Abstract
The common mechanisms by which members of the G protein–coupled receptor (GPCR) family respond to neurotransmitters in the brain have been well studied. However, it is becoming increasingly clear that GPCRs show great diversity in their intracellular location, interacting partners and effectors, and signaling consequences. Here we will discuss recent studies on the diversity of location, effectors, and signaling of GPCRs, and how these could interact to generate specific spatiotemporal patterns of GPCR signaling in cells.
Keywords: GPCR trafficking, Spatial encoding, Localization, Spatiotemporal regulation, Microenvironment
Introduction
Neurotransmission relies heavily on the function of G protein-coupled receptors (GPCRs), the largest and most diverse family of signaling receptors in mammals [1]. GPCRs serve as receptors for the large majority of known neurotransmitters in the brain. GPCRs are “metabotropic” receptors, in that they respond to neurotransmitters typically by initiating metabolic changes that result in signaling cascades in neurons. GPCRs differ in many ways from “ionotropic” neurotransmitter receptors, which are ion channels that respond to neurotransmitters primarily by opening and allowing specific ions to cross the membrane. GPCRs recognize and respond to a variety of small molecule, neuropeptide, hormone, and lipid neurotransmitters, in contrast to ion channels that respond primarily to small molecules. Although GPCRs can couple to their immediate effectors at millisecond and sub-millisecond timescales [2,3], GPCR signaling outputs are sustained over minutes [4]. This is in contrast to ion channels that open and close typically within milliseconds to seconds [5]. Many neurotransmitters that activate GPCRs are not restricted to the synaptic cleft and stay longer in the extracellular environment which also contributes to prolonged signaling. Therefore, GPCR signals primarily set the tone of neuronal responses by regulating overall excitability and contributing to long-term plasticity of neurons, although GPCRs can activate ion channels and generate rapid responses [4,6,7].
Our understanding of how GPCR signaling pathways are spatiotemporally organized in cells, and the trafficking mechanisms that regulate this organization, is still evolving. Traditional studies largely focused on the initial response of GPCRs after they are activated by neurotransmitters on the cell surface. However, it is now widely appreciated that GPCRs localize to various intracellular locations all over the cell, and that GPCRs can signal from arguably all these locations [8-11]. The varied locations allow GPCRs to access a diverse repertoire of effectors, as well as specific microenvironments. These differences in the immediate effectors and the microenvironment could dictate specific receptor interaction patterns, generating enormous spatiotemporal diversity in signaling for any given GPCR (Figure 1). By precisely determining the distribution of GPCRs between these different locations, membrane trafficking mechanisms could therefore serve as a master regulator of signaling.
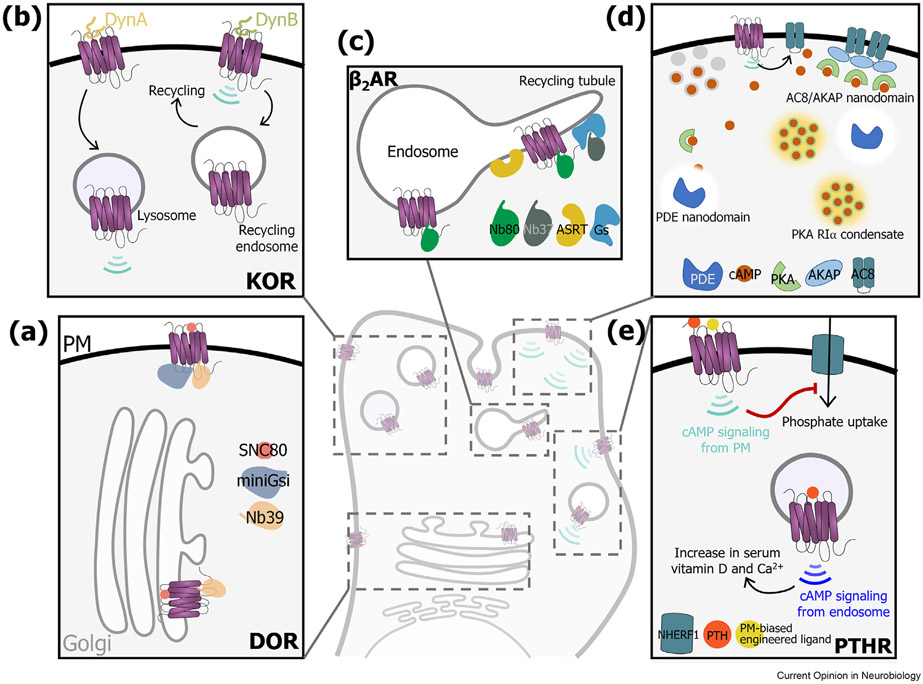
Figure 1.
Diversity in location, interaction, signaling, and functional outcomes in GPCRs. GPCRs exhibit enormous diversity in their localization and interaction profiles, and consequently generate spatiotemporally distinct signaling outcomes. This figure highlights a few examples. (a) The subcellular location of DOR determines receptor active states. Although receptors at both the Golgi and the plasma membrane (PM) inhibit cAMP upon activation by a cell permeable agonist, receptors at the PM recruit Nb39 (biosensor for active DOR) as well as miniGsi (biosensor for Gi binding to active DOR), but only Nb39 at the Golgi. (b) KOR signaling is selectively modulated by different dynorphin peptides in a compartment-specific manner. DynA sorts KOR to the lysosomal pathway and induces sustained cAMP signaling from lysosomal compartments, while DynB drives recycling of KOR without activating receptors on the endosome. Therefore, DynA predominantly drives signaling from endosomes, while DynB predominantly drives signaling from the surface. (c) Sub-organellar microdomains accessed by β2AR allow distinct spatiotemporal control over signaling. Active β2AR (marked by Nb80) is present on bulk as well as receptor C-terminal sequence-dependent recycling tubules marked by actin/sorting nexin/retromer tubular (ASRT) microdomains. However, the receptor stimulates Gs (visualized by Nb37) only on ASRT microdomains. PKA-driven localization of β2AR on ASRT domains biases downstream transcriptional responses to genes controlled by signals from endosomes. (d) Multiple mechanisms operate to maintain spatiotemporally confined responses from the highly dynamic second messenger cAMP. In an unstimulated state, most of the cAMP is sequestered allowing the generation of cAMP-free nanodomains around phosphodiesterase (PDE) which ‘protect’ cAMP effectors. Upon stimulation the high concentrations of cAMP saturate their binding sites and ultimately deplete the ‘protected’ zone around PDE thereby exposing cAMP effectors. Parallelly, under stimulated conditions, cAMP is sequestered by the RIa subunit of PKA in biomolecular condensates formed by liquid-liquid phase separation. In oscillatory circuits triggered by Ca2+, the relative phase of cAMP and Ca2+ are maintained by the formation of adenylyl cyclase 8 (AC8) and PKA recruiting A-kinase anchoring protein (AKAP) nanoclusters on the plasma membrane. (e) Activation of the parathyroid hormone receptor type I (PTHR) triggers distinct cAMP mediated physiological responses based on receptor localization. Prior to endocytosis, transient activation of the receptor on the PM leads to Na+/H+ exchanger regulatory factor-1 (NHERF1)-dependent reduction in serum phosphate levels. On the other hand, sustained activation of the receptor in endosomes upon endocytosis leads to increase in serum vitamin D and Ca2+ levels. Unlike PTH that activates PTHR at both locations with a transient PM response and a sustained endosomal response, a PM-biased ligand could generate sustained cAMP signals from the plasma membrane thus biasing the physiological outcome.
