Abstract
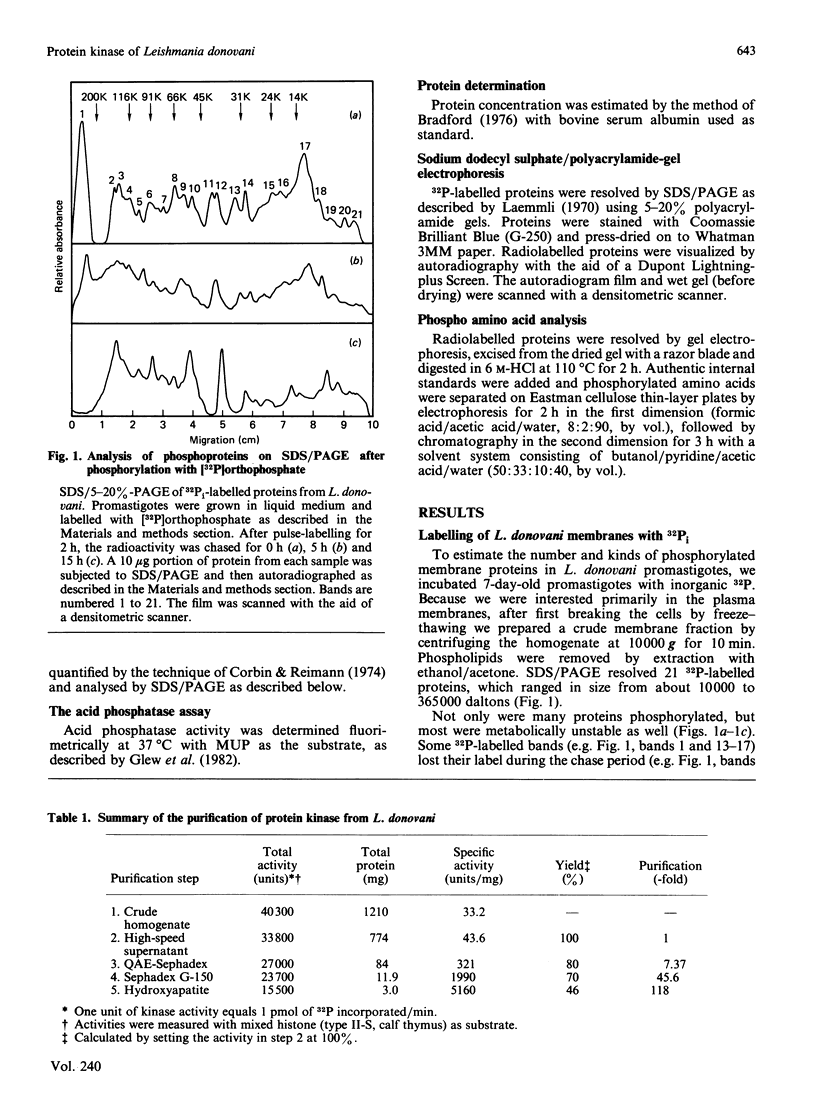
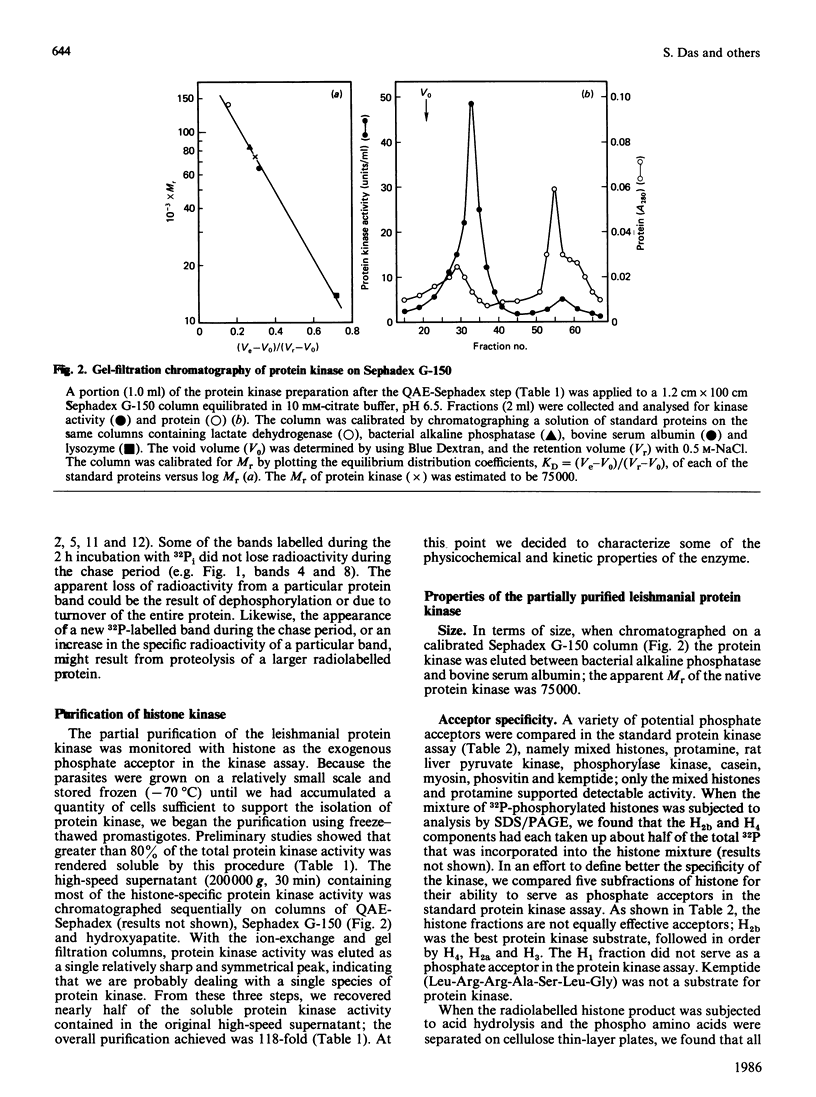
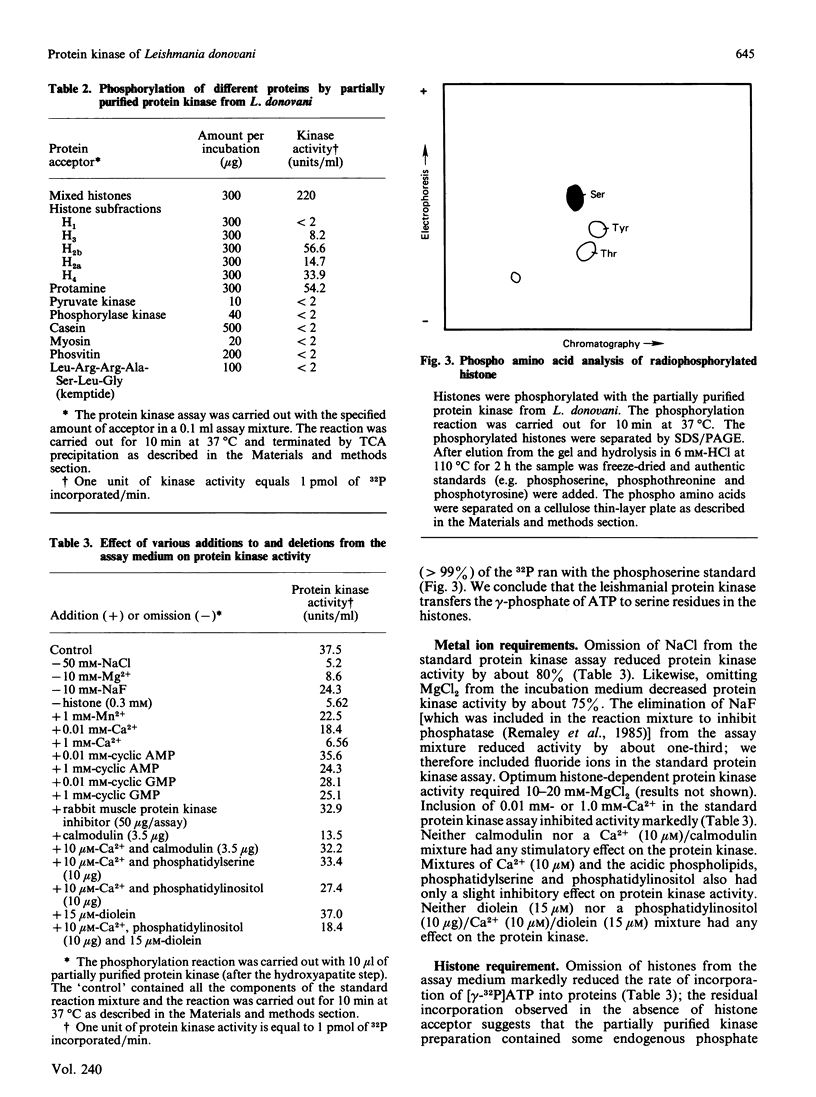
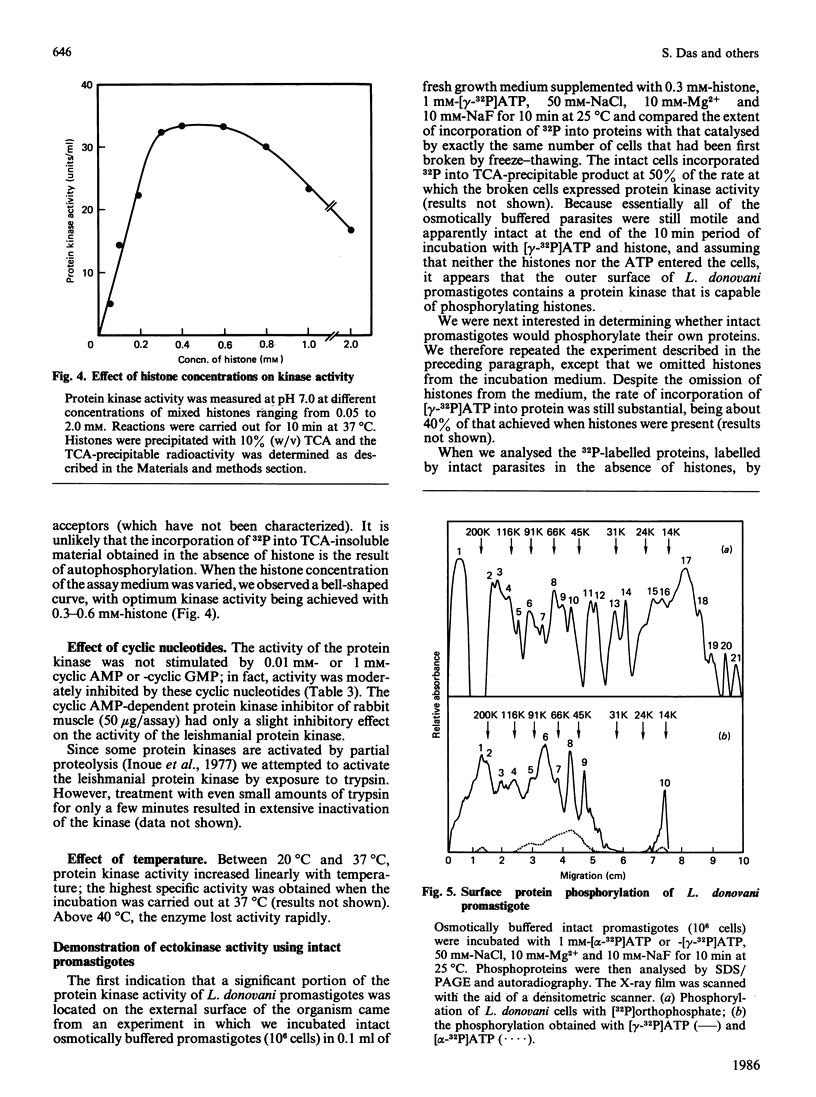
Leishmania donovani promastigotes labelled for 2 h with 32Pi incorporated radioactivity into at least 21 different proteins, as determined by SDS/polyacrylamide-gel electrophoresis. Pulse-chase studies with 32Pi demonstrated that the labelled proteins were in a dynamic state: some radiolabelled proteins rapidly disappeared and others appeared after the chase. The possibility of an ectokinase on the parasite was examined; incubation of intact parasites for 10 min at 25 degrees C in an osmotically buffered medium containing [gamma-32P]ATP, but not [alpha-32P]ATP, resulted in the labelling of 10 different protozoal proteins, presumably localized to the surface of the organism's plasma membrane. Intact promastigotes also catalysed the transfer of 32P from [gamma-32P]ATP to histones. The histone-dependent kinase was solubilized by repeated freezing and thawing, and sonication, and purified 118-fold by chromatographing the high-speed (200,000 g, 1 h) supernatant fraction on QAE-Sephadex, Sephadex G-150 and hydroxyapatite columns. The kinase eluted as a single activity peak from all three columns. The partially purified histone-dependent kinase had the following properties: