
The hematological abnormalities observed in human immunodeficiency virus (HIV)-infected patients appear to be mainly due to bone marrow dysfunction. A macaque models of AIDS could greatly facilitate an in vivo approach to the pathogenesis of such dysfunction. Here, we evaluated in this model the impact of infection with a pathogenic simian/human immunodeficiency virus (SHIV) on bone marrow hematopoiesis. Three groups of macaques were inoculated with 50 50% median infective doses of pathogenic SHIV 89.P, which expresses env of dual-tropic HIV type 1 (HIV-1) 89.6 primary isolate. During the primary phase of infection, animals were treated with either a placebo or highly active antiretroviral therapy (HAART) combining zidovudine, lamivudine, and indinavir, initiated 4 or 72 h postinfection (p.i.) and administered twice a day until day 28 p.i. In both placebo-treated and HAART-treated animals, bone marrow colony-forming cells (CFC) progressively decreased quite early, during the first month p.i. One year p.i., both placebo- and HAART-treated animals displayed decreases in CFC to about 56% of preinfection values. At the same time, a dramatic decrease (greater than 77%) of bone marrow CD34+ long-term culture-initiating cells was noted in all animals were found. No statistically significant differences between placebo- and HAART-treated monkeys were found. These data argue for an early and profound alteration of myelopoiesis at the level of the most primitive CD34+ progenitor cells during SHIV infection, independently of the level of viremia, circulating CD4+ cell counts, or antiviral treatment.
Understanding the fundamental mechanisms of human immunodeficiency virus (HIV) pathogenesis is a key issue for developing new antiviral strategies and improving the efficacy of current highly active antiretroviral therapy (HAART). Hematological abnormalities are frequent during HIV infection and probably contribute to the complexity of the disorders of diverse origins that characterize infection and the development of AIDS. Thrombocytopenia, anemia, lymphopenia, monocytopenia, and neutropenia are found in most AIDS patients, and pancytopenia appears as a rule in advanced disease. Anemia occurs in 18% of asymptomatic HIV-positive subjects and in more than 90% of AIDS patients (30). Although the mechanisms involved are probably multifactorial, the majority of cytopenias most likely reflect bone marrow dysfunction. Intercurrent infections and antiviral drugs or antibiotics commonly used in AIDS patients are factors that may affect hematopoiesis; however, hematopoietic cells may also be directly damaged by HIV in addition to being inhibited by HIV-related proteins and proinflammatory cytokines or chemokines, whose production is dysregulated in response to HIV infection.
Animal models are powerful tools for understanding the complexity of the pathogenic mechanisms of HIV infection and disease. Today, macaques infected with pathogenic strains of the simian immunodeficiency virus (SIV) or related chimeras expressing the envelope of HIV-1 (simian/human immunodeficiency virus [SHIV]) are relevant models of human HIV infection and AIDS. SIV and SHIV have biological properties similar to those of HIV, and infection of macaques with pathogenic isolates reliably induces in macaques an immunodeficiency syndrome strikingly mimicking human AIDS (33). In the same manner as in HIV-positive patients, hematological alterations are commonly found in SIV-infected macaques (16, 17).
We recently reported that treatment of macaques with a combination of zidovudine, lamivudine, and indinavir, initiated as early as 4 h after intravenous exposure to SHIV 89.6P and maintained for 4 weeks, failed to prevent infection but has long-lasting beneficial effects on the plasma viral load and blood CD4+ cell counts (21). Here, we extended our study to the consequences on bone marrow hematopoiesis of early HAART in macaques infected with pathogenic SHIV 89.6P.
MATERIALS AND METHODS
Animals.
Adult male cynomolgus macaques (Macaca fascicularis) weighing 3.5 to 6.5 kg were imported from Mauritius Island and housed in single cages within level 3 biosafety facilities according to national guidelines as reported previously (21). All experimental procedures were conducted according to the European guidelines for animal care (Journal Officiel des Communautés Européennes, L358, 18 December 1986).
Virus and infection of animals.
The animals were inoculated into the saphenous vein with 50 50% median infective doses of a SHIV 89.6P stock kindly provided by Anne Marie Aubertin (Université Louis Pasteur, Strasbourg, France). This virus is susceptible in vitro to zidovudine, lamivudine, and indinavir (21).
Treatment of animals.
As previously reported (21), eight animals were treated with a combination of zidovudine (4.5 mg/kg of body weight), lamivudine (2.5 mg/kg), and indinavir (20 mg/kg) administered twice a day through a nasogastric catheter (8Fr,Cat31; J. Frankle Co.). Treatment was initiated 4 (n = 4) or 72 h (n = 4) after inoculation of SHIV 89.6P, and it was continued until day 28 postinfection (p.i.). Three other animals were treated with a placebo. One noninfected, untreated animal (PR102B) was used as a control (Fig. 3). He was subjected to sedation and blood and bone marrow collections with the same frequency as the other macaques. Three other noninfected male cynomolgus macaques that did not experience repeated bleedings were used as controls.
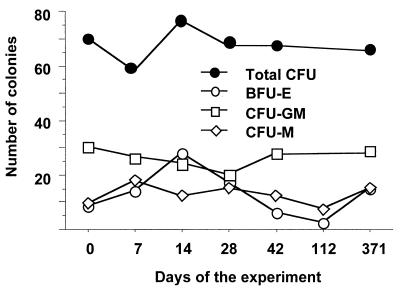
FIG. 3.
Evolution of total CFU, BFU-E , CFU-GM, and CFU-M in cultures of bone marrow cells of noninfected, nontreated control macaque PR102B. This animal was subjected to sedation and blood and bone marrow collections with the same frequency as the other macaques.
Plasma viral load.
Viral RNA in plasma was quantitated by an SIV-specific branched DNA amplification assay (Bayer Diagnostics, Amsterdam, The Netherlands).
Detection of viral DNA in mononucleated cells.