In this review, we will highlight selected recent studies on the importance of diversity in localization, interaction, and signaling profiles of GPCRs, in the context of general principles of GPCR function [12]. These are important considerations for both understanding specificity in GPCR function and for developing novel strategies to target GPCRs for therapeutics.
Diversity in location
GPCRs are localized to a wide variety of subcellular compartments by membrane trafficking. GPCR localization to the plasma membrane, endoplasmic reticulum, Golgi, mitochondria, nucleus, lysosomes, peroxisomes, and endosomes, have been discussed extensively in recent reviews [8-11,13]. The trafficking processes that move GPCRs to and from the plasma membrane can be considered under two broad classes of intracellular transport - endocytic trafficking, which internalizes and cycles receptors that are activated on the surface, and biosynthetic trafficking, which delivers newly synthesized receptors through the secretory pathway.
Endocytic trafficking of GPCRs activated on the cell surface has been studied extensively, because of the early focus on understanding how GPCRs on the plasma membrane are regulated once they are activated by drugs of interest. GPCRs internalize primarily via clathrin-mediated endocytosis [14,15]. Some GPCRs, such as the M2 muscarinic receptor (M2) in nonneuronal cells [16], can localize to caveolae and internalize via clathrin-independent mechanisms under certain conditions [17,18]. However, even for these receptors, the main mode of internalization is likely to be clathrin-mediated, as has been observed for M2 in neurons [19]. Once internalized, GPCRs can be either recycled to the cell surface or sorted to the lysosome to be degraded, based on whether they contain specific sequences or modifications. Mutating these sequences or depleting the protein machinery that recognize these sequences can disrupt the post-endocytic sorting of receptors, as reviewed in detail previously [14,15,20]. This sorting can determine the fate of GPCRs and the long-term signaling consequences of receptor activation [10].
Interestingly, endogenous neurotransmitters might take advantage of this sorting to generate diversity in location. One example is in the endogenous opioid system, where there are over 25 opioid peptides which can activate four opioid receptors [21]. In the dynorphin family of opioid peptides, highly related peptides DynA and DynB activate the kappa opioid receptor (KOR) to a similar extent, but induce distinct receptor endocytic trafficking fates. While DynA drives KOR to late endosomes and lysosomes, DynB causes recycling of the receptor to the plasma membrane [22]. These distinct trafficking itineraries cause marked differences in signaling responses from the receptor, as discussed later. Therefore, differential post-endocytic sorting might be a mechanism by which endogenous neurotransmitters generate different spatiotemporal patterns of signaling, even though they activate identical pathways at the biochemical level adjacent to the receptor.
The membrane lipid microenvironment is emerging as an important regulator of receptor endocytosis. In case of the serotonin1A receptor (5-HT1AR), reduction in membrane cholesterol levels by inhibition of cellular cholesterol biosynthesis using statin led to a switch in agonist-mediated endocytosis of the receptor from clathrin- to caveolin-mediated endocytosis. In addition, the receptor was routed to lysosomes instead of recycling [18]. On the other hand, the steady state levels of 5-HT1AR on the plasma membrane exhibited reduction along with accumulation of the receptor in late endosomal/lysosomal compartments in a cellular model of Smith-Lemli-Opitz syndrome, an autosomal recessive defect in cholesterol biosynthesis that leads to the accumulation of the immediate biosynthetic precursor of cholesterol called 7-dehydrocholesterol [23]. The role of the membrane microenvironment in the subcellular organization of GPCRs is likely driven by a combination of the specific interaction of lipids with receptors [24] and the biophysical nature of the membrane bilayer in the vicinity of the receptor [25,26]. Quantitation of receptor density and membrane curvature in the plasma membrane of neuron-like cells showed that the neuropeptide Y2 receptor, and β1- and β2-adrenergic receptors, all class A GPCRs, exhibited differential sorting into curved vs. flat regions of the membrane in a manner dependent on agonist-induced receptor activation [27].
