Abstract
Background
The nationwide HUN-CANCER EPI study examined cancer incidence and mortality rates in Hungary from 2011 to 2019.
Methods
Using data from the National Health Insurance Fund (NHIF) and Hungarian Central Statistical Office (HCSO), our retrospective study analyzed newly diagnosed malignancies between Jan 1, 2011, and Dec 31, 2019. Age-standardized incidence and mortality rates were calculated for all and for different tumor types using both the 1976 and 2013 European Standard Populations (ESP).
Findings
The number of newly diagnosed cancer cases decreased from 60,554 to 56,675 between 2011–2019. Age-standardized incidence rates were much lower in 2018, than previously estimated (475.5 vs. 580.5/100,000 person-years [PYs] in males and 383.6 vs. 438.5/100,000 PYs in females; ESP 1976). All-site cancer incidence showed a mean annual decrease of 1.9% (95% CI: 2.4%-1.4%) in men and 1.0% (95% CI:1.42%-0.66%) in women, parallel to mortality trends (-1.6% in males and -0.6% in females; ESP 2013). In 2018, the highest age-standardized incidence rates were found for lung (88.3), colorectal (82.2), and prostate cancer (62.3) in men, and breast (104.6), lung (47.7), and colorectal cancer (45.8) in women. The most significant decreases in incidence rates were observed for stomach (4.7%), laryngeal (4.4%), and gallbladder cancers (3.5%), with parallel decreases in mortality rates (3.9%, 2.7% and 3.2%, respectively).
Interpretation
We found a lower incidence of newly diagnosed cancer cases for Hungary compared to previous estimates, and decreasing trends in cancer incidence and mortality, in line with global findings and the declining prevalence of smoking.
Keywords: cancer burden, cancer incidence, cancer mortality, financial health insurance database, real-wold data, Hungary
Introduction
Cancer is the second leading cause of mortality after cardiovascular disease in developed countries (1). Monitoring cancer incidence and mortality is of paramount importance for estimating the burden of the disease as well as for planning and evaluating public health programs and healthcare services (2–4). Although, most of the cancer registries are following the ENCR and IACR rules and work hard to produce incidence figures that are comparable between countries, minor methodological, quality and accuracy differences still exist which limit cross-country comparisons. This is especially an issue in some Central Eastern European countries where cancer registries are underfunded. Hungary has been reported to have the highest cancer incidence rates in Europe (5, 6). Although the National Cancer Registry has been operating according to international guidelines since 2000, its registered data have not been used for such estimates. The overestimation of cancer incidence in Hungary may partly be due to one of the highest reported cancer mortality rates among European countries, since most studies calculate incidence rates based on mortality data (5). However, a recent publication suggested that mortality rates may be significantly influenced by the high autopsy rate of the country resulting in a significant number of post-mortem diagnoses for cancers which may remain asymptomatic for a long time such as lung, colorectal and pancreatic cancers (7). The Hungarian Undiagnosed Lung Cancer (HULC) study showed that 11.1% of reported lung cancer deaths were diagnosed post-mortem by a pathologist in 2019, which is in line with Hungary’s outstandingly high autopsy rate compared to other European countries (8).
The main objective of our nationwide, retrospective study (HUN-CANCER EPI-study – Multiple Cancer Epidemiology study) was to estimate the incidence of all cancer types for the first time in Hungary, using data from the Hungarian National Health Insurance Fund (NHIF) database. Furthermore, we sought to examine the incidence of the top ten most frequent cancer types by sex and age and describe trends in incidence rates for cancers frequently included in European cancer incidence reports, mostly by Ferlay et al. (3) Finally, we aimed to compare our results to findings from other European countries.
Materials and methods
Data sources
Our nationwide, retrospective study was performed using the databases of the Hungarian National Health Insurance Fund (NHIF) and the Hungarian Central Statistical Office (HCSO). The NHIF database encompasses almost the entire Hungarian population, including details on drug prescriptions, hospital admissions, outpatient consultations, and medical interventions. It also contains medical information related to diagnosis codes, according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) (9). The HCSO database is the official source of statistics on annual mortality rates among Hungarian citizens, stratified by the reported cause of death, age, and sex.
Our current study focused on patients diagnosed with any type of cancer (ICD-10 codes C00-97, excluding C44) between January 1, 2011, and December 31, 2019. The identification of records from different sources was based on social security numbers (‘TAJ’ number in Hungarian). To calculate annual cancer incidence rates, the NHIF database was queried for individuals having a cancer-related ICD-10 code in at least two separate reimbursement records. Patients who died within 60 days of the first reported ICD-10 code of interest were also included. If a patient had two or more different cancer-related ICD-10 codes, the ICD-10 code group with a higher number of associated occurrences was considered. For instance, if both the breast cancer-related ICD-10 code C50 and the lung cancer-related C34 code appeared in the reports, but more reimbursement entries were related to C50, the patient was defined as having breast cancer. This approach helped to exclude coding mistakes (e.g., metastasis of breast cancer in the lung coded as primary lung cancer, as the NHIF database is not a medical registry but a reimbursement-focused database). The date of diagnosis was defined as the first appearance of the identified cancer-related ICD-10 code. Second or multiple primary malignancies were excluded from further analysis, the consequences of which are detailed in the limitation section. When defining the ‘dominant’ tumor type in patients with multiple cancer types, only ICD-10 codes with at least two occurrences were considered. To allow for international comparisons, we clustered patients into the following groups in line with Ferlay’s publications (2, 3): lip, oral cavity and pharynx (C00-14), esophagus (C15), stomach (C16), colorectum (C18-21), liver (C22), gallbladder (C23-24), pancreas (C25), larynx (C32), lung (C33-34), melanoma of the skin (C43), breast (C50), cervix (C53), corpus uteri (C54), ovarian (C56), prostate (C61), testicular (C62), kidney (C64-65), bladder (C67), cancer of brain and CNS (C70-72), thyroid (C73), Hodgkin tumor (C81), non-Hodgkin lymphoma (C82-86, C96), multiple myeloma (C88+C90), and leukemia (C91-95).
Patients who did not have any of the previously described ICD-10 codes but had an ICD-10 code starting with C (except for C44) were classified as having other cancer. ICD-10 codes of C44 (non-melanoma skin cancer) were excluded in accordance with international cancer epidemiology studies (2, 3). We also calculated the incidence of any type of cancer for cross-country comparisons as the sum of the number of individuals in all categories (excluding C44).
A screening period was set from 2009 to 2010 to accurately identify newly diagnosed cancer patients from 2011 and to exclude patients with prevalent cancers at the start of the time window who had a prior cancer diagnosis code. To test the sensitivity of our definitions in the query, we carried out multiple calculations based on slightly modified alternative definitions of patients with cancer and compared the results. Incidence rates estimated based on these alternative definitions are included as Supplementary Material ( Supplementary Table 1 ). The tested definitions included more stringent conditions, specifying a maximum gap between the two closest visits (30-180 days or 30-365 days), as well as less strict requirements, such as requiring only one occurrence of a cancer-related ICD-10 code for the most liberal definition. To identify the most accurate definition for cancer incidence, we investigated all reimbursed cancer-related interventions (systemic anticancer therapy, radiotherapy, morphology code, and cancer-related surgical intervention) for all the previously detailed scenarios ( Supplementary Table 2 ). Based on these sensitivity analyses, we prioritized the scenario for cancer definition which required the occurrence of minimum two cancer-related ICD-10 codes of a given cancer type (except for cervical cancer, where a stricter definition was prioritized with a minimum of two cancer-related ICD-10 codes repeated within 30 to 180 days based on the sensitivity analyses in Supplementary Tables 2 - 4 ).
Hungarian population sizes for incidence calculations by age and sex, as well as dates and numbers of cause-specific mortality among cancer patients were obtained from the HCSO (10). For the calculation of incidence rates, annual numbers of newly diagnosed patients with any type of cancer were given as crude numbers (n); new cases were counted for each calendar year (between January 1 and December 31). Annual incidence rates are expressed as standardized rates (per 100,000 person-years [PYs]). Age-standardized incidence per 100,000 PYs were also calculated by sex using the cohort weights from European Standard Population (ESP) 2013 (11) for local trend analyses and ESP 1976 for cross-country comparisons (as most of the prior publications used this standard population) (12). Where crude numbers of any parameter were recorded below ten, we indicated “<10” as the NHIF data protection rule does not allow the presentation of case numbers below ten in a stratum. In these cases, calculations were run on the exact crude numbers.