Cellular DNA was extracted using the High Pure PCR Template Preparation kit according to the manufacturer's instructions (Boehringer GmbH, Mannheim, Germany). DNA was quantified by measuring optical density (Pharmacia Biotech Ltd., Cambridge, England). The method consisted of a primary PCR amplification using primers specific for the gag gene (1386N [5′-GAAACTATGCCAAAAACAAGT] and 2129 [5′-TAATCTAGCCTTCTGTCCTGG]). Amplification cycles were performed with an automated thermocycler (Crocodile III; Appligene, Illkirch, France) as follows: 1 cycle of denaturation for 3 min at 94°C; then 40 cycles of denaturation for 45 s at 94°C, annealing for 2 min at 56°C, and extension for 1 min 30 s at 72°C; then 1 cycle for 10 min at 72°C. The reaction mixture was composed of 1 μg of test DNA, 1 U of Taq polymerase (Appligene), 10 μl of Taq polymerase buffer (10 mM Tris-HCl [pH 8.3], 1.5 mM MgCl2, 50 mM KCl, 0.1% Triton X-100), 5 mM deoxynucleoside triphosphates, and 30 pmol of each primer in a total volume of 100 μl. A second nested-PCR amplification was performed with 3 μl of amplimer inner primers 1731N (5′-CCGTCAGGATCAGATATTGCAGGAA) and 2042C (3′-CACTAGCTGCAATCTGGGTT) under the following conditions: 1 cycle of denaturation for 3 min at 94°C; then 25 cycles of denaturation for 45 s at 94°C, annealing for 1 min 30 s at 56°C, and extension for 1 min at 72°C; and 1 cycle for 10 min at 72°C. The PCR products were visualized by 1.5% agarose gel electrophoresis with ethidium bromide. The complete PCR was run on limiting dilutions of the initial stock of DNA. Copy numbers were determined by comparison to a standard stock of DNA from CEMX174 cells containing known copies of the integrated SIVmac251 DNA. The sensitivity of the method was estimated to be 1 copy of viral DNA in 105 cells.
Characterization of blood cells.
Blood cells were assessed with an automated hemacytometer (Microdiff II; Coultronics, Miami, Fla.). To characterize T lymphocytes, blood from the femoral vein was collected on EDTA and centrifuged at 300 × g; pelleted cells were then resuspended in calcium-free phosphate-buffered saline (PBS) (Gibco, Cergy Pontoise, France) and centrifuged at 400 × g for 45 min on Ficoll (MSL 2000; Eurobio). Peripheral blood mononuclear cells (PBMC) were collected and washed twice in PBS (400 × g, 10 min), and 105 cells were then incubated for 30 min at 4°C with fluorescein isothiocyanate (FITC)-conjugated anti-CD4+ (CD4 Leu-3a; Becton Dickinson, San Jose, Calif.), phycoerythrin (PE)-conjugated anti-CD8 (CD8 Leu-3a; Becton Dickinson), or FITC-conjugated anti-CD3 (FN-18; Biosource International, Camarillo, Calif.) monoclonal antibodies. FITC- and PE-conjugated immunoglobulins G1 (Immunotech, Marseille, France) were used as the controls. Stained cells were washed twice in PBS–5% fetal calf serum (FCS; Boehringer GmbH) and fixed (Cell-Fix; Becton Dickinson). T-lymphocyte subsets were analyzed with a FACScan cytometer and CellQuest software (Becton Dickinson).
Expansion of bone marrow hematopoietic progenitors.
Bone marrow was obtained by aspiration at the iliac crest. Bone marrow mononuclear cells were separated by centrifugation over Ficoll as described for peripheral blood mononuclear cells. Cells were washed once in PBS–3% FCS (Boehringer GmbH) and suspended (5 × 104 cells) in 1 ml of Methocult HF4434 medium (Stem-Cell Technologies, Meylan, France) in 35-mm-diameter petri dishes. Cells were incubated at 37°C for 14 days and scored under the inverted microscope for granulocyte-macrophage CFU (CFU-GM), granulocyte CFU (CFU-G), macrophage CFU (CFU-M), and burst-forming units–erythrocytes (BFU-E).
Characterization of long-term culture-initiating cells (LTC-IC).
Bone marrow mononuclear cells were washed once in PBS–3% FCS and suspended in PBS for subsequent staining. Cells were incubated with 10 μg of a mouse anti-CD34 monoclonal antibody (clone 5.63; kindly provided by T. Egeland, National Hospitalet, Oslo, Norway)/ml at 4°C for 20 min. Cells in PBS were sorted using magnetic beads coated with secondary rat anti-mouse immunoglobulin G1 antibody (MACS-Miltenyi Biotech, Paris, France) according to the manufacturer's instruction.
Feeder MS-5 stromal cells were cultured in 96-well plates at 6 × 103 cells/well in 100 μl of long-term culture medium (Myelocult GF4434; Stem Cell Technologies) to which 10−6 M 21-hemisuccinate hydrocortisone (sodium salt) (Stem Cell Technologies) had been added. MS-5 cultures were incubated for 24 h at 37°C in 5% CO2 before initiating coculture with CD34+ bone marrow cells.
CD34+ cells were distributed in limiting dilutions in MS-5 cultures, for which 200, 100, 50, or 20 cells were seeded per well in 20 wells for each dilution point. Cocultures were initiated in long-term Myelocult H5100 medium (Stem Cell Technologies) supplemented with 10−6 M 21-hemisuccinate hydrocortisone. One-half of the medium was changed once a week, and cultures were maintained for 5 weeks. The entire content of each well was then collected after trypsin treatment (Eurobio, Les Ulis, France) for 5 min and plated in 35-mm-diameter petri dishes in 1 ml of Methocult GF4434 medium. Cells were incubated at 37°C in 5% CO2 for 14 days and scored for CFU under an inverted microscope.
Statistical analysis.
Paired or unpaired comparisons were performed using the nonparametric Wilcoxon rank or Mann-Whitney tests for small samples; correlations were determined by the Spearman test, using StatView software (SAS Institute Inc., Cary, N.C.).
RESULTS
Impact of SHIV on bone marrow hematopoietic progenitors.