Unlike endocytic trafficking, whether newly synthesized receptors are localized to specific compartments in the biosynthetic pathway, and whether receptor delivery is regulated, are much less explored [28]. One family of proteins that plays complex roles in steady-state delivery of GPCRs to the surface are Rab GTPases, which cycle between an active GTP-bound form and an inactive GDP-bound form. For example, Rab43, likely interacts with the intracellular loop 3 of the a2B-adrenergic receptor to drive surface export of the receptor [29]. Inhibiting Rab43, using CRISPR-mediated knockout and dominant negative mutants that could not bind GTP, impaired anterograde trafficking of the receptor in neurons [29]. Another Rab GTPase, Rab11a, which is involved in cargo recycling from endosomes to the plasma membrane, is required for exporting newly synthesized platelet-activating factor receptors from the trans-Golgi network [30]. In this case, however, the receptors are transported to the nucleus by a nuclear transport protein, importin-5 [30]. In this context, the delta opioid receptor (DOR) provides an interesting example to study biosynthetic trafficking, as its surface delivery in neurons is regulated by extracellular signaling pathways. Unlike in fibroblasts, DOR is retained in an intracellular compartment that roughly overlaps with the Golgi apparatus in neurons, due to a neuron-specific checkpoint that ‘holds’ newly synthesized DOR in the Golgi. In neuroendocrine PC12 cells, this checkpoint can be activated by nerve growth factor signaling which inhibits the activity of the Golgi-specific enzyme, class 2 phosphatidylinositol-3-kinase α (PI3KC2A). PI3KC2A converts the phosphoinositide lipid PI(4)P to PI(3,4)P2 which normally promotes DOR export from the Golgi [31]. Conversely, the opposing enzyme, PI3K phosphatase and tensin homolog (PTEN), induces DOR retention in the Golgi [32]. The retention signal for DOR was mapped to a conserved dual RXR motif in the receptor C-terminal tail [33]. In nociceptive neurons, physiological and pathological cues such as inflammation and activation by agonists drive the translocation of newly synthesized receptors from intracellular structures that overlap with the Golgi to the plasma membrane [32,34,35]. At present, it is not clear if these physiological cues act by modifying the phospholipid balance in the Golgi to regulate DOR export.
Diversity in receptor-effector interactions and active states
The diverse locations to which GPCRs traffic could enable diverse and specific interaction profiles of receptors and their effectors, separated both in time and space. Taking DOR as an example, in HEK293 cells, three different agonists - ARM390, SNC80 and DADLE - all activate Gai/o at the plasma membrane, irrespective of their ability to cause receptor endocytosis, as measured by enhanced bystander bioluminescence energy transfer (ebBRET) changes [36]. However, agonists that induce strong receptor internalization (SNC80 and DADLE) engaged the receptor with both Gai/o and β-arrestin at the plasma membrane as well as endosomes [36]. The related KOR, which localizes to lysosomes upon stimulation with DynA, recruits Nb39 (a conformation-specific nanobody biosensor which recognizes the active conformation of opioid receptors through residues conserved across opioid receptor subtypes) and causes reduction in cAMP levels [22]. However, upon stimulation with DynB, KOR localizes to recycling endosomes and does not recruit Nb39 or reduce cAMP levels [22]. A more striking difference in activation was shown for DOR in the biosynthetic pathway, using Nb39 and miniGsi, which mimics Gi binding to active DOR [37]. When activated by the permeable agonist SNC80, the plasma membrane pool of DOR recruited Nb39 as well as miniGsi at the plasma membrane, but the Golgi pool recruited only Nb39, although both pools decreased cAMP [37]. These observations raise the interesting possibility that the receptor microenvironments could stabilize distinct receptor activation states induced by the same ligand.
Local concentrations of effectors, such as cyclic AMP (cAMP), are an important aspect of the microenvironment that could regulate GPCR signaling at specific intracellular locations. Much of the research on how cAMP is compartmentalized has focused on the local functions of effectors and regulators, such as adenylate cyclase enzymes, phosphodiesterases, PKA, and kinase anchoring proteins [38-40]. For example, the generation of cAMP at different locations was found to be specific to different adenylyl cyclase (AC) subtypes. Both AC1 and AC9 were activated on the plasma membrane, but only AC9 trafficked to the early endosomes and generated cAMP in endosomes upon activation by a Gs-coupled receptor [41].
How gradients of cAMP are maintained in response to compartmentalized activation of ACs is still not fully understood. One possibility is that cAMP diffusion is restricted to specific domains by cAMP binding sites and phosphodiesterase (PDE) enzymes that degrade cAMP [39]. In unstimulated conditions, the diffusion of a fluorogenic cell-permeable cAMP analog is “buffered” by cAMP-binding sites, causing low concentrations of free cAMP in unstimulated conditions. Genetically encoded FRET-based cAMP sensors tagged to phosphodiesterase (PDE) showed “nanodomains” of low cAMP around PDE which prevented baseline stimulation of cAMP effectors in these regions [42]. Upon GPCR-mediated cAMP stimulation, the cAMP binding sites are saturated, increasing free cAMP, which floods the cAMP-free zones around PDE and activates effectors [42]. Another possibility is that cAMP is sequestered through effector proteins. The regulatory type I (RIα) subunit of the cAMP effector protein kinase A (PKA) undergoes liquid-liquid phase separation to form biomolecular condensates in a cAMP activity dependent manner. These phase-separated compartments are enriched in cAMP and PKA activity [43]. At the membrane, biosensors measuring compartment-specific cAMP and Ca2+ oscillations revealed a shift in the phase of cAMP oscillations in PKA-recruiting A-kinase anchoring protein (AKAP) domains relative to the rest of the plasma membrane [44]. These oscillations were found to be regulated by the relative effects of Ca2+-dependent cAMP synthesis through AC8 and reduction in cAMP levels through PDE1. Interestingly, AKAP and AC8 form nanodomains on the plasma membrane. A computational model revealed that these domains are necessary to maintain the oscillatory circuit [44]. Such oscillatory changes could encode signaling information in systems such as neurons and pancreatic β cells. Overall, the data suggest a model where a highly coordinated network of membrane-associated and cytosolic assemblies maintain precise concentrations of second messengers to spatiotemporally regulate signaling within cells.
Receptor function could become specialized even on the same membrane compartments, generating diversity in function. One interesting example of this diversity is seen with the dopamine D2 receptor. The short and the long isoforms of this receptor were initially thought to localize to presynaptic and postsynaptic terminals respectively, based on functional assays [45,46]. However, later studies showed that these isoforms might play overlapping roles at both presynaptic and postsynaptic terminals in basal conditions, but that upon cocaine treatment, these receptors show different functions in the presynaptic terminal [47-49]. The exact downstream components that allow for functional specialization of these receptors is still not fully understood.