Aggregated cause-specific mortality data by age and sex was obtained from the HCSO for the disease groups described previously. We considered the number of patients who died of any type of cancer (excluding C44) between January 1 and December 31 of a given year as the number of cancer-related cause-specific deaths. Cancer-specific mortality was expressed as crude numbers (n) and standardized rates per 100,000 PYs. We used standardized incidence and cause-specific mortality rates to evaluate temporal trends in incidence and mortality. Where the crude number of individuals in a category was below three (no more detail can be disclosed to protect the privacy of these patients), the numerical value was imputed to be 1.5 in calculations concerning the data point. The size of the Hungarian population by year, age and sex was also provided by the HCSO.
The study was approved by the National Ethical Committee (IV/298-2/2022/EKU).
Statistical analysis
Age-standardized incidence and mortality rates per 100,000 PYs by sex were calculated using ESP 2013 (11) cohort weights for local trend analyses and ESP 1976 weights (12) for comparability with Ferlay’s publications (3). Annual trends for incidence and mortality were estimated based on a Poisson-regression model incorporating age and sex as explanatory variables in addition to the year and the log of the population size as offsets. To compensate for the bias potentially introduced by the interdependence of population sizes in consecutive years, 95% confidence intervals (95% CI) were estimated using the robust sandwich method offered by the R package sandwich (13). Differences were considered statistically significant when p-value proved to be lower than 0.05. All calculations were carried out in R v4.2.1 (Available from: https://www.r-project.org).
Results
Number of newly diagnosed cases
We identified 60,554 and 56,675 newly diagnosed cancer cases in the NHIF database in 2011 and in 2019, respectively (30,154 in males and 30,400 in females in 2011; 27,970 in males and 28,705 in females in 2019) ( Table 1 ). The five most common tumor types in men were as follows (based on the number of newly diagnosed cases in 2019): colorectal cancer (C18-21: n=5,293), lung cancer (C33-34: n=4,787), prostate cancer (C61: n=4,022), bladder cancer (C67: n=1,797), and lip, oral cavity, and pharyngeal cancers (C00-14: n=1,509). In women, the most common tumor types were breast cancer (C50: n=7,305), colorectal cancer (C18-21: n=3,978), lung cancer (C33-34: n=3,711), uterine cancer (C54: n=1,463), and melanoma of the skin (C43: 1,180). The number of newly diagnosed cancers (a) and the number of cause-specific deaths (b) by different age groups are shown in Supplementary Table 3 . For the sensitivity analyses, newly diagnosed cancer cases based on alternative cancer definitions are shown in Supplementary Tables 1A–D .
Table 1.
Number of newly diagnosed cases and deaths by cancer type, sex, and year in Hungary, HCSO: Hungarian Central Statistical Office; NHIF: National Health Insurance Fund.
| A) Incidence of cancer in Hungary | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | |
| All sites (C00-97 excl. C44) | 30,154 | 30,400 | 60,554 | 28,971 | 29,409 | 58,380 | 29,584 | 30,133 | 59,717 | 29,529 | 30,015 | 59,544 | 29,069 | 29,961 | 59,030 | 29,251 | 30,241 | 59,492 | 28,969 | 29,358 | 58,327 | 28,014 | 29,075 | 57,089 | 27,970 | 28,705 | 56,675 |
| Bladder cancer (C67) | 1,906 | 896 | 2,802 | 1,969 | 907 | 2,876 | 1,862 | 898 | 2,760 | 1,968 | 868 | 2,836 | 1,922 | 880 | 2,802 | 1,821 | 890 | 2,711 | 1,896 | 855 | 2,751 | 1,814 | 815 | 2,629 | 1,797 | 866 | 2,663 |
| Breast cancer (C50) | 72 | 7,775 | 7,847 | 81 | 7,363 | 7,444 | 72 | 7,447 | 7,519 | 96 | 7,565 | 7,661 | 71 | 7,491 | 7,562 | 72 | 7,694 | 7,766 | 85 | 7,511 | 7,596 | 80 | 7,540 | 7,620 | 92 | 7,305 | 7,397 |
| Cancer of brain and CNS (C70-72) | 585 | 550 | 1,135 | 536 | 530 | 1,066 | 563 | 509 | 1,072 | 548 | 538 | 1,086 | 518 | 480 | 998 | 501 | 446 | 947 | 492 | 483 | 975 | 504 | 475 | 979 | 489 | 424 | 913 |
| Cancer of lip, oral cavity and pharynx (C00-14) | 1,883 | 615 | 2,498 | 1,813 | 588 | 2,401 | 1,787 | 616 | 2,403 | 1,720 | 618 | 2,338 | 1,647 | 635 | 2,282 | 1,749 | 590 | 2,339 | 1,560 | 582 | 2,142 | 1,490 | 580 | 2,070 | 1,509 | 552 | 2,061 |
| Cancer of the corpus uteri (C54) | 1,370 | 1,370 | 1,277 | 1,277 | 1,297 | 1,297 | 1,368 | 1,368 | 1,364 | 1,364 | 1,534 | 1,534 | 1,367 | 1,367 | 1,431 | 1,431 | 1,463 | 1,463 | |||||||||
| Cervical cancer (C53) | 1,067 | 1,067 | 978 | 978 | 997 | 997 | 1,042 | 1,042 | 1,043 | 1,043 | 1,032 | 1,032 | 889 | 889 | 863 | 863 | 822 | 822 | |||||||||
| Colorectum cancer (C18-21) | 5,221 | 4,396 | 9,617 | 5,016 | 4,218 | 9,234 | 5,203 | 4,236 | 9,439 | 5,101 | 4,111 | 9,212 | 5,064 | 4,126 | 9,190 | 5,198 | 4,068 | 9,266 | 5,171 | 3,982 | 9,153 | 4,951 | 3,961 | 8,912 | 5,293 | 3,978 | 9,271 |
| Gallbladder cancer (C23-24) | 205 | 399 | 604 | 226 | 400 | 626 | 246 | 380 | 626 | 263 | 390 | 653 | 232 | 370 | 602 | 231 | 361 | 592 | 235 | 324 | 559 | 250 | 305 | 555 | 199 | 286 | 485 |