In HIV-positive patients, hematopoietic abnormalities are common at advanced stages of disease and may result, in part, from inhibition of hematopoietic progenitor cell growth and differentiation (30), especially CFU-GM and BFU-E. Here, we first examined whether a SHIV with the envelope of a pathogenic HIV-1 could induce similar dysmyelopoiesis. Macaques were inoculated intravenously with cell-free SHIV 89.6P, which expresses env of the HIV-1 89.6 primary isolate, uses both CCR-5 and CXCR-4 coreceptors like many other primary isolates, and induces AIDS in macaques. We investigated the colony-forming capacity of progenitor cells from bone marrow collected at different time points, from primary infection to 1 year p.i. Interestingly, in animals infected with the SHIV 89.6P and treated with the placebo, total CFU numbers decreased early after virus inoculation (Fig. 1a); differences from preinfection values became significant as soon as the third week p.i. (P = 0.0503), coincident with high plasma viral load and initial decrease of peripheral CD4+ lymphocytes (Fig. 2). This observation was never reported for humans, probably because of the difficulty of access to a bone marrow sample during the early phase of infection. Thereafter, as in humans, low values persisted during chronic infection: 1 year p.i. the number of total CFU (36 ± 10) was approximately 59% (P = 0.0152) that of the baseline value (61 ± 18). Such reduction of bone marrow CFU could not be attributed to depletion of CD34+ progenitors, inasmuch as between 6 months and 1 year p.i. bone marrow CD34+ cell fractions did not change (0.87% ± 0.09% and 0.86% ± 0.27%, respectively) and did not differ from those of uninfected control animals (0.70% ± 0.07%; P = 0.1824). We verified in one healthy animal which was not inoculated with SHIV and which experienced the same regular blood and bone marrow collections as the infected animals that the growth and/or expansion of the different types of CFU were not affected over time (Fig. 3).
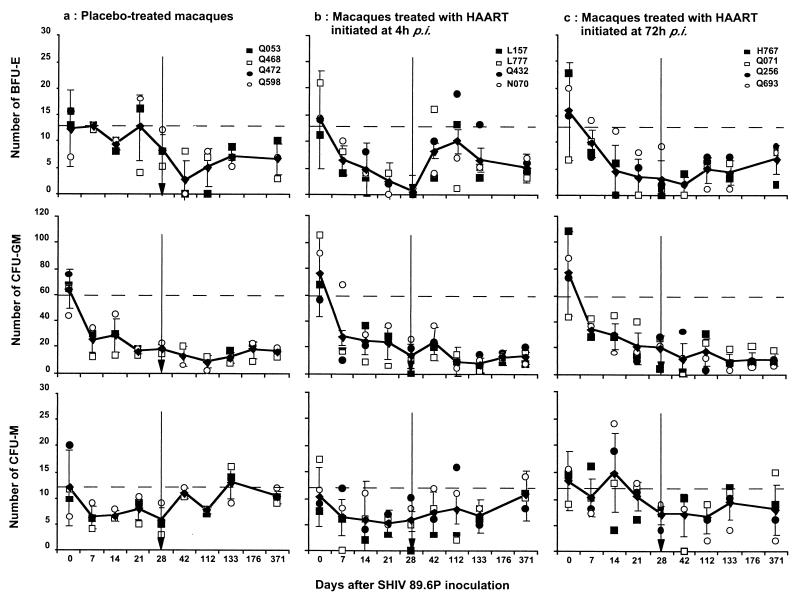
FIG. 1.
Evolution of total CFU numbers in cultures of bone marrow cells of macaques infected with SHIV 89.6P. (a) Placebo-treated macaques; (b) animals treated with HAART initiated 4 h p.i.; (c) animals treated with HAART initiated 72 h p.i. Diamonds, means of values for the different groups; error bars, SD; vertical arrow, end of treatment; dashed line, mean of more than 41 baseline values of noninfected, nontreated cynomolgus macaques.
FIG. 2.
Plasma viremia and evolution of circulating CD4+ cells. (a) Plasma viral load estimated by the number of copies of viral RNA. (b) Numbers of CD4+ circulating T lymphocytes. Solid circles, placebo-treated macaques (means of three animals ± SD); squares, animals treated with HAART (means for four animals ± SD) between days 0 (4 h p.i.) and 28 p.i.; open circles, animals treated with HAART (means for four animals ± SD between days 3 and 28 p.i.. Vertical arrow, end of treatment period.
As reported for AIDS patients and SIV-infected macaques, decrease of total CFU was associated here with decreased BFU-E (Fig. 4), which was observed in placebo-treated animals after the viral replication peak that characterizes primary infection (P = 0.0015 on day 42 p.i.). BFU-E then remained at low levels until 1 year p.i. (Fig. 4). Decreased CFU-GM counts were also noted early (Fig. 4), with differences from the baseline values becoming significant by day 21 p.i. (P = 0.0076) and persisting up to 1 year p.i. At that time, CFU-GM numbers were reduced to about 33% of baseline values (P = 0.0077). Finally, we did not confirm previous observations of an inhibition of CFU-M in HIV-infected patients (42), since we found (Fig. 4) in placebo-treated animals that CFU-M varied within the normal range until the end of the study (P = 0.4802 at 1 year p.i.).
FIG. 4.
Evolution of BFU-E, CFU-GM, and CFU-M in cultures of bone marrow cells of macaques infected with SHIV 89.6P. (a) Placebo-treated macaques; (b) animals treated with HAART initiated 4 h p.i.; (c) animals treated with HAART initiated 72 h p.i. Means (diamonds) and SD (error bars) of values for the different groups are shown. Vertical arrow, end of treatment; dashed line, mean of more than 40 baseline values of noninfected, nontreated cynomolgus macaques.
Early HAART does not prevent early myelopoiesis dysfunction.
Initiating HAART as early as hours or days after virus inoculation significantly reduces the viral load during primary infection. We therefore analyzed whether early HAART could also prevent the hematopoietic abnormalities we found early after infection in placebo-treated control animals. Starting 4 or 72 h p.i., 8 animals were treated by the oral route for 28 days with HAART (zidovudine, lamivudine, and indinavir). As previously reported (21), all animals became infected, demonstrating that initiating HAART as early as 4 h p.i. could not prevent infection after intravenous inoculation of cell-free virus. However, the number of viral RNA copies in plasma during primary infection in HAART-treated macaques was significantly lower than in placebo-treated controls (Fig. 2a): log10 copy numbers were 5.64 ± 0.59 (mean ± standard deviation [SD]) and 8.01 ± 0.14 at the peak of viremia, respectively (P = 0.0143). In association with the reduction of viremia, HAART also prevents the early and persistent decrease of CD4+ circulating T lymphocytes observed in the placebo-treated control macaques. Indeed, no significant change in blood CD4+ T-lymphocyte percentages was observed in either HAART-treated group, although a mild decrease of CD4+ T-cell counts occurred between days 14 and 28 p.i. (Fig. 2b).