The dynamic and selective nature of receptor-effector interactions and diverse receptor activation states can be appreciated even at the ‘sub-organellar’ scale. Single molecule imaging of receptor dynamics and interactions on the plasma membrane at a high spatiotemporal resolution revealed ‘hot spots‘ for the interaction of receptors and G proteins that were defined by the actin cytoskeleton and clathrin-coated pits [50]. In the endosome, although the β2-adrenergic receptor (β2AR) is present in an active conformation all over the endosomal membrane, it activates Gαs only on specialized actin/sorting nexin/retromer tubular (ASRT) microdomains [51,52]. Exploring the molecular nature of such ‘active domains’ on organelles could uncover previously unidentified or underappreciated factors in receptor-effector interactions and receptor and effector activation states.
Diversity in signaling outcomes: how can we leverage it?
The diversity in GPCR localization, interaction with effectors, and location-based signaling outcomes raises the exciting possibility that we can manipulate signaling at specific locations to fine-tune specific outcomes in therapeutics. For instance, activation of DOR in endosomes of nociceptors was found to be necessary for sustained DOR-mediated antinociception. Delivering the DOR agonist DADLE specifically to its endosomal pool by encapsulating it in a liposomal formulation enhanced DOR-mediated antinociception in dorsal root ganglion neurons from DOR-eGFP knock-in mice [36]. In the case of the parathyroid hormone (PTH) receptor type 1 (PTHR), distinct cAMP-mediated cellular responses were generated from PTHR activation on the plasma membrane and endosomes [53]. Receptor-mediated homeostatic control of serum calcium and vitamin D required sustained endosomal signaling, while cAMP generated from the plasma membrane receptor pool contributed to reduction in serum phosphate levels by PKA-dependent phosphorylation of Na+/H+ exchanger regulatory factor-1 (NHERF1). An engineered location-biased peptide ligand for PTHR caused sustained cAMP signaling from the plasma membrane, but not from endosomes [53]. This is unlike activation of PTHR by endogenous PTH that induces cAMP production transiently from the plasma membrane and a sustained cAMP response from endosomes. In protease activated receptor 2 (PAR2)-muGFP knock-in mice with colitis, endocytosis of PAR2, induced by receptor activation by proinflammatory proteases, mediated sustained colonic inflammation and pain [54]. This was possibly due to the formation of an endosomal signaling complex of the receptor with Gαq, Gαi and β-arrestin. Restricting endosomal signaling by PAR2 by specifically targeting endosomal PAR2 could therefore serve as a potential therapeutic strategy in this case [54]. Similar paradigms might also apply to the biosynthetic pathway. As described earlier, the phosphatase PTEN imposes a ‘hold’ on the biosynthetic traffic of DOR in the Golgi. Disengaging this hold using PTEN inhibitors improved the availability of the receptor at the plasma membrane of trigeminal ganglion neurons allowing effective DOR-mediated antihyperalgesia [32]. Overall, targeting the subcellular localization of GPCRs in cells could provide exciting therapeutic avenues in the future.
Perspectives for the future
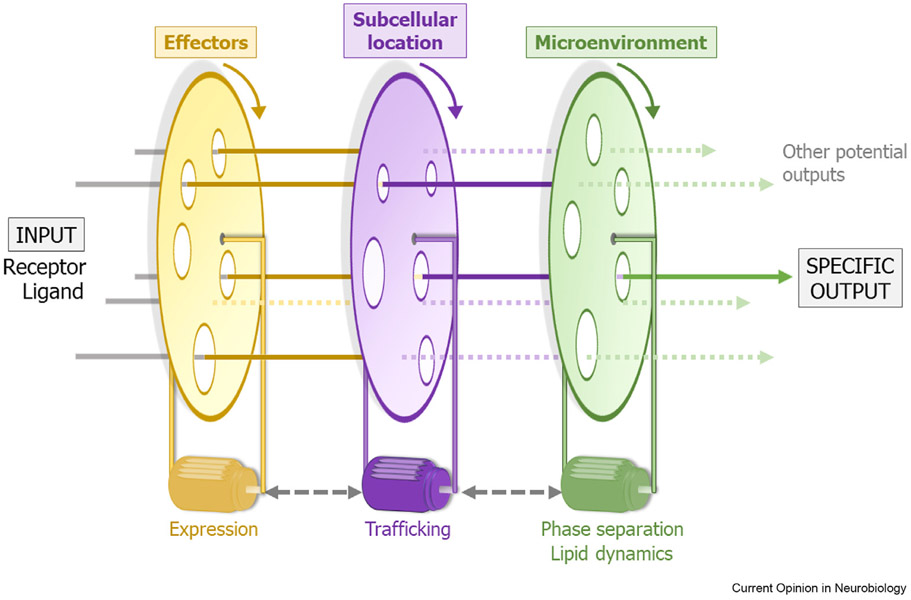
Our understanding of GPCR signaling has come a long way from the initial linear views of a receptor activating an effector to generate one outcome. We now know that each receptor can generate a variety of signaling outcomes, and that the outcome generated depends on the alignment of a number of factors, regulated by complex cellular dynamics. For any combination of GPCR and ligands, the signaling output generated depends on interactions with effectors, subcellular localization of interacting partners, and the microenvironment enabling these interactions. Interestingly, these factors are constantly remodeled by cellular dynamics through changes in expression, changes in relative localization and changes in physicochemical nature of the microenvironment. Although such diversity, complexity, and individuality discourages a simple generalization across the GPCR family, one way to visualize a simplified model is to consider transmission of light through a series of rotating discs with holes that allow light to pass (Figure 2). An output is generated only if a combination of holes on all the discs align with the incident light beam, and the location of the output depends on the position of the holes. As our knowledge of receptor-effector interactions and expression profiles across different cell types, subcellular locations, and sub-organellar microenvironments evolves, the number and identity of holes on each wheel, their relative motion, and feedback regulation will be refined in the future.
Figure 2.