| Hodgkin tumor (C81) | 143 | 131 | 274 | 119 | 126 | 245 | 121 | 115 | 236 | 157 | 132 | 289 | 126 | 116 | 242 | 147 | 109 | 256 | 113 | 103 | 216 | 116 | 129 | 245 | 113 | 91 | 204 |
| Kidney cancer (C64-65) | 1,095 | 855 | 1,950 | 1,137 | 811 | 1,948 | 1,105 | 869 | 1,974 | 1,103 | 819 | 1,922 | 1,142 | 834 | 1,976 | 1,176 | 803 | 1,979 | 1,125 | 796 | 1,921 | 1,149 | 783 | 1,932 | 1,081 | 817 | 1,898 |
| Larynx cancer (C32) | 742 | 142 | 884 | 671 | 121 | 792 | 700 | 126 | 826 | 677 | 125 | 802 | 629 | 117 | 746 | 652 | 111 | 763 | 587 | 95 | 682 | 580 | 89 | 669 | 546 | 89 | 635 |
| Leukemia (C91-95) | 967 | 952 | 1,919 | 952 | 888 | 1,840 | 991 | 920 | 1,911 | 975 | 964 | 1,939 | 990 | 859 | 1,849 | 958 | 906 | 1,864 | 1,034 | 915 | 1,949 | 950 | 829 | 1,779 | 950 | 817 | 1,767 |
| Liver cancer (C22) | 615 | 346 | 961 | 612 | 336 | 948 | 616 | 310 | 926 | 590 | 315 | 905 | 647 | 338 | 985 | 626 | 342 | 968 | 623 | 335 | 958 | 624 | 301 | 925 | 659 | 326 | 985 |
| Lung cancer (C33-34) | 6,226 | 3,681 | 9,907 | 5,936 | 3,698 | 9,634 | 5,643 | 3,809 | 9,452 | 5,747 | 3,703 | 9,450 | 5,525 | 3,821 | 9,346 | 5,515 | 3,788 | 9,303 | 5,515 | 3,875 | 9,390 | 5,263 | 3,748 | 9,011 | 4,787 | 3,711 | 8,498 |
| Melanoma of the skin (C43) | 958 | 1,018 | 1,976 | 896 | 1,113 | 2,009 | 975 | 1,125 | 2,100 | 1,054 | 1,180 | 2,234 | 1,128 | 1,268 | 2,396 | 1,112 | 1,241 | 2,353 | 1,154 | 1,209 | 2,363 | 1,020 | 1,185 | 2,205 | 1,122 | 1,181 | 2,303 |
| Multiple myeloma (C88+C90) | 239 | 324 | 563 | 242 | 359 | 601 | 269 | 323 | 592 | 293 | 331 | 624 | 262 | 331 | 593 | 264 | 328 | 592 | 296 | 344 | 640 | 317 | 343 | 660 | 268 | 327 | 595 |
| Non-Hodgkin lymphoma (C82-86, C96) | 717 | 838 | 1,555 | 717 | 799 | 1,516 | 783 | 967 | 1,750 | 778 | 995 | 1,773 | 781 | 841 | 1,622 | 759 | 923 | 1,682 | 775 | 863 | 1,638 | 800 | 924 | 1,724 | 855 | 1,018 | 1,873 |
| Oesophagus cancer (C15) | 476 | 127 | 603 | 471 | 133 | 604 | 444 | 124 | 568 | 494 | 124 | 618 | 456 | 116 | 572 | 469 | 108 | 577 | 404 | 99 | 503 | 442 | 90 | 532 | 430 | 100 | 530 |
| Ovarian cancer (C56) | 1,044 | 1,044 | 1,052 | 1,052 | 1,117 | 1,117 | 1,024 | 1,024 | 1,062 | 1,062 | 1,036 | 1,036 | 1,022 | 1,022 | 1,005 | 1,005 | 936 | 936 | |||||||||
| Pancreas cancer (C25) | 981 | 986 | 1,967 | 955 | 992 | 1,947 | 998 | 1,118 | 2,116 | 964 | 1,043 | 2,007 | 1,007 | 1,070 | 2,077 | 1,024 | 1,122 | 2,146 | 1,082 | 1,085 | 2,167 | 991 | 1,082 | 2,073 | 1,014 | 1,080 | 2,094 |
| Prostate cancer (C61) | 3,934 | 3,934 | 3,582 | 3,582 | 4,239 | 4,239 | 4,108 | 4,108 | 3,982 | 3,982 | 3,996 | 3,996 | 3,971 | 3,971 | 3,876 | 3,876 | 4,022 | 4,022 | |||||||||
| Stomach cancer (C16) | 1,112 | 865 | 1,977 | 1,074 | 841 | 1,915 | 1,073 | 828 | 1,901 | 980 | 762 | 1,742 | 986 | 774 | 1,760 | 978 | 746 | 1,724 | 965 | 698 | 1,663 | 823 | 662 | 1,485 | 862 | 598 | 1,460 |
| Testicular cancer (C62) | 659 | 659 | 606 | 606 | 530 | 530 | 580 | 580 | 576 | 576 | 594 | 594 | 539 | 539 | 576 | 576 | 510 | 510 | |||||||||
| Thyroid cancer (C73) | 130 | 465 | 595 | 122 | 454 | 576 | 144 | 546 | 690 | 133 | 536 | 669 | 167 | 528 | 695 | 180 | 597 | 777 | 181 | 519 | 700 | 156 | 538 | 694 | 199 | 607 | 806 |
| Other | 1,288 | 1,558 | 2,846 | 1,238 | 1,425 | 2,663 | 1,220 | 1,456 | 2,676 | 1,200 | 1,462 | 2,662 | 1,211 | 1,497 | 2,708 | 1,229 | 1,466 | 2,695 | 1,166 | 1,407 | 2,573 | 1,242 | 1,397 | 2,639 | 1,173 | 1,311 | 2,484 |
| B) Mortality of cancer in Hungary | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | ||||||||||||||||||
| Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | |
| All sites (C00-97 excl. C44) | 17,875 | 14,607 | 32,482 | 18,166 | 14,846 | 33,012 | 17,728 | 14,843 | 32,571 | 17,653 | 14,889 | 32,542 | 17,552 | 15,047 | 32,599 | 17,900 | 14,862 | 32,762 | 17,596 | 15,019 | 32,615 | 17,517 | 14,875 | 32,392 | 17,131 | 14,648 | 31,779 |
| Bladder cancer (C67) | 655 | 268 | 923 | 714 | 269 | 983 | 637 | 262 | 899 | 586 | 320 | 906 | 679 | 280 | 959 | 683 | 292 | 975 | 667 | 316 | 983 | 706 | 302 | 1,008 | 713 | 320 | 1,033 |
| Breast cancer (C50) | 21 | 2,138 | 2,159 | 27 | 2,096 | 2,123 | 27 | 2,167 | 2,194 | 26 | 2,107 | 2,133 | 30 | 2,220 | 2,250 | 25 | 2,223 | 2,248 | 15 | 2,123 | 2,138 | 22 | 2,127 | 2,149 | 26 | 2,174 | 2,200 |
| Cancer of brain and CNS (C70-72) | 271 | 320 | 591 | 344 | 296 | 640 | 343 | 322 | 665 | 330 | 332 | 662 | 352 | 336 | 688 | 342 | 326 | 668 | 348 | 314 | 662 | 349 | 339 | 688 | 348 | 276 | 624 |
| Cancer of lip, oral cavity and pharynx (C00-14) | 1,213 | 281 | 1,494 | 1,225 | 311 | 1,536 | 1,146 | 285 | 1,431 | 1,157 | 303 | 1,460 | 1,167 | 305 | 1,472 | 1,122 | 273 | 1,395 | 1,035 | 298 | 1,333 | 1,037 | 279 | 1,316 | 1,018 | 293 | 1,311 |
| Cancer of the corpus uteri (C54) | 292 | 292 | 287 | 287 | 275 | 275 | 326 | 326 | 264 | 264 | 317 | 317 | 315 | 315 | 310 | 310 | 331 | 331 | |||||||||
| Cervical cancer (C53) | 414 | 414 | 426 | 426 | 405 | 405 | 420 | 420 | 476 | 476 | 396 | 396 | 377 | 377 | 408 | 408 | 386 | 386 | |||||||||
| Colorectum cancer (C18-21) | 2,835 | 2,219 | 5,054 | 2,810 | 2,274 | 5,084 | 2,865 | 2,242 | 5,107 | 2,848 | 2,202 | 5,050 | 2,825 | 2,183 | 5,008 | 2,845 | 2,214 | 5,059 | 2,816 | 2,169 | 4,985 | 2,836 | 2,198 | 5,034 | 2,837 | 2,096 | 4,933 |