During the treatment period, a significant (P = 0.0028) decrease of total CFU numbers occurred as early as the first week p.i. despite HAART (Fig. 1). This decrease persisted until the end of the treatment period (P < 0.0001 on day 28 p.i.), and no difference between the two groups of HAART-treated animals was detected. Abnormalities of BFU-E were also observed (Fig. 4) as early as the first week p.i. (P = 0.002), and they persisted until the end of treatment (P < 0.0001 on day 28 p.i.). At that time, numbers of BFU-E from the bone marrow of HAART-treated animals were significantly lower than those in placebo-treated controls (P = 0.0412). This probably reflects the combined effect of nucleoside analogs and infection on myelopoiesis. Indeed, zidovudine alone is known to affect erythropoiesis in HIV-positive patients (3). A decrease of CFU-GM similar to that in placebo-treated animals was also observed in HAART-treated macaques during primary infection (Fig. 4), and it became statistically significant by day 21 p.i. (P = 0.0339). There was no difference in CFU-GM formation between the two groups of HAART-treated macaques or between HAART-treated animals and placebo-treated control animals. A transient decrease of CFU-M was observed in all animals (Fig. 4) at the end of the treatment period that was statistically significant only for animals that received HAART (P = 0.0303).
Long-lasting effects of early antiviral treatment.
Starting HAART as early as hours or days after virus inoculation not only significantly reduces the viral load during primary infection but also improves the antiviral immune response and delays the onset of disease. Indeed, during chronic infection and until the end of the study (1 year p.i.), plasma viral load remained low, under the detection threshold (<1,500 copies/ml) in both groups of HAART-treated macaques, whereas it was persistently detectable in placebo-treated animals (Fig. 2). In the control group, an important reduction of circulating CD4+ T-lymphocyte numbers and percentages was noted as early as the second week p.i., and T-lymphocyte numbers were very low up to the end of the study, 12 months later (183 ± 94 cells/μl). By contrast, at 1 year p.i., i.e., 11 months after the end of treatment, circulating CD4+ T-cell numbers remained high (686 ± 180 cells/μl) in all macaques previously treated with HAART (Fig. 2).
We therefore analyzed whether early HAART could also prevent the long-lasting hematopoietic abnormalities we found in placebo-treated control animals. At an advanced stage of infection (1 year p.i.) no significant modification of CD34+ bone marrow cell percentages was detected in HAART-treated animals relative to placebo-treated animals (P > 0.9999) or uninfected controls (P = 0.2948).
Nevertheless, after the end of treatment, total CFU numbers remained at low levels (Fig. 1). One year p.i., these numbers remained significantly reduced to about 58% (P < 0.0001) in all the macaques (72 ± 31 CFU before infection versus 30 ± 9 CFU 1 year p.i.), with no detectable difference between controls and HAART-treated monkeys (P = 0.4705). By the same time, the mean number of BFU-E was 6 ± 2 (Fig. 4), a 57% reduction from baseline values (14 ± 8; P <0.0001) with no statistical difference between animals that had received HAART and those that had received the placebo (P = 0.6810). The CFU-GM decrease persisted until the end of the study. One year p.i., a reduction of 55% relative to baseline values (P < 0.0001) was noted (Fig. 4), with no differences between HAART-treated and placebo-treated monkeys (P = 0.2207). The decrease of CFU-M numbers persisted for several months (P = 0.0048 at 4 months p.i.). However, 1 year after infection, this decrease was no longer significant compared to baseline values (Fig. 4). No difference from placebo-treated monkeys was found (P = 0.6815).
Alteration of LTC-IC in SHIV-infected macaques.
Quantitative evaluation of LCT-IC is considered a relevant method for characterizing the most immature bone marrow stem cells. We therefore used this method to study whether the altered growth and/or differentiation of CFU progenitors we observed in SHIV-infected macaques was a consequence of failure of immature bone morrow precursors.
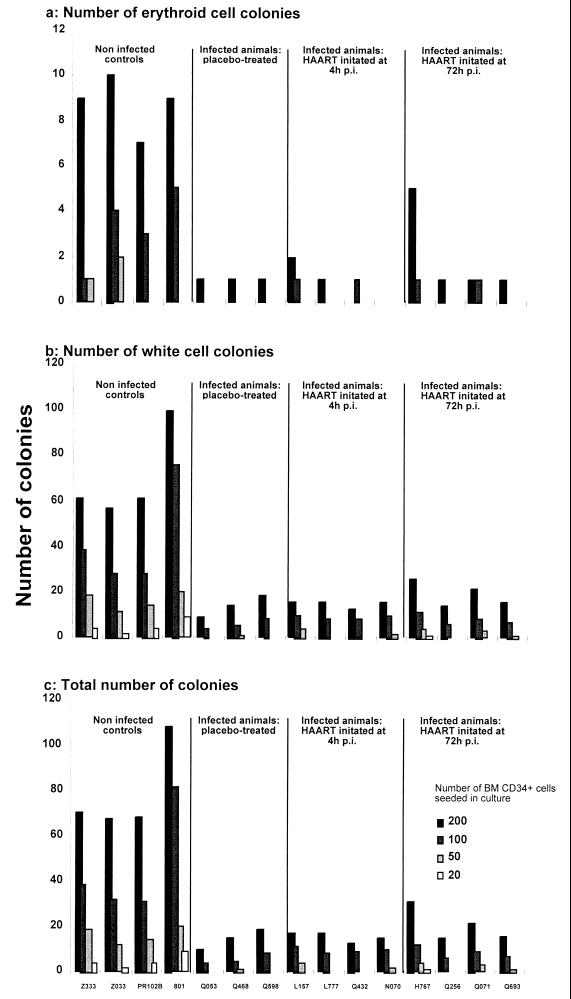
One year p.i., we purified CD34+ bone marrow cells from infected macaques initially treated with HAART or placebo, and we compared their expansion capacities with those of similar cells obtained from noninfected controls (one of which, PR102B, had been subjected to the same regular sampling of bone marrow and peripheral blood as infected macaques). A reduction of about 76% in the clonogenicity of LTC-IC, which affected the generation of both red and white cell colonies, was observed in the three groups of infected macaques (Fig. 5). The comparison of the slopes of the regression lines obtained from the analysis of LTC-IC limiting-dilution cultures did not reveal any statistically significant difference between infected macaques initially treated with HAART and those initially treated with a placebo (P = 0.1836) or between the two groups of HAART-treated animals (P = 0.5637). In contrast, a highly significant difference between infected and noninfected animals was noted (P = 0.0049).