pH optimum, 7.0; optimum temperature, 37 degrees C; Km for mixed calf thymus histone, 0.15 mM; Km for ATP, 0.8 mM; preferred fractionated histone acceptors, H2b greater than H4 greater than H2a greater than H3 (H1 does not serve as an acceptor); optimum activity required 10-20 mM-Mg2+; inhibited 50-80% by 0.01 mM- and 1 mM-Ca2+; activity was not stimulated by calmodulin, cyclic AMP (1 mM) or cyclic GMP (1 mM) nor inhibited by a cyclic AMP-dependent protein kinase inhibitor (50 micrograms/assay); apparent Mr 75,000, as determined by Sephadex G-150 gel filtration chromatography; phosphorylated exclusively serine residues. Protein kinase activity was low in the early exponential phase of the growth curve and increased 6-fold upon entry into the stationary phase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cardoso de Almeida M. L., Turner M. J. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature. 1983 Mar 24;302(5906):349–352. doi: 10.1038/302349a0. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Glew R. H., Czuczman M. S., Diven W. F., Berens R. L., Pope M. T., Katsoulis D. E. Partial purification and characterization of particulate acid phosphatase of Leishmania donovani promastigotes. Comp Biochem Physiol B. 1982;72(4):581–590. doi: 10.1016/0305-0491(82)90510-7. [DOI] [PubMed] [Google Scholar]