A simple visual model for diversity and specificity in GPCR signaling. The cascade of events required for signal transduction via GPCRs could be considered analogous to transmission of light through a series of rotating discs with holes. Each disc represents a diverse set of factors in each family (indicated by holes on the discs) that contribute to effective signal transduction. The discs are set in motion by dynamic cellular factors (motors rotating the discs). Each set of factors could influence the dynamics of the other sets (for example, effectors depend on the subcellular location, and lipid dynamics could change trafficking). The diversity in factors depend on the size and position of the holes. Akin to light passing through the discs when a set of holes across each disc aligns, for a combination of receptor and ligand inputs, a given signaling output is generated if specific combinations of effectors at the optimal subcellular location and microenvironment ‘align’.
In this context, while recent work has illuminated membrane-specific receptor activation and compartmentalized cAMP domains adjacent to the GPCR, whether other signaling outputs downstream of GPCRs also show a similar level of spatial regulation is still being investigated. For example, opioid receptors can activate a variety of other outputs, including G protein-coupled inwardly-rectifying potassium (GIRK) channels [7,55], and Ca2+ channels [56]. While activation of GIRK channels is likely to be restricted to the surface, opioid receptors can differentially inhibit voltage-gated Ca2+ channels on the surface and activate intracellular (store-operated) Ca2+ channels [57,58]. Opioid receptors can also activate ERK either directly or via arrestin [59-61], presumably on the plasma membrane [62,63]. However, the mechanism of activation of ERK might dictate the final site of action, as arrestin-mediated activation of ERK causes nuclear translocation [64]. The functional significance of location-based activity of many of these outputs are still not fully understood.
One aspect of GPCR biology that remains poorly understood is the interaction of GPCRs with membrane lipids at different organelles [65,66]. Although these are likely transient and low affinity interactions, they could have significant influence on receptor conformation and interactions. For example, GPCRs show specific requirements of membrane phosphoinositides as an allosteric modulator of receptor-β-arrestin interaction [67]. Understanding these low affinity and transient interactions is critical to fully understand location-based diversity in GPCR trafficking and signaling.
A second aspect that is less explored is the differential cellular expression profiles of GPCR isoforms [68] and receptor variants [69]. Receptor isoforms arising from tissue-specific alternative splicing of a gene encoding any given GPCR can generate distinct trafficking and signaling outcomes. Traditional views suggested that cells expressed a predominant GPCR with minor ‘alternate’ isoforms. However, recent large scale bioinformatics studies of tissue-specific expression have shown that the extent of expression of multiple isoforms is much higher than anticipated, especially in some tissues. Importantly, combinatorial expression of multiple GPCR isoforms in cells generates different patterns of signaling, compared to expression of single isoforms [68]. Moreover, single nucleotide polymorphisms are widely prevalent, but how these variations change modalities of receptor and effector localization, trafficking, and signaling is still not well studied [69]. Between isoforms and variants, a single gene could therefore generate many functional versions of the same receptor. These are important considerations not just for understanding GPCR function, but also for drug development, as a significant fraction of GPCRs that are drug targets have more than one isoform expressed in different tissues [68].
Overall, defining the mechanistic basis of how location determines specificity of receptor-effector interactions is the next frontier in understanding GPCR signaling. GPCRs are the major stakeholders in the current drug market [70,71]. However, significant knowledge gaps remain in our understanding of the complexity and diversity of their function that limit our ability to utilize their full potential in therapy. In this context, it is important to understand the spatiotemporal organization of signaling pathways that is maintained by the distribution and behavior of GPCRs, their effectors, their isoforms, and environments in cells. Understanding this is challenging, as receptor-effector interactions are highly dynamic, with differing degrees of transience or persistence based on the microenvironment, which could affect cellular physiology. The development and application of highly sensitive tools such as live cell imaging and conformation-specific biosensors [37], ebBRET [72], protein fragment complementation [73], and proximity labeling proteomics [74], as well as molecular dynamics simulations and modeling [75], have allowed real time, compartment-specific and high throughput analysis of receptor localization and interaction with effectors. These methods could lead us to a more complete understanding of the subtle but important differences in expression, location, and microenvironments that harbor and stabilize receptor interactions that are critical for diversity in receptor signaling, physiology, and pharmacology.
Acknowledgements
MAP was supported by NIH GM117425 and by NSF 1935926. We thank Ian Chronis for helpful comments and suggestions.
Footnotes
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Pierce KL, Premont RT, Lefkowitz RJ: Seven-transmembrane receptors. Nat Rev Mol Cell Biol 2002, 3:639–650. [DOI] [PubMed] [Google Scholar]
- 2.Vuong TM, Chabre M, Stryer L: Millisecond activation of transducin in the cyclic nucleotide cascade of vision. Nature 1984, 311:659–661. [DOI] [PubMed] [Google Scholar]
- 3.Hein P, Frank M, Hoffmann C, Lohse MJ, Bünemann M: Dynamics of receptor/G protein coupling in living cells. EMBO J 2005, 24:4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG: Desensitization of G protein–coupled receptors and neuronal functions. Annu Rev Neurosci 2004, 27:107–144. [DOI] [PubMed] [Google Scholar]