FIG. 5.
Evolution of LTC-IC obtained after 7 weeks of culture from immunopurified bone marrow (BM) CD34+ cells. (a) Numbers of red cell colonies; (b) numbers of white cell colonies; (c) total numbers of colonies. Shading of bars indicates the numbers of mononucleated cells seeded in culture (200, 100, 50, and 20).
Absence of correlation of hematological disorders with classical parameters of infection.
By the Spearman rank test, no clear-cut correlation between classical parameters of infection of placebo-treated animals such as plasma viral load, numbers or percentages of blood CD4+ T-cell load, and total CFU, BFU-E, CFU-GM, or CFU-M levels could be established, but maybe too few animals were used in this group for powerful statistical analysis. A similar absence of correlation with viral load and CD4+ circulating T cells was obtained for animals that received HAART.
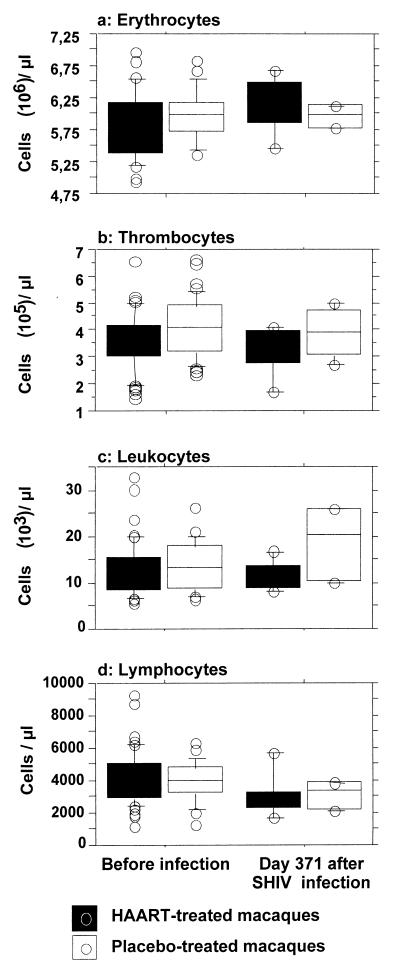
By a nested-PCR assay, viral gag DNA could not be detected in 1 μg of total DNA from CD34+ immunosorted bone marrow cells (over 98% pure) obtained from infected macaques, indicating that if infection of hematopoietic stem cells occurs it is a very rare event and could not account for the hematopoiesis failure we observed (data not shown). Finally, in this experiment, it is noteworthy that at 1 year p.i. the number of peripheral blood cells did not appear to be affected by infection or initial treatment, with the exception of total lymphocyte counts (P < 0.0001), the decline of which is a common feature during primate lentivirus infection (Fig. 6).
FIG. 6.
Comparison of 1-year-p.i. blood cell counts in the different groups of animals infected with SHIV. Tops and bottoms of boxes, 75th and 25th percentiles, respectively; horizontal lines between the box limits, medians; upper and lower error bars, 90th and 10th percentiles, respectively; circles, individual values outside the 90th and 10th percentiles. Before infection, 13 to 34 baseline values obtained before infection with SHIV 89.6P.
DISCUSSION
In this study, we demonstrated that expansion and/or differentiation of bone marrow hematopoietic progenitors is impaired in macaques infected with a pathogenic lentivirus expressing the envelope of a HIV-1 primary isolate, similar to what is observed in AIDS patients.
Abnormal numbers of CFU were detected as early as the primary phase of infection and persisted until the development of severe immunodeficiency. The strongest growth inhibition (over 75%) was observed for bone marrow CD34+ LTC-IC, emphasizing alterations of the most immature progenitor cells. Remarkably, no significant changes in blood cell counts taken 1 year p.i. were noted, with the exception of the lymphopenia characteristic of pathogenic lentivirus infection of primates. This suggests that compensatory mechanisms, such as accelerated expansion and differentiation of committed myeloid precursors, developed in order to maintain normal circulating blood cell levels. Indeed, mild changes in the more-differentiated CFU-M progenitors were observed in 1-year-infected macaques, whereas in vitro generation of less-differentiated CFU-GM and BFU-E was reduced at least 50% relative to preinfection values.
Although long-term antiviral therapy in chronically infected patients could partially restore hematopoietic functions (1, 11), in our experiment early HAART during primary infection did not prevent bone marrow abnormalities. Nevertheless, the combination of zidovudine, lamivudine, and indinavir reduced more than 100-fold the initial viremia and maintained normal blood CD4+ lymphocyte counts until 11 months after the end of treatment. However, at that time all animals, whether previously treated or not, exhibited comparable reductions of CFU and LTC-IC, which did not correlate with surrogate markers of infection progression such as viral load and CD4+ lymphopenia.
Hematological abnormalities in HIV-positive patients are certainly of diverse origin.
Although the viral gp120 envelope glycoprotein could bind to the CD4 receptor and the CXCR4 coreceptor, which are expressed at the membranes of very early bone marrow progenitors (2, 7, 23), there are conflicting data regarding the susceptibility of human bone marrow CD34+ cells to HIV infection (2, 7, 41). Most studies indicate that progenitor cells are not a major viral reservoir in HIV-1-infected patients, even at advanced stages of the disease (43, 44). Here, the SHIV 89.6P we used is dual-tropic and uses both CCR5 and CXCR4 like the HIV-1 primary isolate from which it derives (33). Therefore, this virus is potentially infectious for an extended range of target cells. However, we did not detect viral DNA in highly purified CD34+ bone marrow cells, which is in accordance with previously reported data for HIV-infected patients and for simian models of AIDS (17, 24). Absence of infection of CD34+ bone marrow cells is consistent with two of our major observations for SHIV 89.6P-infected macaques: (i) there is no significant reduction of the percentage of CD34+ bone marrow cells during infection, which confirms previous reports for HIV-1-infected humans (27), and (ii) there is an apparent lack of incidence of plasma viremia on bone marrow-derived CFU and LCT-IC.