| Gallbladder cancer (C23-24) | 215 | 428 | 643 | 229 | 434 | 663 | 219 | 418 | 637 | 227 | 384 | 611 | 219 | 365 | 584 | 240 | 352 | 592 | 214 | 355 | 569 | 240 | 347 | 587 | 230 | 308 | 538 |
| Hodgkin tumor (C81) | 30 | 13 | 43 | 9 | 22 | 31 | 21 | 18 | 39 | 25 | 10 | 35 | 24 | 22 | 46 | 20 | 15 | 35 | 18 | 21 | 39 | 13 | 24 | 37 | 20 | 15 | 35 |
| Kidney cancer (C64-65) | 459 | 352 | 811 | 487 | 267 | 754 | 456 | 352 | 808 | 486 | 313 | 799 | 466 | 281 | 747 | 475 | 311 | 786 | 469 | 342 | 811 | 470 | 304 | 774 | 458 | 311 | 769 |
| Larynx cancer (C32) | 490 | 68 | 558 | 484 | 56 | 540 | 476 | 68 | 544 | 445 | 65 | 510 | 389 | 74 | 463 | 409 | 61 | 470 | 398 | 67 | 465 | 395 | 68 | 463 | 471 | 52 | 523 |
| Leukaemia (C91-95) | 474 | 474 | 948 | 480 | 435 | 915 | 456 | 453 | 909 | 482 | 404 | 886 | 489 | 470 | 959 | 460 | 439 | 899 | 461 | 404 | 865 | 476 | 479 | 955 | 496 | 397 | 893 |
| Liver cancer (C22) | 516 | 280 | 796 | 538 | 283 | 821 | 553 | 279 | 832 | 625 | 279 | 904 | 565 | 297 | 862 | 581 | 269 | 850 | 590 | 305 | 895 | 565 | 290 | 855 | 578 | 281 | 859 |
| Lung cancer (C33-34) | 5,558 | 2,975 | 8,533 | 5,763 | 3,133 | 8,896 | 5,418 | 3,173 | 8,591 | 5,456 | 3,277 | 8,733 | 5,356 | 3,397 | 8,753 | 5,542 | 3,341 | 8,883 | 5,393 | 3,447 | 8,840 | 5,341 | 3,375 | 8,716 | 4,997 | 3,450 | 8,447 |
| Melanoma of the skin (C43) | 214 | 154 | 368 | 210 | 169 | 379 | 186 | 163 | 349 | 196 | 186 | 382 | 216 | 135 | 351 | 201 | 142 | 343 | 190 | 147 | 337 | 178 | 141 | 319 | 193 | 143 | 336 |
| Multiple myeloma (C88+C90) | 120 | 153 | 273 | 142 | 157 | 299 | 135 | 140 | 275 | 116 | 142 | 258 | 134 | 170 | 304 | 134 | 141 | 275 | 162 | 160 | 322 | 126 | 149 | 275 | 152 | 158 | 310 |
| Non-Hodgkin lymphoma (C82-86, C96) | 295 | 256 | 551 | 288 | 293 | 581 | 304 | 272 | 576 | 274 | 290 | 564 | 322 | 286 | 608 | 300 | 278 | 578 | 299 | 327 | 626 | 280 | 301 | 581 | 255 | 287 | 542 |
| Oesophagus cancer (C15) | 467 | 129 | 596 | 457 | 118 | 575 | 522 | 105 | 627 | 432 | 112 | 544 | 464 | 113 | 577 | 471 | 87 | 558 | 427 | 104 | 531 | 459 | 89 | 548 | 450 | 103 | 553 |
| Ovarian cancer (C56) | 700 | 700 | 700 | 700 | 739 | 739 | 729 | 729 | 727 | 727 | 696 | 696 | 744 | 744 | 716 | 716 | 660 | 660 | |||||||||
| Pancreas cancer (C25) | 942 | 908 | 1,850 | 950 | 1,053 | 2,003 | 943 | 1,033 | 1,976 | 958 | 1,041 | 1,999 | 946 | 1,032 | 1,978 | 1,056 | 1,122 | 2,178 | 1,084 | 1,144 | 2,228 | 1,071 | 1,082 | 2,153 | 982 | 1,137 | 2,119 |
| Prostate cancer (C61) | 1,198 | 1,198 | 1,125 | 1,125 | 1,211 | 1,211 | 1,280 | 1,280 | 1,258 | 1,258 | 1,301 | 1,301 | 1,389 | 1,389 | 1,314 | 1,314 | 1,319 | 1,319 | |||||||||
| Stomach cancer (C16) | 955 | 746 | 1,701 | 1,000 | 732 | 1,732 | 942 | 677 | 1,619 | 904 | 698 | 1,602 | 853 | 647 | 1,500 | 930 | 649 | 1,579 | 845 | 627 | 1,472 | 819 | 614 | 1,433 | 779 | 554 | 1,333 |
| Testicular cancer (C62) | 53 | 53 | 37 | 37 | 36 | 36 | 58 | 58 | 40 | 40 | 38 | 38 | 43 | 43 | 48 | 48 | 47 | 47 | |||||||||
| Thyroid cancer (C73) | 32 | 71 | 103 | 40 | 65 | 105 | 26 | 61 | 87 | 27 | 60 | 87 | 24 | 55 | 79 | 42 | 43 | 85 | 39 | 74 | 113 | 28 | 46 | 74 | 31 | 56 | 87 |
| Other | 862 | 968 | 1,830 | 807 | 970 | 1,777 | 806 | 934 | 1,740 | 715 | 889 | 1,604 | 734 | 912 | 1,646 | 683 | 875 | 1,558 | 694 | 839 | 1,533 | 744 | 887 | 1,631 | 731 | 860 | 1,591 |
Bold values indicate total case numbers, considering all tumor types included in the study.
Age-standardized incidence and mortality rates
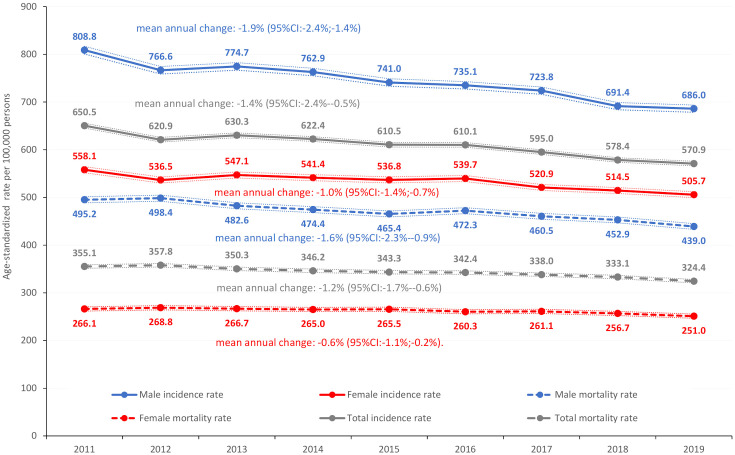
Age-standardized incidence rates of all cancers excluding C44 (ESP 2013) decreased from 808.8 in 2011 to 686.0/100,000 PYs in 2019 in men, with a mean annual decrease of 1.86% (95% CI: 1.36-2.35; decrease for the whole study period: 15.18%) ( Figure 1 ). Corresponding numbers in females were 558.1 in 2011 (31.0% lower than in males) and 505.7 in 2019, showing a mean annual decrease of 1.04% (95% CI: 0.66-1.42). Age-standardized overall cause-specific mortality rates (excluding C44) were 495.2/100,000 PYs in 2011 and 439.0/100,000 PYs in 2019 in males, with a mean annual decrease of 1.63% (95% CI: 0.92-2.34) ( Figure 1 ). Similarly, mortality rates were 266.1/100,000 PYs in 2011 and 250.98/100,000 PYs in 2019 among females, corresponding to a significant mean annual decrease of 0.64% (95% CI: 0.15-1.13). On the other hand, the annual number of registered cancer deaths did not significantly change during the study period, remaining between 32,000–33,000 in both genders. The overall, combined incidence rate of cancer has increased from 650.5 onto 570.9/100,000 PYs with a yearly average of 1.4% (95%CI: 2.4%-0.5%), while the overall mortality rate has change from 355.1 onto 324.4/100,000 PYs, showing also significant yearly average change (-1.2%; 95%CI:-1.7%–0.6%).
Figure 1.
Age-standardized overall cancer incidence rates and overall cause-specific cancer mortality rates in Hungary between 2011 and 2019 (ESP 2013) (per 100,000 PYs; dotted lines represent 95% CI). CI, confidence interval; ESP, European Standard Population; PYs, person-years.