- 5.Grutter T, de Carvalho LP, Dufresne V, Taly A, Edelstein SJ, Changeux JP: Molecular tuning of fast gating in pentameric ligand-gated ion channels. Proc Natl Acad Sci USA 2005, 102:18207–18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan TM, Henderson G, North RA, Williams JT: Noradrenaline-mediated synaptic inhibition in rat locus coeruleus neurones. J Physiol 1983, 345:477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.North RA, Williams JT, Surprenant A, Christie MJ: Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA 1987, 84:5487–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jong YJI, Harmon SK, O’Malley KL: GPCR signalling from within the cell. Br J Pharmacol 2018, 175:4026–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobingier BT, von Zastrow M: When trafficking and signaling mix: how subcellular location shapes G protein-coupled receptor activation of heterotrimeric G proteins. Traffic 2019, 20:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunselman JM, Lott J, Puthenveedu MA: Mechanisms of selective G protein-coupled receptor localization and trafficking. Curr Opin Cell Biol 2021, 71:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crilly SE, Puthenveedu MA: Compartmentalized GPCR signaling from intracellular membranes. J Membr Biol 2020, 254:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Q, Yang D, Wu M, Guo Y, Guo W, Zhong L, Cai X, Dai A, Jang W, Shakhnovich EI, Liu Z-J, Stevens RC, Lambert NA, Babu MM, Wang M-W, Zhao S: Common activation mechanism of class A GPCRs. eLife 2019, 8, e50279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nezhady MA, Rivera JC, Chemtob S: Location bias as emerging paradigm in GPCR biology and drug discovery. iScience 2020, 23, 101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanyaloglu AC, von Zastrow M: Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 2008, 48:537–568. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe BL, Trejo J: Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 2008, 8:462–470. [DOI] [PubMed] [Google Scholar]
- 16.Wan M, Zhang W, Tian Y, Xu C, Xu T, Liu J, Zhang R: Unraveling a molecular determinant for clathrin-independent internalization of the M2 muscarinic acetylcholine receptor. Sci Rep 2015, 5, 11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucrot E, Ferreira A, Almeida-Souza L, Debard S, Vallis Y, Howard G, Bertot L, Sauvonnet N, McMahon HT: Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 2015, 517:460–465. [DOI] [PubMed] [Google Scholar]
- 18.Kumar GA, Chattopadhyay A: Statin-induced chronic cholesterol depletion switches GPCR endocytosis and trafficking: insights from the serotonin1A receptor. ACS Chem Neurosci 2020, 11:453–465. [DOI] [PubMed] [Google Scholar]
- 19.Lambert L, Dubayle D, Fafouri A, Herzog E, Csaba Z, Dournaud P, El Mestikawy S, Bernard V: Endocytosis of activated muscarinic M2 receptor (M2R) in live mouse hippocampal neurons occurs via a clathrin-dependent pathway. Front Cell Neurosci 2018, 12:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberg ZY, Puthenveedu MA: Regulation of G protein-coupled receptor signaling by plasma membrane organization and endocytosis. Traffic 2019, 20:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes I, Sierra S, Lueptow L, Gupta A, Gouty S, Margolis EB, Cox BM, Devi LA: Biased signaling by endogenous opioid peptides. Proc Natl Acad Sci USA 2020, 117:11820–11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.*. Kunselman JM, Gupta A, Gomes I, Devi LA, Puthenveedu MA: Compartment-specific opioid receptor signaling is selectively modulated by different dynorphin peptides. eLife 2021, 10, e60270. This paper shows that related endogenous peptides of the dynorphin family, which bind and activate KOR to similar extents on the plasma membrane, drive different KOR trafficking and signaling profiles after the initial phase. Dynorphin A leads to KOR localization in lysosomes and induces sustained signaling from these compartments, while Dynorphin B causes recycling of the receptor to the plasma membrane.
- 23.Sharma A, Kumar GA, Chattopadhyay A: Late endosomal/lysosomal accumulation of a neurotransmitter receptor in a cellular model of Smith-Lemli-Opitz syndrome. Traffic 2021, 22:332–344. [DOI] [PubMed] [Google Scholar]
- 24.Kumar GA, Sarkar P, Stepniewski TM, Jafurulla M, Singh SP, Selent J, Chattopadhyay A: A molecular sensor for cholesterol in the human serotonin1A receptor. Sci Adv 2021, 7, eabh2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soubias O, Teague WE, Hines KG, Gawrisch K: The role of membrane curvature elastic stress for function of rhodopsinlike G protein-coupled receptors. Biochimie 2014, 107:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sych T, Levental KR, Sezgin E: Lipid-protein interactions in plasma membrane organization and function. Annu Rev Biophys 2022, 51:135–156. [DOI] [PubMed] [Google Scholar]
- 27.Rosholm KR, Leijnse N, Mantsiou A, Tkach V, Pedersen SL, Wirth VF, Oddershede LB, Jensen KJ, Martinez KL, Hatzakis NS, Bendix PM, Callan-Jones A, Stamou D: Membrane curvature regulates ligand-specific membrane sorting of GPCRs in living cells. Nat Chem Biol 2017, 13:724–729. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Wu G: Mechanisms of the anterograde trafficking of GPCRs: regulation of AT1R transport by interacting proteins and motifs. Traffic 2019, 20:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.*. Wei Z, Xu X, Fang Y, Khater M, Naughton SX, Hu G, Terry AV, Wu G: Rab43 GTPase directs postsynaptic trafficking and neuron-specific sorting of G protein-coupled receptors. J Biol Chem 2021, 296, 100517. This paper shows that Rab43 selectively regulates the surface trafficking of α2B-adrenergic receptors (α2BAR) but not M3 muscarinic acetylcholine receptors (M3), in neuronal cells. Rab43 potentially mediates surface export via interaction with ICL3 of α2BAR.
- 30.Bhosle VK, Rivera JC, Zhou TE, Omri S, Sanchez M, Hamel D, Zhu T, Rouget R, Al Rabea A, Hou X, Lahaie I, Ribeiro-da-Silva A, Chemtob S: Nuclear localization of platelet-activating factor receptor controls retinal neovascularization. Cell Discov 2016, 2, 16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiwarski DJ, Darr M, Telmer CA, Bruchez MP, Puthenveedu MA: PI3K class II α regulates δ-opioid receptor export from the trans-Golgi network. Mol Biol Cell 2017, 28:2202–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiwarski DJ, Tipton A, Giraldo MD, Schmidt BF, Gold MS, Pradhan AA, Puthenveedu MA: A PTEN-regulated checkpoint controls surface delivery of δ opioid receptors. J Neurosci 2017, 37:3741–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiwarski DJ, Crilly SE, Dates A, Puthenveedu MA: Dual RXR motifs regulate nerve growth factor-mediated intracellular retention of the delta opioid receptor. Mol Biol Cell 2019, 30:680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM: Bradykinin-induced functional competence and trafficking of the δ-opioid receptor in trigeminal nociceptors. J Neurosci 2005, 25:8825–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettinger L, Gigout S, Linley JE, Gamper N: Bradykinin controls pool size of sensory neurons expressing functional δ-opioid receptors. J Neurosci 2013, 33:10762–10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.*. Jimenez-Vargas NN, Gong J, Wisdom MJ, Jensen DD, Latorre R, Hegron A, Teng S, DiCello JJ, Rajasekhar P, Veldhuis NA, Carbone SE, Yu Y, Lopez-Lopez C, Jaramillo-Polanco J, Canals M, Reed DE, Lomax AE, Schmidt BL, Leong KW, Vanner SJ, Halls ML, Bunnett NW, Poole DP: Endosomal signaling of delta opioid receptors is an endogenous mechanism and therapeutic target for relief from inflammatory pain. Proc Natl Acad Sci USA 2020, 117:15281–15292. This work shows that signaling from endosomal DOR is required for sustained pain relief in nociceptive neurons. Specifically targeting endosomal delta opioid receptors using an encapsulated liposomal formulation of the DOR agonist DADLE enhanced DOR-mediated antinociception in dorsal root ganglia neurons.