Therefore, studies of human and animal models, including the huSCID mouse, emphasize that mechanisms other than direct infection of progenitor cells should contribute to HIV-related hematological disorders (19, 24). Direct toxicity of HIV-1 proteins for bone marrow cells, such as gp120, p24, Nef, and Tat, has been documented (5, 26, 32). Interaction of gp120 with bone marrow cells independently of an infectious process (45) results in the accumulation of CD34+ cells in G0/G1 and a progressive increase of cells with subdiploid DNA content, characteristic of apoptosis (46). This suppression of CD34+ cell growth mediated by viral proteins (gp120 and Tat) may also be attributed to an upregulation of endogenous transforming growth factor β (TGF-β), which is a strong inhibitor of hematopoiesis (46). The high level of viremia observed in HIV-infected patients (36) and in SIV- or SHIV-infected macaques, particularly during primary infection (34), strengthens the in vivo relevance of the possible suppression of hematopoiesis by viral factors.
The generation of host factors that inhibit hematopoiesis during HIV infection should also be considered. Indeed, cytokines and chemokines elaborated in response to HIV infection (22, 28, 35, 40) could have a significant negative influence on hematopoiesis (30). The chemokine macrophage inhibitory protein 1α has been reported to specifically inhibit erythropoiesis (4, 25), and several of the cytokines produced during the course of HIV infection (interleukin-1 [IL-1] and IL-2, interferons including alpha interferon [13], tumor necrosis factor alpha [29], and TGF-β [46]) may adversely affect hematopoiesis (12, 20, 38).
It is conceivable that the inhibitory effects of cytokines and growth factors could be obtained as early as primary infection, which is characterized in monkey models by an important inflammatory process (8–10, 18, 37).
The harmful effect of inhibitory cytokines could be worsened by the infection and/or dysfunction of bone marrow stromal cells (6). In particular, HIV-infected macrophages and bone marrow microvascular endothelial cells, a key element of the stroma, could account for the regenerative failure and the reduced capacity to support on-demand myelopoiesis. This could result from the release of inappropriate growth factors or the upregulation of inhibitory cytokines such as tumor necrosis factor alpha and TGF-β (31, 39).
It is noteworthy that in our experiment neither the plasma viral load nor HAART had any effect on the expansion defect of progenitor cells and LTC-IC. Consistent with this finding, we have previously reported that, despite a dramatic reduction in the acute viral load in macaques treated with antiviral drugs, early profiles of inflammatory cytokine mRNA were not significantly changed compared to those for untreated SIV-infected controls. This indicates that lymphokine expression patterns may not strictly depend on the virus load (14).
The observations reported here for a relevant model of HIV infection emphasize the in vivo damage of bone marrow cells, which could not be explained only by a direct interaction between the virus and hematopoietic progenitors. Whether bone marrow failure in infected individuals has any effect on T lymphopoiesis is questionable (15). The mechanisms involved in suppressing hematopoiesis during HIV infection remain to be determined in order to improve the currently used antiviral therapies and the design of new therapeutic strategies.
ACKNOWLEDGMENTS
We acknowledge T. Egeland (National Hospitalet, Oslo, Norway) for providing the mouse anti-CD34 monoclonal antibody. We also gratefully acknowledge J.-C. Gluckman (Hôpital Saint Antoine, Paris) for helpful discussions and his kind review of this paper. We thank R. Bidault and D. Lapierre from Glaxo Wellcome and M.-C. Gervais and P. Duprat from Merck Sharp & Dohme-Chibret for their efficient collaboration. We thank P. Ducouret (Université de Caen, France) for helpful discussions. We acknowledge B. Boson, B. Delache, C. Aubenque, V. Cordette, D. Renault, P. Pochard, J. C. Wilk, and R. Rioux for excellent technical assistance.
This work was supported by the Agence Nationale de Recherches sur le SIDA (ANRS, Paris, France), the Centre de Recherches du Service de Santé des Armées Emile Pardé (CRSSA, La Tronche, France), the association Tous ensemble contre le SIDA (SIDACTION, Paris, France), the Conseil Régional de Basse Normandie, and the Commissariat à l'Energie Atomique (CEA; Fontenay aux Roses, France).
REFERENCES
- 1.Adams G B, Pym A S, Poznansky M C, McClure M O, Weber J N. The in vivo effects of combination antiretroviral drug therapy on peripheral blood CD34+ cell colony-forming units from HIV type 1-infected patients. AIDS Res Hum Retroviruses. 1999;15:551–559. doi: 10.1089/088922299311079. [DOI] [PubMed] [Google Scholar]
- 2.Aiuti A, Turchetto L, Cota M, Cipponi A, Brambilla A, Arcelloni C, Paroni R, Vicenzi E, Bordignon C, Poli G. Human CD34(+) cells express CXCR4 and its ligand stromal cell-derived factor-1. Implications for infection by T-cell tropic human immunodeficiency virus. Blood. 1999;94:62–73. [PubMed] [Google Scholar]
- 3.Brogan K L, Zell S C. Hematologic toxicity of zidovudine in HIV-infected patients. Am Fam Physician. 1990;41:1521–1528. [PubMed] [Google Scholar]
- 4.Broxmeyer H E, Sherry B, Cooper S, Lu L, Maze R, Beckmann M P, Cerami A, Ralph P. Comparative analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. Interacting effects involving suppression, synergistic suppression, and blocking of suppression. J Immunol. 1993;150:3448–3458. [PubMed] [Google Scholar]