Age-standardized incidence and mortality rates by most common tumor types in 2018
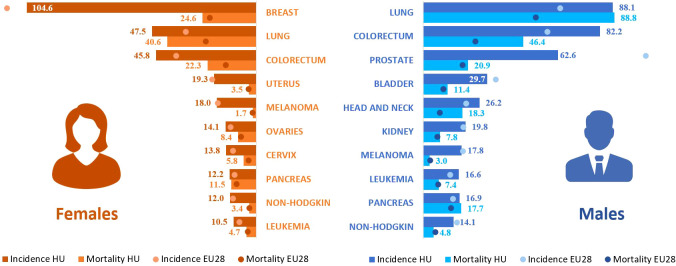
In 2018, the most common cancer type among men was lung cancer, with an age-standardized incidence rate of 88.3/100,000 PYs, followed by colorectal cancer (82.2/100,000 PYs) and prostate cancer (62.3/100,000 PYs). Mortality rates were 88.8, 46.5, and 20.9 per 100,000 PYs, respectively, using ESP 1976 ( Figure 2 ). In 2019, the age-standardized incidence of colorectal cancer exceeded that of lung cancer (87.2 vs. 79.3/100,000 PYs, respectively) ( Supplementary Table 4 ). Incidence and mortality rates of lung cancer and colorectal cancer were well above the European Union (EU) average.(3) However, the incidence of prostate cancer was lower.
Figure 2.
Age-standardized incidence and mortality rates for the 10 most common cancer types in Hungary in 2018 using ESP 1976. Incidence and mortality rates are compared with the European Union (28) average based on Ferlay’s report from 2018 (3). ESP, European Standard Population.
In women, the three most common cancer types were the breast cancer (104.6/100,000 PYs), lung cancer (47.7/100,000 PYs), and colorectal cancer (45.8/100,000 PYs). The highest mortality rate was found for lung cancer (40.6/100,000 PYs), followed by breast cancer (24.6/100,000 PYs), and colorectal cancer (22.6/100,000 PYs) in 2018. Incidences of colorectal cancer and lung cancer were higher, than the EU average; however, breast cancer incidence was similar. Figure 2 provides a comprehensive overview of the other most common cancer types among males and females, comparing their incidence rates and rankings to the EU average. For international comparisons, age-standardized incidence and mortality rates of different cancer types are shown for the whole study period with ESP 1976 ( Supplementary Table 4 ) as well as with ESP 2013 ( Supplementary Table 5 ).
Incidence and mortality of all cancer types in Hungary compared to other European countries
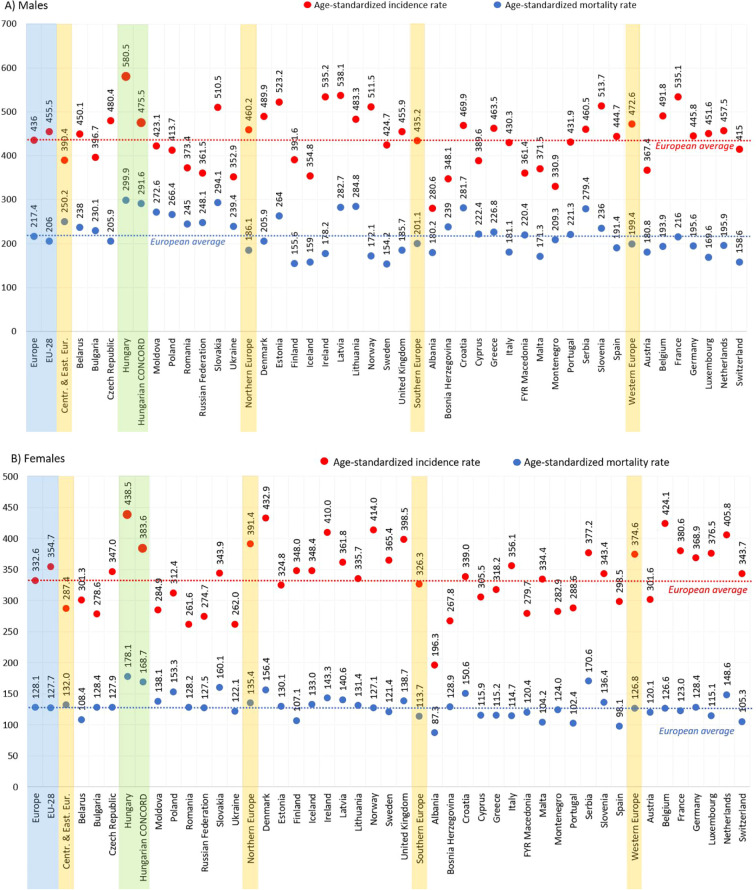
In the current study, the age-standardized incidence of all cancer types (excluding C44) was 475.5/100,000 PYs in 2018 in males, which was higher, than the EU-27 average (436.0/100,000 PYs), but significantly lower, than previously estimated for Hungary (580.5/100,000 PYs using ESP 1976) ( Figure 3A ). Incidence rates found in the current study were comparable to those from the Czech Republic, Slovakia, Lithuania, and Estonia, and higher than in Austria, a neighboring but more developed country. The age-standardized mortality rate of all cancer types was 291.7/100,000 PYs in 2018 in males, one of the highest among European countries (average: 217.4/100,000 PYs), but somewhat lower, than in Ferlay’s report (299.9/100,000 PYs) ( Figure 3A ) (3).
Figure 3.
Age-standardized incidence (red) and mortality (blue) rates in males (A) and females (B) for all cancer types (excluding C44) per 100,000 PYs in European countries and Hungary based on Ferlay’s report (2) and in the HUN-CANCER EPI study using the NHIF/HCSO databases in 2018 (ESP 1976). ESP, European Standard Population; NHIF, National Health Insurance Fund; HCSO, Hungarian Central Statistical Office; PYs, person-years. Description: EU-28: 28 European Union member states in 2018.
In women, the age-standardized incidence of all cancer types (excluding C44) was 383.6/100,000 PYs in 2018, which was higher, than the EU-27 average (332.6/100,000 PYs), but significantly lower, than previously estimated for Hungary (438.5/100,000 PYs using ESP 1976). The age-standardized mortality rate for all cancer types was 168.7/100,000 PYs compared to the European average of 128.1/100,000 PYs in 2018, considerably lower, than the rate estimated by Ferlay et al. (178.1/100,000 PYs) ( Figure 3B ). (3) Mortality rates remained among the highest in Europe in both sexes and were higher than in other Central Eastern European countries.
Change in incidence and mortality rates of different cancer types between 2011 and 2019
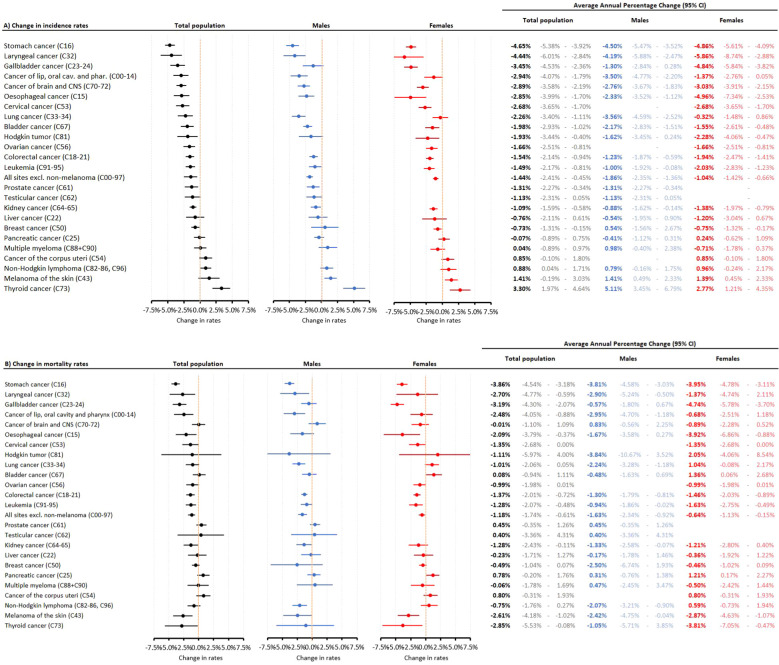
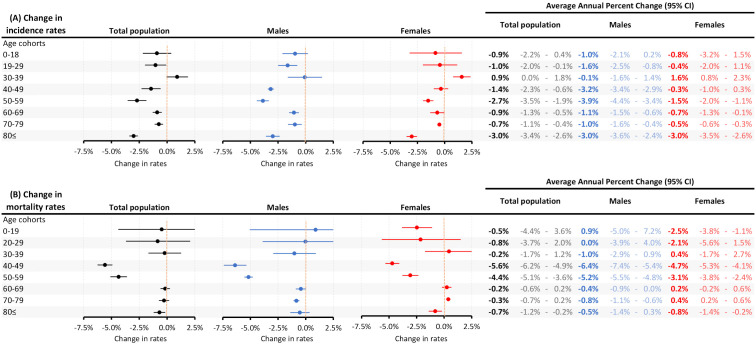
Figure 4 shows the changes in incidence and cause-specific mortality rates of all cancer types (excluding C44) among the most common cancer types between 2011 and 2019.