- 37.**. Crilly SE, Ko W, Weinberg ZY, Puthenveedu MA: Conformational specificity of opioid receptors is determined by subcellular location irrespective of agonist. eLife 2021, 10, e67478. This paper uses differential recruitment of nanobody- and G-protein-based fluorescent conformational biosensors to show that the same ligand can induce different conformational states of the same opioid receptor at different intracellular locations. The conformational states of the opioid receptors induced on both the plasma membrane and the Golgi were able to inhibit cAMP, but only the plasma membrane-localized receptors recruited arrestins and G-protein-based sensors.
- 38.Smith FD, Esseltine JL, Nygren PJ, Veesler D, Byrne DP, Vonderach M, Strashnov I, Eyers CE, Eyers PA, Langeberg LK, Scott JD: Local protein kinase A action proceeds through intact holoenzymes. Science 2017, 356:1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blair CM, Baillie GS: Reshaping cAMP nanodomains through targeted disruption of compartmentalised phosphodiesterase signalosomes. Biochem Soc Trans 2019, 47:1405–1414. [DOI] [PubMed] [Google Scholar]
- 40.Coghlan VM, Langeberg LK, Fernandez A, Lamb NJ, Scott JD: Cloning and characterization of AKAP 95, a nuclear protein that associates with the regulatory subunit of type II cAMP-dependent protein kinase. J Biol Chem 1994, 269:7658–7665. [PubMed] [Google Scholar]
- 41.Lazar AM, Irannejad R, Baldwin TA, Sundaram AB, Gutkind JS, Inoue A, Dessauer CW, von Zastrow M: G protein-regulated endocytic trafficking of adenylyl cyclase type 9. eLife 2020, 9, e58039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.*. Bock A, Annibale P, Konrad C, Hannawacker A, Anton SE, Maiellaro I, Zabel U, Sivaramakrishnan S, Falcke M, Lohse MJ: Optical mapping of cAMP signaling at the nanometer scale. Cell 2020, 182:1519–1530. This paper provides a mechanism for spatiotemporal control over cAMP dynamics in live cells. The mechanism is based on the sequestration of fast diffusing cAMP which generates cAMP-free nanodomains around phosphodiesterases that ‘protect’ cAMP effectors from activation. These protected domains are diminished upon stimulation of cAMP production leading to localized activation.
- 43.Zhang JZ, Lu TW, Stolerman LM, Tenner B, Yang JR, Zhang JF, Falcke M, Rangamani P, Taylor SS, Mehta S, Zhang J: Phase separation of a PKA regulatory subunit controls cAMP compartmentation and oncogenic signaling. Cell 2020, 182:1531–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.*. Tenner B, Getz M, Ross B, Ohadi D, Bohrer CH, Greenwald E, Mehta S, Xiao J, Rangamani P, Zhang J: Spatially compartmentalized phase regulation of a Ca2+-cAMP-PKA oscillatory circuit. eLife 2020, 9, e55013. This study describes how nanodomains of Ca2+-sensitive adenylate cyclase drive cAMP level oscillations that are in sync with Ca2+ oscillations, while phosphodiesterases maintain out-of-sync oscillations outside the nanodomain. Disruption of these cAMP phases disrupted Ca2+ oscillations, suggesting that the compartmentalized phases are important for normal signaling.
- 45.Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E: Distinct functions of the two isoforms of dopamine D2 receptors. Nature 2000, 408:199–203. [DOI] [PubMed] [Google Scholar]
- 46.Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hökfelt T, Borrelli E, Fisone G: Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci USA 2003, 100:4305–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radl D, Chiacchiaretta M, Lewis RG, Brami-Cherrier K, Arcuri L, Borrelli E: Differential regulation of striatal motor behavior and related cellular responses by dopamine D2L and D2S isoforms. Proc Natl Acad Sci USA 2018, 115:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gantz SC, Robinson BG, Buck DC, Bunzow JR, Neve RL, Williams JT, Neve KA: Distinct regulation of dopamine D2S and D2L autoreceptor signaling by calcium. eLife 2015, 4, e09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neve KA, Ford CP, Buck DC, Grandy DK, Neve RL, Phillips TJ: Normalizing dopamine D2 receptor-mediated responses in D2 null mutant mice by virus-mediated receptor restoration: comparing D2 and D2. Neuroscience 2013, 248:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sungkaworn T, Jobin M-L, Burnecki K, Weron A, Lohse MJ, Calebiro D: Single-molecule imaging reveals receptor-G protein interactions at cell surface hot spots. Nature 2017, 550:543–547. [DOI] [PubMed] [Google Scholar]
- 51.Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, Taunton J, Weiner OD, Parton RG, Von Zastrow M: Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 2010, 143:761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowman SL, Shiwarski DJ, Puthenveedu MA: Distinct G protein-coupled receptor recycling pathways allow spatial control of downstream G protein signaling. J Cell Biol 2016, 214:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.**. White AD, Peña KA, Clark LJ, Maria CS, Liu S, Jean-Alphonse FG, Lee JY, Lei S, Cheng Z, Tu CL, Fang F, Szeto N, Gardella TJ, Xiao K, Gellman SH, Bahar I, Sutkeviciute I, Chang W, Vilardaga J-P: Spatial bias in cAMP generation determines biological responses to PTH type 1 receptor activation. Sci Signal 2021, 14, eabc5944. This study showed that subcellular location of the parathyroid hormone (PTH) receptor type 1 was critical for the biological outcome of receptor activation. An engineered plasma membrane biased ligand could generate sustained cAMP signaling from the plasma membrane, but could not mimic the Ca2+ and Vitamin D responses generated by PTH which causes a transient response from the plasma membrane and a sustained endosomal cAMP signaling.