- 5.Calenda V, Chermann J C. HIV Tat protein potentiates in vitro granulomonocytic progenitor cell growth. Eur J Haematol. 1995;54:180–185. doi: 10.1111/j.1600-0609.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 6.Canque B, Marandin A, Rosenzwajg M, Louache F, Vainchenker W, Gluckman J C. Susceptibility of human bone marrow stromal cells to human immunodeficiency virus (HIV) Virology. 1995;208:779–783. doi: 10.1006/viro.1995.1211. [DOI] [PubMed] [Google Scholar]
- 7.Chelucci C, Casella I, Federico M, Testa U, Macioce G, Pelosi E, Guerriero R, Mariani G, Giampaolo A, Hassan H J, Peschle C. Lineage-specific expression of human immunodeficiency virus (HIV) receptor/coreceptors in differentiating hematopoietic precursors: correlation with susceptibility to T- and M-tropic HIV and chemokine-mediated HIV resistance. Blood. 1999;94:1590–1600. [PubMed] [Google Scholar]
- 8.Cheret A, Caufour P, Le Grand R, Theodoro F, Boussin F, Vaslin B, Dormont D. Macrophage inflammatory protein-1 alpha mRNA expression in mononuclear cells from different tissues during acute simian immunodeficiency virus strain mac251 infection of macaques. AIDS. 1997;11:257–258. [PubMed] [Google Scholar]
- 9.Cheret A, Le Grand R, Caufour P, Dereuddre-Bosquet N, Matheux F, Neildez O, Theodoro F, Maestrali N, Benveniste O, Vaslin B, Dormont D. Cytokine mRNA expression in mononuclear cells from different tissues during acute SIVmac251 infection of macaques. AIDS Res Hum Retroviruses. 1996;12:1263–1272. doi: 10.1089/aid.1996.12.1263. [DOI] [PubMed] [Google Scholar]
- 10.Cheret A, Le Grand R, Caufour P, Neildez O, Matheux F, Theodoro F, Boussin F, Vaslin B, Dormont D. Chemoattractant factors (IP-10, MIP-1alpha, IL-16) mRNA expression in mononuclear cells from different tissues during acute SIVmac251 infection of macaques. J Med Primatol. 1997;26:19–26. doi: 10.1111/j.1600-0684.1997.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 11.Dam Nielsen S, Kjaer Ersboll A, Mathiesen L, Nielsen J O, Hansen J E. Highly active antiretroviral therapy normalizes the function of progenitor cells in human immunodeficiency virus-infected patients J. Infect Dis. 1998;178:1299–1305. doi: 10.1086/314464. [DOI] [PubMed] [Google Scholar]
- 12.Francis M L, Maltzer M S, Gendelman H E. Interferons in the persistence, pathogenesis and treatment of HIV infection. AIDS Res Hum Retroviruses. 1992;8:199–207. doi: 10.1089/aid.1992.8.199. [DOI] [PubMed] [Google Scholar]
- 13.Ganser A, Carlo-Stella C, Greher J, Volkers B, Hoelzer D. Effect of recombinant interferons alpha and gamma on human bone marrow-derived megakaryocytic progenitor. cells. Blood. 1987;70:1173–1179. [PubMed] [Google Scholar]
- 14.Gigout L, Vaslin B, Matheux F, Caufour P, Neildez O, Cheret A, Lebel-Binay S, Theodoro F, Dilda P, Benveniste O, Clayette P, Le Grand R, Dormont D. Consequences of ddI-induced reduction of acute SIVmac251 virus load on cytokine profiles in cynomolgus macaques. Res Virol. 1998;149:341–354. doi: 10.1016/s0923-2516(99)80002-8. [DOI] [PubMed] [Google Scholar]
- 15.Hellerstein M K, McCune J M. T cell turnover in HIV-1 disease. Immunity. 1997;7:583–589. doi: 10.1016/s1074-7613(00)80379-9. [DOI] [PubMed] [Google Scholar]
- 16.Hillyer C D, Klumpp S A, Hall J M, Lackey III D A, Ansari A A, McClure H M. Multifactorial etiology of anemia in SIV-infected rhesus macaques: decreased BFU-E formation, serologic evidence of autoimmune hemolysis, and an exuberant erythropoietin response. J Med Primatol. 1993;22:253–256. [PubMed] [Google Scholar]
- 17.Hillyer C D, Lackey III D A, Villinger F, Winton E F, McClure H M, Ansari A A. CD34+ and CFU-GM progenitors are significantly decreased in SIVsmm9 infected rhesus macaques with minimal evidence of direct viral infection by polymerase chain reaction. Am J Hematol. 1993;43:274–278. doi: 10.1002/ajh.2830430409. [DOI] [PubMed] [Google Scholar]
- 18.Khatissian E, Tovey M G, Cumont M C, Monceaux V, Lebon P, Montagnier L, Hurtrel B, Chakrabarti L. The relationship between the interferon alpha response and viral burden in primary SIV infection. AIDS Res Hum Retroviruses. 1996;12:1273–1278. doi: 10.1089/aid.1996.12.1273. [DOI] [PubMed] [Google Scholar]
- 19.Koka P S, Jamieson B D, Brooks D G, Zack J A. Human immunodeficiency virus type 1-induced hematopoietic inhibition is independent of productive infection of progenitor cells in vivo. J Virol. 1999;73:9089–9097. doi: 10.1128/jvi.73.11.9089-9097.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane H C. Interferons in HIV and related diseases. AIDS. 1994;8:S19–S23. doi: 10.1097/00002030-199409001-00005. [DOI] [PubMed] [Google Scholar]
- 21.Le Grand R, Vaslin B, Larghero J, Neidez O, Thiebot H, Sellier P, Clayette P, Dereuddre-Bosquet N, Dormont D. Post-exposure prophylaxis with highly active antiretroviral therapy could not protect macaques from infection with SIV/HIV chimera. AIDS. 2000;14:1864–1866. doi: 10.1097/00002030-200008180-00029. [DOI] [PubMed] [Google Scholar]
- 22.Locati M, Murphy P M. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu Rev Med. 1999;50:425–440. doi: 10.1146/annurev.med.50.1.425. [DOI] [PubMed] [Google Scholar]
- 23.Louache F, Debili N, Marandin A, Coulombel L, Vainchenker W. Expression of CD4 by human hematopoietic progenitors. Blood. 1994;84:3344–3355. [PubMed] [Google Scholar]
- 24.Louache F, Henri A, Bettaieb A, Oksenhendler E, Raguin G, Tulliez M, Vainchenker W. Role of human immunodeficiency virus replication in defective in vitro growth of hematopoietic progenitors. Blood. 1992;80:2991–2999. [PubMed] [Google Scholar]