Figure 4.
Changes in incidence (A) and cause-specific mortality rates (B) between 2011 and 2019 (prior to the Covid-19 pandemic) among the most common cancer types in the total study population and according to sex. CI, confidence interval.
The highest annual decreases in incidence rates were found in stomach cancer (4.65%), laryngeal cancer (4.44%) and gallbladder cancer (3.45%), while there were significant increases in the incidence of non-Hodgkin lymphoma (0.88%), melanoma of the skin (1.41%) and thyroid cancer (3.30%). The most pronounced decreases in cause-specific mortality rates were found in stomach cancer (3.85%), gallbladder cancer (3.20%), thyroid cancer (2.91%), laryngeal cancer (2.71%), and melanoma of the skin (2.66%). The only significant increases in mortality rates were found among women for bladder cancer (1.38%) and pancreatic cancer (1.22%).
Change in overall incidence and mortality rates by different age cohorts between 2011 and 2019
Figure 5 shows the changes in overall incidence and cause-specific mortality rates of all cancer types (excluding C44) among the different age cohorts by different sex, between 2011 and 2019. The most relevant decrease of cancer incidence was found in the 40-49, 50-59 and in 80≤ age cohorts, especially in case of males, where we found a 3.9% average annual decrease (4.4%-3.6%) at the 50-59 cohorts. On the other hand, the increase of average annual incidence was significant in the female 30-39 cohort (1.6%; 95%CI:0.8%-2.4%). The age and sex related change of mortality rates were mostly similar to the incidence rate trends, the highest decrease in the mortality rate was found in the 40-49 and 50-59 male cohorts. The only significant increase in mortality rate was found in the 70-79 cohort of females.
Figure 5.
Age and sex related average annual percent changes in incidence (A) and cause-specific mortality rates (B) between 2011 and 2019 (prior to the Covid-19 pandemic). CI, confidence interval.
Discussion
This nationwide, retrospective study was performed as part of the Hungarian HUN-CANCER EPI Multiple Cancer Epidemiology program to assess incidence and mortality rates of all cancer types between 2011 and 2019 in Hungary. Besides the epidemiological data of the Hungarian National Cancer Registry (14, 15), this study provides real-world data on all cancer types in Hungary, based on the analysis of the national health insurance fund database.
The main results of our analysis were as follows:
1. Age-standardized incidence rates of all cancer types were significantly lower in both sexes in 2018, than estimates reported by Ferlay et al. (18.1% lower in men and 12.4% lower in women; HUN-CANCER EPI vs. Ferlay et al.: 475.5 vs. 580.5/100,000 PYs in males and 383.6 vs. 438.5/100,000 PYs in females in 2018).
2. Age-standardized incidence of all cancer types was higher in males, and significantly decreased by 1.46% on average per year (by 1.86% in males and by 1.04% in females). Age-standardized mortality rates showed a mean annual decrease of 1.16% in males and 0.57% in females between 2011 and 2019. Both decrease of incidence and mortality rates were most pronounced at 40-59 age cohorts of males.
3. In 2018, the five most common cancer types were lung, colon, prostate, bladder and head and neck cancers in males, and breast, colon, lung, uterine cancer, and melanoma of the skin in females.
4. Age-standardized incidence rates (ESP 1976) were generally higher, than the EU average, except for prostate cancer, where the Hungarian incidence rates were significantly lower.
Recent cancer incidence reports, such as those from the European Cancer Information System (ECIS), Globocan, and Cancer Incidence in Five Continents (CI5), provide comprehensive and up-to-date data on the global burden of cancer. The ECIS focuses on detailed cancer statistics across Europe, highlighting regional variations and trends within the continent. Globocan offers a global perspective, estimating cancer incidence and mortality worldwide, with a particular emphasis on variations between high- and low-income countries. The CI5 series, published by the International Agency for Research on Cancer (IARC), compiles data from cancer registries across five continents, enabling comparisons of cancer patterns and trends over time and across different populations. Together, these sources underscore the importance of continuous monitoring and international collaboration in understanding and addressing the global cancer burden.
However, previous GLOBOCAN estimates on overall cancer incidence in Hungary were solely based on the methodology applied by Ferlay et al. for 2018. Namely, cancer mortality rates reported by the HCSO were used for the calculation of incidence rates. Mortality data from the 2006–2015 period served as a basis for the calculation of age-standardized mortality rates in 2018, already resulting in a slight overestimation. Then, incidence and mortality data from the Czech Republic and Slovakia were derived from their respective cancer registries. As a final step, Hungarian incidence rates were estimated using mortality-per-incidence rate ratios from these two neighboring countries and HCSO-based mortality data. In other words, incidence and mortality rates reported in the Czech Republic and Slovakia were ‘translated’ into Hungarian incidence data (3).
Our recent publication on the epidemiology of lung cancer in Hungary demonstrated the high proportion of only post-mortem diagnosed lung cancer cases in Hungary, accounting for 11.1% of cancer-related cause-specific mortality. This may be due to the much higher autopsy rate compared to other European countries (7, 8). Lower autopsy rates for hospital deaths decrease the likelihood of post-mortem cancer diagnoses, especially for cancers commonly diagnosed in late stages. Therefore, cause-specific mortality reported from countries with low autopsy rates may be lower than the actual numbers. The Czech Republic and Slovakia were reported to have 16.6% and 10.9% autopsy rates of hospital deaths in 2018, respectively, compared to the Hungarian rate of 35.8% (8). Traditionally, international comparisons of mortality rates have used HCSO-derived data from Hungary, which contains post-mortem diagnosed cases. Therefore, we propose using NHIF-based cancer incidence data from Hungary for comparisons of cancer burden with other European countries, at the same time acknowledging that actual cancer incidence may be higher due to the significant proportion of post-mortem diagnosed cases. Of note, autopsy rates significantly decreased during the study period in Hungary, which may partly explain the decrease of standardized mortality. On the other hand, the change in age distribution in Hungary during the study period may also have contributed to the observed decrease in standardized mortality rates, which is supported by the fact that the number of deaths did not change during the study period. Incidence rates found in our study are much more in line with those reported from other post-socialist countries in the region, than shown by previous estimates. Cancer incidence in Hungary was found to be in a similar range to that reported from the Czech Republic, Slovakia, and Serbia based on their respective cancer registries, and much higher, than incidence rates reported from Austria, a neighboring but more developed country. This similarity can be attributed to the comparable prevalence of cancer risk factors in these countries. Smoking is one of the main risk factors for the development of cancer (16). The estimated proportion of regular daily smokers in the population aged 15 years and over was 25.8% in 2014 in Hungary, which only slightly exceeds the EU average (22.3%) and proportions in the Czech Republic (22.3%), Slovakia (22.9%), and Austria (24.3%), and is lower than in Croatia (27.5%), Bulgaria (28.2%), and Serbia (29.2% in 2013) (WHO) (17). In terms of obesity, which is another important risk factor for cancer development (18), Hungary ranks among the countries with the highest prevalence of overweight (58.4% in 2014 vs. 51.2% EU average), with similar rates observed in the Czech Republic (58.4%), Slovakia (57.8%), Poland (56.7%), and Croatia (63.8%). Regular alcohol consumption is also known to increase the risk of cancer (19). In Hungary, the proportions of daily and weekly alcohol consumers were 6.3% and 19.1% in 2019, respectively, lower than the EU average (8% and 28.4%, respectively), Czech Republic (7.8% and 33.8%), and Bulgaria (10.2% and 23.8%), but similar to Slovakia (4.1% and 19.3%), Croatia (10.2% and 17.9%), and Romania (2.9% and 19.0%) (20).