- 54.Latorre R, Hegron A, Peach CJ, Teng S, Tonello R, Retamal JS, Klein-Cloud R, Bok D, Jensen DD, Gottesman-Katz L, Rientjes J, Veldhuis NA, Poole DP, Schmidt BL, Pothoulakis CH, Rankin C, Xie Y, Koon HW, Bunnett NW: Mice expressing fluorescent PAR2 reveal that endocytosis mediates colonic inflammation and pain. Proc Natl Acad Sci USA 2022, 119, e2112059119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams JT, Egan TM, North RA: Enkephalin opens potassium channels on mammalian central neurones. Nature 1982, 299:74–77. [DOI] [PubMed] [Google Scholar]
- 56.Gross RA, Macdonald RL: Dynorphin A selectively reduces a large transient (N-type) calcium current of mouse dorsal root ganglion neurons in cell culture. Proc Natl Acad Sci USA 1987, 84:5469–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bourinet E, Soong TW, Stea A, Snutch TP: Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci USA 1996, 93:1486–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spencer RJ, Jin W, Thayer SA, Chakrabarti S, Law PY, Loh HH: Mobilization of Ca2+ from intracellular stores in transfected neuro2a cells by activation of multiple opioid receptor subtypes. Biochem Pharmacol 1997, 54:809–818. [DOI] [PubMed] [Google Scholar]
- 59.Li LY, Chang KJ: The stimulatory effect of opioids on mitogen-activated protein kinase in Chinese hamster ovary cells transfected to express mu-opioid receptors. Mol Pharmacol 1996, 50:599–602. [PubMed] [Google Scholar]
- 60.Fukuda K, Kato S, Morikawa H, Shoda T, Mori K: Functional coupling of the delta-, mu-, and kappa-opioid receptors to mitogen-activated protein kinase and arachidonate release in Chinese hamster ovary cells. J Neurochem 1996, 67:1309–1316. [DOI] [PubMed] [Google Scholar]
- 61.Cassier E, Gallay N, Bourquard T, Claeysen S, Bockaert J, Crépieux P, Poupon A, Reiter E, Marin P, Vandermoere F: Phosphorylation of β-arrestin2 at Thr383 by MEK underlies β-arrestin-dependent activation of Erk1/2 by GPCRs. eLife 2017, 6, e23777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kramer HK, Simon EJ: μ and δ-opioid receptor agonists induce mitogen-activated protein kinase (MAPK) activation in the absence of receptor internalization. Neuropharmacology 2000, 39:1707–1719. [DOI] [PubMed] [Google Scholar]
- 63.Weinberg ZY, Zajac AS, Phan T, Shiwarski DJ, Puthenveedu MA: Sequence-specific regulation of endocytic lifetimes modulates arrestin-mediated signaling at the μ opioid receptor. Mol Pharmacol 2017, 91:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng H, Loh HH, Law PY: β-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol Pharmacol 2008, 73:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arumugam S, Kaur A: The lipids of the early endosomes: making multimodality work. Chem Bio Chem 2017, 18:1053–1060. [DOI] [PubMed] [Google Scholar]
- 66.van Meer G, de Kroon AIPM: Lipid map of the mammalian cell. J Cell Sci 2011, 124:5–8. [DOI] [PubMed] [Google Scholar]
- 67.Janetzko J, Kise R, Barsi-Rhyne B, Siepe DH, Heydenreich FM, Masureel M, Kawakami K, Garcia KC, Von Zastrow M, Inoue A, Kobilka BK: Membrane phosphoinositides stabilize GPCR-arrestin complexes and offer temporal control of complex assembly and dynamics. bioRxiv 2021:2021, 10.1101/2021.10.09.463790. [DOI] [Google Scholar]
- 68.*. Marti-Solano M, Crilly SE, Malinverni D, Munk C, Harris M, Pearce A, Quon T, Mackenzie AE, Wang X, Peng J, Tobin AB, Ladds G, Milligan G, Gloriam DE, Puthenveedu MA, Babu MM: Combinatorial expression of GPCR isoforms affects signalling and drug responses. Nature 2020, 587:650–656. This study shows that GPCR isoforms are widely expressed in a tissue-specific manner, and that that co-expression of isoforms are key determinants of receptor signaling outcomes. Combinatorial expression of protein isoforms arising from a single GPCR gene result in diverse signaling and trafficking readouts, thereby highlighting the importance of receptor isoforms in the development of drugs targeting GPCRs.
- 69.Joshi M, Nikte SV, Sengupta D: Molecular determinants of GPCR pharmacogenetics: deconstructing the population variants in β2-adrenergic receptor. Adv Prot Chem Struct Biol 2022, 128:361–396. [DOI] [PubMed] [Google Scholar]
- 70.Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE: Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 2017, 16:829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sriram K, Insel PA: G protein-coupled receptors as targets for approved drugs: how many targets and how many drugs? Mol Pharmacol 2018, 93:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Namkung Y, Le Gouill C, Lukashova V, Kobayashi H, Hogue M, Khoury E, Song M, Bouvier M, Laporte SA: Monitoring G protein-coupled receptor and β-arrestin trafficking in live cells using enhanced bystander BRET. Nat Commun 2016, 7, 12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dixon AS, Schwinn MK, Hall MP, Zimmerman K, Otto P, Lubben TH, Butler BL, Binkowski BF, Machleidt T, Kirkland TA, Wood MG: NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem Biol 2016, 11:400–408. [DOI] [PubMed] [Google Scholar]
- 74.Chandan NR, Abraham S, SenGupta S, Parent CA, Smrcka AV: A network of Gai signaling partners is revealed by proximity labeling proteomics analysis and includes PDZ-RhoGEF. Sci Signal 2022, 15, eabi9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sengupta D, Joshi M, Athale CA, Chattopadhyay A: What can simulations tell us about GPCRs: integrating the scales. Methods Cell Biol 2016, 132:429–452. [DOI] [PubMed] [Google Scholar]