- 25.Lu L, Xiao M, Grigsby S, Wang W X, Wu B, Shen R N, Broxmeyer H E. Comparative effects of suppressive cytokines on isolated single CD34(3+) stem/progenitor cells from human bone marrow and umbilical cord blood plated with and without serum. Exp Hematol. 1993;21:1442–1446. [PubMed] [Google Scholar]
- 26.Maciejewski J P, Weichold F F, Young N S. HIV-1 suppression of hematopoiesis in vitro mediated by envelope glycoprotein and TNF-alpha. J Immunol. 1994;153:4303–4310. [PubMed] [Google Scholar]
- 27.Marandin A, Katz A, Oksenhendler E, Tulliez M, Picard F, Vainchenker W, Louache F. Loss of primitive hematopoietic progenitors in patients with human immunodeficiency virus infection. Blood. 1996;88:4568–4578. [PubMed] [Google Scholar]
- 28.McKenzie S W, Dallalio G, North M, Frame P, Means R T., Jr Serum chemokine levels in patients with non-progressing HIV infection. AIDS. 1996;10:F29–F33. doi: 10.1097/00002030-199610090-00001. [DOI] [PubMed] [Google Scholar]
- 29.Molina J M, Scadden D T, Byrn R, Dinarello C A, Groopman J E. Production of tumor necrosis factor alpha and interleukin 1 beta by monocytic cells infected with human immunodeficiency virus. J Clin Investig. 1989;84:733–737. doi: 10.1172/JCI114230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moses A, Nelson J, Bagby G. The influence of human immunodeficiency virus-1 on hematopoiesis. Blood. 1998;91:1479–1495. [PubMed] [Google Scholar]
- 31.Moses A V, Williams S, Heneveld M L, Strussenberg J, Rarick M, Loveless M, Bagby G, Nelson J A. Human immunodeficiency virus infection of bone marrow endothelium reduces induction of stromal hematopoietic growth factors. Blood. 1996;87:919–925. [PubMed] [Google Scholar]
- 32.Rameshwar P, Denny T N, Gascon P. Enhanced HIV-1 activity in bone marrow can lead to myelopoietic suppression partially contributed by gag p24 J. Immunol. 1996;157:4244–4250. [PubMed] [Google Scholar]
- 33.Reimann K A, Li J T, Veazey R, Halloran M, Park I W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate Env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reimann K A, Watson A, Dailey P J, Lin W, Lord C I, Steenbeke T D, Parker R A, Axthelm M K, Karlsson G B. Viral burden and disease progression in rhesus monkeys infected with chimeric simian-human immunodeficiency viruses. Virology. 1999;256:15–21. doi: 10.1006/viro.1999.9632. [DOI] [PubMed] [Google Scholar]
- 35.Reinhold D, Wrenger S, Kahne T, Ansorge S. HIV-1 Tat: immunosuppression via TGF-beta1 induction. Immunol Today. 1999;20:384–385. doi: 10.1016/s0167-5699(99)01497-8. [DOI] [PubMed] [Google Scholar]
- 36.Rodman T C, To S E, Hashish H, Manchester K. Epitopes for natural antibodies of human immunodeficiency virus (HIV)-negative (normal) and HIV-positive sera are coincident with two key functional sequences of HIV Tat protein. Proc Natl Acad Sci USA. 1993;90:7719–7723. doi: 10.1073/pnas.90.16.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg Y J, Cafaro A, Brennan T, Greenhouse J G, Villinger F, Ansari A A, Brown C, McKinnon K, Bellah S, Yalley-Ogunro J, Elkins W R, Gartner S, Lewis M G. Virus-induced cytokines regulate circulating lymphocyte levels during primary SIV infections. Int Immunol. 1997;9:703–712. doi: 10.1093/intimm/9.5.703. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg Z F, Fauci A S. Immunopathogenic mechanisms of HIV infection: cytokine induction of HIV expression Immunol. Today. 1990;11:176–181. doi: 10.1016/0167-5699(90)90070-p. [DOI] [PubMed] [Google Scholar]
- 39.Rosenthal G J, Stranahan III R P, Thompson M, Blair P, Germolec D R, Comment C E, Schwab K, Luster M I. Organ-specific hematopoietic changes induced by a recombinant human interferon-alpha in mice. Fundam Appl Toxicol. 1990;14:666–675. doi: 10.1016/0272-0590(90)90292-r. [DOI] [PubMed] [Google Scholar]
- 40.Shearer G M, Clerici M. Cytokine profiles in HIV type 1 disease and protection. AIDS Res Hum Retroviruses. 1998;14:S149–S152. [PubMed] [Google Scholar]
- 41.Shen H, Cheng T, Preffer F I, Dombkowski D, Tomasson M H, Golan D E, Yang O, Hofmann W, Sodroski J G, Luster A D, Scadden D T. Intrinsic human immunodeficiency virus type 1 resistance of hematopoietic stem cells despite coreceptor expression. J Virol. 1999;73:728–737. doi: 10.1128/jvi.73.1.728-737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sloand E M, Young N S, Sato T, Kumar P, Kim S, Weichold F F, Maciejewski J P. Secondary colony formation after long-term bone marrow culture using peripheral blood and bone marrow of HIV-infected patients. AIDS. 1997;11:1547–1553. doi: 10.1097/00002030-199713000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Stanley S K, Kessler S W, Justement J S, Schnittman S M, Greenhouse J J, Brown C C, Musongela L, Musey K, Kapita B, Fauci A S. CD34+ bone marrow cells are infected with HIV in a subset of seropositive individuals. J Immunol. 1992;149:689–697. [PubMed] [Google Scholar]
- 44.von Laer D, Hufert F T, Fenner T E, Schwander S, Dietrich M, Schmitz H, Kern P. CD34+ hematopoietic progenitor cells are not a major reservoir of the human immunodeficiency virus. Blood. 1990;76:1281–1286. [PubMed] [Google Scholar]
- 45.Zauli G, Re M C, Visani G, Furlini G, La Placa M. Inhibitory effect of HIV-1 envelope glycoproteins gp120 and gp160 on the in vitro growth of enriched (CD34+) hematopoietic progenitor cells. Arch Virol. 1992;122:271–280. doi: 10.1007/BF01317189. [DOI] [PubMed] [Google Scholar]
- 46.Zauli G, Vitale M, Gibellini D, Capitani S. Inhibition of purified CD34+ hematopoietic progenitor cells by human immunodeficiency virus 1 or gp120 mediated by endogenous transforming growth factor beta 1. J Exp Med. 1996;183:99–108. doi: 10.1084/jem.183.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]