After conducting a thorough validation of the HUN-CANCER EPI study data on incidence and mortality, we can state that the incidence of diagnosed cancer in Hungary is higher, than the European average in both sexes, albeit not exceptionally high compared to other Central European countries. Of note, based on analysis, these rates showed a decreasing trend over the past decade, even prior to the onset of the Covid-19 pandemic (21, 22). Our results highlight a fundamental shift in perception about cancer burden in Hungary, showing similar cancer incidence rates to other countries in the region, rather than exceptionally high incidence rates in Europe. These findings support the need for the unification of data collection and adjustment guidelines which may establish more accurate and comprehensive cancer registries in post-socialist countries. Achieving this goal would allow for the more accurate analysis of cancer burden in the region, since the role of population-based registries is essential to provide reliable epidemiological data. Until then, currently available cancer incidence estimates, including our current study, should be interpreted with caution, acknowledging their limitations (3).
According to the population-based data of GLOBOCAN, in the majority of EU member states the standardized incidence rates of all malignant tumors excluding C44 stagnated or decreased in 2011-2018 or in part of the period for which data were reported (23). This is in line with our findings. Nevertheless, the studied period was relatively short, and the future availability of longer time series will allow for the observation of more reliable trends over time. Beside the already implemented prevention and screening programs, to ensure the positive trends in cancer incidence, more focus should be put on the Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer by the World Cancer Research Fund (WCRF), the European code against cancer guidelines and the American Institute for Cancer Research (AICR), which offers a comprehensive analysis of current research in cancer prevention and survivorship (24). It provides a global framework for reducing cancer burden, enhancing health, and improving the quality of life for survivors, while guiding healthcare practitioners on diet and lifestyle changes. Additionally, it outlines research priorities to improve evidence-based recommendations for public health and personalized strategies for at-risk individuals and cancer survivors.
Our study has important strengths and limitations that need to be considered when interpreting the results. One of the notable strengths lies in the robust number of identified cancer patients, which increases the statistical reliability of our findings. The data were thoroughly cleaned, ensuring their accuracy and validity. Additionally, the extended 9-year follow-up period provided a comprehensive view of cancer trends over time. The nationwide coverage of the NHIF database and its comparison to HCSO mortality data allowed for a more comprehensive assessment of cancer outcomes in the country. Furthermore, the inclusion of cancer-related interventions helped to exclude cases where cancer-associated ICD codes were used mistakenly, and the interventions did not align with the ICD code and the patient did not die shortly after the ICD code assignment. These methodological considerations contribute to the soundness of our conclusions.
However, there are certain limitations, as well. Our reported incidence rates are generally lower than those published by the Hungarian National Cancer Registry, which is due to the different data cleaning and processing methods. For example, one reason for the difference is that the registry contains each reported case until the rapporteur withdraws it in case of false reporting (i.e., the diagnosis of the case changes), whereas our study used the rules for case definition described in the Methods section. Furthermore, the NHIF database lacks information on post-mortem diagnosed cases, which could have resulted in the underestimation of cancer incidence. The impact of post-mortem diagnosed cancer cases highly depends on the autopsy rate for hospital deaths. While this limitation exists, it is worth noting that the autopsy rate in most other European countries is generally below 10%. Therefore, the potential underestimation of cancer incidence due to the exclusion of post-mortem identified cases might be of a similar magnitude, rendering our results still comparable to those of other countries. Another limitation is related to the methodology of identifying cancer patients based on the majority of cancer-related ICD code records. Although a sensitivity analysis was performed, this approach might lead to the exclusion of patients who could have had secondary or multiple primary tumors, potentially resulting in a certain underestimation of cancer incidence. Moreover, our retrospective database analyses span a 9-year period, during which patients initially diagnosed with a certain primary tumor could have developed another type of primary cancer during the follow-up period. This temporal aspect should be considered when interpreting the results and understanding the potential changes in cancer diagnoses over time. Previous analyses from the SEER Program in 1995 reported that multiple primary neoplasms accounted for 13.1% of cancers in men and 13.7% in women (25, 26). In a study from Portugal, the 5-year cumulative incidence of secondary primary cancer was 3.0% (27). In our 9-year study, we estimated approximately 14,000 cases of secondary primary cancer over a median follow-up of 4.5 years in a prevalent cancer population of 459,204 individuals in 2019 (including incident cases in 2019). This may have led to an underestimation of around 1,500 new cancer cases annually (2.7%). Moreover, the NHIF database lacked information on molecular histology, TNM stage, and ECOG status of the patients, preventing us from assessing cancer incidence by specific histological and molecular subtypes and examining the impact of patient-related factors on cancer mortality. We are also emphasizing, that there is a cancer registry for childhood malignancies available in Hungary led by Semmelweis University, which reports cancer incidence and survival estimations for 0-18 year cohort on a regular basis.
Given that cancer incidence trends can be sensitive to the duration of the screening period (2 years from 2009 and 2010), we conducted a trend analysis with the use of a longer, six-year screening period. We calculated the average yearly incidence change from 2015 to 2019 and observed a significant decrease, greater than that seen in the 2011-2019 period, for both sexes. For males, the average yearly decrease was 2.10% (95% CI: 1.47%-2.72%-), and for females, it was 1.67% (95% CI: 1.18%-2.17%-). Regarding mortality rates, the 2015-2019 period also showed a significant decrease, slightly higher than the previous period, with a yearly average of 1.67% (95% CI: 0.48%-2.85%-) for males and 1.14% (95% CI: 0.33%-1.94%-) for females. Therefore, the longer screening period confirmed that cancer incidence and mortality rates have significantly decreased for both sexes in Hungary over the last decade.
Conclusion
The HUN-CANCER EPI study represents an effort to provide more accurate estimates of the incidence of newly diagnosed cancer cases in Hungary using the database of the Hungarian National Health Insurance Fund in a pioneering way. Our results show a significantly lower number of diagnosed cancer cases compared to previous GLOBOCAN estimates which were based on cause-specific cancer mortality data and mortality-to-incidence rate ratios from neighboring countries. One crucial factor contributing to the previous overestimation of cancer incidence in Hungary is the exceptionally high autopsy rate for hospital deaths in the country which led to a high number of post-mortem diagnosed cancer cases coded as the underlying cause of death, thus inflating incidence estimates. Our newly calculated cancer incidence rates are consistent with data from other Central Eastern European countries in line with the similar prevalence of major cancer risk factors in these countries. The highest incidence rates were found for lung, colon, and prostate cancer in men, and for breast, colon, and lung cancer in women, which is consistent with international findings. Furthermore, our study revealed decreasing trends in cancer incidence and mortality for almost all cancer types. These trends can largely be attributed to the decreasing prevalence of smoking, driven by European and Hungarian initiatives and regulations aimed at tobacco control. Nevertheless, the burden of cancer is still very high in Hungary, and its control should be a public health priority. The means are known and should be implemented.
Acknowledgments
We would like to thank Zsófia Barcza of Syntesia Medical Communications for medical writing support. We also express our gratitude for the Central Statistical Office of Hungary for providing detailed mortality data for our calculations.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work of CP, PN, IK, and AW was implemented with the support from the National Research, Development and Innovation Fund of the Ministry of Culture and Innovation under the National Laboratories Program (National Tumor Biology Laboratory [2022-2.1.1-NL-2022-00010]) and the Hungarian Thematic Excellence Program (under project TKP2021-EGA-44) Grant Agreements with the National Research, Development and Innovation Office. This project has received funding from the HUN-REN Hungarian Research Network (grant 1500207).
Data availability statement
All data presented in the manuscript can be found directly in the Supplementary Material .
Ethics statement
The studies involving humans were approved by TUKEB - National Ethical Committee (IV/298-2/2022/EKU). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZKi: Conceptualization, Methodology, Visualization, Writing – original draft. TS: Conceptualization, Methodology, Visualization, Writing – original draft. CP: Conceptualization, Supervision, Validation, Writing – review & editing. ZH: Conceptualization, Writing – review & editing. PN: Conceptualization, Validation, Writing – review & editing. IF: Data curation, Formal analysis, Methodology, Writing – review & editing. VK: Data curation, Formal analysis, Methodology, Writing – review & editing. GS: Conceptualization, Supervision, Writing – review & editing. ZB: Writing – review & editing. IK: Conceptualization, Validation, Writing – review & editing. AW: Conceptualization, Validation, Writing – review & editing. IW: Conceptualization, Writing – review & editing. GM: Conceptualization, Writing – review & editing. EG: Visualization, Writing – review & editing, Project administration. AB: Writing – review & editing, Conceptualization. EK: Conceptualization, Writing – review & editing. ZA: Conceptualization, Writing – review & editing, Data curation, Methodology. RB: Conceptualization, Writing – review & editing. DF: Conceptualization, Writing – review & editing. KB: Conceptualization, Writing – review & editing, Supervision. JM: Conceptualization, Supervision, Writing – review & editing. GG: Conceptualization, Supervision, Writing – review & editing. LiT: Conceptualization, Writing – review & editing. VM: Conceptualization, Writing – review & editing. ZKr: Conceptualization, Writing – review & editing. GO: Conceptualization, Writing – review & editing. ZP: Conceptualization, Writing – review & editing. AM: Conceptualization, Writing – review & editing. GB: Conceptualization, Writing – review & editing, Data curation. LH: Conceptualization, Data curation, Writing – review & editing. La´T: Conceptualization, Writing – review & editing, Validation, Visualization. GR: Conceptualization, Data curation, Methodology, Supervision, Writing – review & editing. ZV: Conceptualization, Methodology, Supervision, Writing – review & editing.
Conflict of interest
Authors ZKi, ZP, EG, MV, AB, TS, EK and KK were employed by the company MSD Pharma Hungary. ZV is an employee of Semmelweis University. Semmelweis University received a grant from MSD Pharma Hungary to contribute to this research. GR, VK, AB-T and IF are employees of RxTarget Ltd. and ZB is employed by Syntesia Ltd. where their contribution to this project was financially compensated.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study received funding from MSD Pharma Hungary. The funder had the following involvement with the study: study design, data collection and analysis, decision to publish, and preparation of the manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1393132/full#supplementary-material
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. (2013) 49:1374–403. doi: 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. (2018) 103:356–87. doi: 10.1016/j.ejca.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 5. Eurostat cancer report (2020). Available online at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Cancer_statistics#Deaths_from_cancer (Accessed January 14, 2024).
- 6. Wéber A, Mery L, Nagy P, Polgár C, Bray F, Kenessey I. Evaluation of data quality at the Hungarian National Cancer Registry, 2000-2019. Cancer Epidemiol. (2023) 82:102306. doi: 10.1016/j.canep.2022.102306 [DOI] [PubMed] [Google Scholar]
- 7. Kiss Z, Bogos K, Tamási L, Ostoros G, Müller V, Bittner N, et al. Underlying reasons for post-mortem diagnosed lung cancer cases - A robust retrospective comparative study from Hungary (HULC study). Front Oncol. (2022) 12:1032366. doi: 10.3389/fonc.2022.1032366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Health Information Gateway: Report on autopsy rate for all deaths. Available online at: https://gateway.euro.who.int/en/indicators/hfa_545-6410-autopsy-rate-for-all-deaths (Accessed January 14, 2024).
- 9. Janca A, Ustün TB, Early TS, Sartorius N. The ICD-10 symptom checklist: a companion to the ICD-10 classification of mental and behavioural disorders. Soc Psychiatry Psychiatr Epidemiol. (1993) 28:239–42. doi: 10.1007/BF00788743 [DOI] [PubMed] [Google Scholar]
- 10. Hungarian Central Statistical Office database. Available online at: https://www.ksh.hu/?lang=hu (Accessed January 14, 2024).
- 11. Eurostat . Revision of the European Standard Population. Report of Eurostat's task force. Available online at: https://ec.europa.eu/eurostat/documents/3859598/5926869/KS-RA-13-028-EN.PDF.pdf/e713fa79-1add-44e8-b23d-5e8fa09b3f8f?t=1414782757000 (Accessed January 14, 2024).
- 12. Waterhouse JAH, Muir CS, Correa P, Powell J. Cancer incidence in five continents. IARC Sci Publ (1971). (1976) 15:1–583. [PubMed] [Google Scholar]
- 13. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. (2006) 16:1–16. doi: 10.18637/jss.v016.i09 [DOI] [Google Scholar]
- 14. Kásler M, Ottó S, Kenessey I. A rákmorbiditás és -mortalitás jelenlegi helyzete a Nemzeti Rákregiszter tükrében [The current situation of cancer morbidity and mortality in the light of the National Cancer Registry. Orv Hetil. (2017) 158:84–89. Hungarian. doi: 10.1556/650.2017.30654 [DOI] [PubMed] [Google Scholar]
- 15. Kenessey I, Nagy P, Polgár C. A rosszindulatú daganatok hazai epidemiológiai helyzete a XXI. század második évtizedében [The Hungarian situation of cancer epidemiology in the second decade of the 21st century. Magy Onkol. (2022) 66:175–184. Hungarian. doi: 10.1556/650.2024.33062 [DOI] [PubMed] [Google Scholar]
- 16. GBD 2019 Cancer Risk Factors Collaborators . The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2022) 400:563–91. doi: 10.1016/s0140-6736(22)01438-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Health Information Gateway . WHO Europe. Age-standardized prevalence of current tobacco smoking, age 15+, WHO estimates (%), males. Available online at: https://gateway.euro.who.int/en/indicators/hfa_421-3010-of-regular-daily-smokers-in-the-population-age-15plus (Accessed January 14, 2024).
- 18. Brown KF, Rumgay H, Dunlop C, Ryan M, Quartly F, Cox A, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. (2018) 118:1130–41. doi: 10.1038/s41416-018-0029-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soerjomataram I, Shield K, Marant-Micallef C, Vignat J, Hill C, Rogel A, et al. Cancers related to lifestyle and environmental factors in France in 2015. Eur J Cancer. (2018) 105:103–13. doi: 10.1016/j.ejca.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 20. Eurostat data browser, Alcohol consumption statistics (2019). Available online at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Alcohol_consumption_statistics#Frequency_of_alcohol_consumption (Accessed January 14, 2024).
- 21. Soerjomataram I, Bardot A, Aitken J, Piñeros M, Znaor A, Steliarova-Foucher E, et al. Impact of the COVID-19 pandemic on population-based cancer registry. Int J Cancer. (2022) 150:273–8. doi: 10.1002/ijc.33792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawler M, Davies L, Oberst S, Oliver K, Eggermont A, Schmutz A, et al. European Groundshot-addressing Europe's cancer research challenges: a Lancet Oncology Commission. Lancet Oncol. (2023) 24:e11–56. doi: 10.1016/S1470-2045(22)00540-X [DOI] [PubMed] [Google Scholar]
- 23. Ervik M, Lam F, Laversanne M, Ferlay J, Bray F. Global Cancer Observatory: Cancer Over Time. Lyon, France: International Agency for Research on Cancer 2021. Available online at: https://gco.iarc.fr/overtime (Accessed January 17, 2024). [Google Scholar]
- 24. Clinton SK, Giovannucci EL, Hursting SD. The world cancer research Fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. (2020) 150:663–71. doi: 10.1093/jn/nxz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rheingold SR, Neugut AI, Meadows AT. Incidence of secondary cancer, in: Holland-Frei Cancer Medicine (2003). Hamilton (ON: BC Decker. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK13212 (Accessed 14 January, 2024). [Google Scholar]
- 26. Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. SEER cancer statistics review 1973-1992: tables and graphs. JAMA. (1996) 276:1293–4. [Google Scholar]
- 27. Pacheco-Figueiredo L, Antunes L, Bento MJ, Lunet N. Incidence of second primary cancers in North Portugal-a population-based study. J Cancer Surviv. (2016) 10:142–52. doi: 10.1007/s11764-015-0460-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in the manuscript can be found directly in the Supplementary Material .