- Gottlieb M., Dwyer D. M. Leishmania donovani: surface membrane acid phosphatase activity of promastigotes. Exp Parasitol. 1981 Aug;52(1):117–128. doi: 10.1016/0014-4894(81)90067-9. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Keates R. A. Cyclic nucleotide-independent protein kinase from pea shoots. Biochem Biophys Res Commun. 1973 Sep 18;54(2):655–661. doi: 10.1016/0006-291x(73)91473-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S. Algorithms in the diagnosis and management of exotic diseases. XXIV. Leishmaniases. J Infect Dis. 1977 Jul;136(1):160–163. doi: 10.1093/infdis/136.1.160. [DOI] [PubMed] [Google Scholar]
- Majumder G. C., Shrago E., Elson C. E. Changes in cyclic AMP-dependent protein dinase activity in Tetrahymena pyriformis during the growth cycle. Biochim Biophys Acta. 1975 Apr 19;384(2):399–412. doi: 10.1016/0005-2744(75)90041-8. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Kemp B. E., Krebs E. G. In vivo phosphorylation of a synthetic peptide substrate of cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1978 Jan;75(1):248–251. doi: 10.1073/pnas.75.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperdoes F. R. Biochemical peculiarities of trypanosomes, African and South American. Br Med Bull. 1985 Apr;41(2):130–136. doi: 10.1093/oxfordjournals.bmb.a072039. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Adenosine 3',5'-phosphate in fungi. Microbiol Rev. 1981 Sep;45(3):462–480. doi: 10.1128/mr.45.3.462-480.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Pai S. H., Ponta H., Herrlich P., Roskoski R., Jr, Schweiger M., Studier F. W. Protein kinase induction in Escherichia coli by bacteriophage T7. Proc Natl Acad Sci U S A. 1974 Feb;71(2):586–589. doi: 10.1073/pnas.71.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaley A. T., Das S., Campbell P. I., LaRocca G. M., Pope M. T., Glew R. H. Characterization of Leishmania donovani acid phosphatases. J Biol Chem. 1985 Jan 25;260(2):880–886. [PubMed] [Google Scholar]
- Remaley A. T., Kuhns D. B., Basford R. E., Glew R. H., Kaplan S. S. Leishmanial phosphatase blocks neutrophil O-2 production. J Biol Chem. 1984 Sep 25;259(18):11173–11175. [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Saha A. K., Dowling J. N., LaMarco K. L., Das S., Remaley A. T., Olomu N., Pope M. T., Glew R. H. Properties of an acid phosphatase from Legionella micdadei which blocks superoxide anion production by human neutrophils. Arch Biochem Biophys. 1985 Nov 15;243(1):150–160. doi: 10.1016/0003-9861(85)90783-0. [DOI] [PubMed] [Google Scholar]
- Sanchez G., Knight S., Strickler J. Nucleotide transport in African trypanosomes. Comp Biochem Physiol B. 1976;53(3):419–421. doi: 10.1016/0305-0491(76)90351-5. [DOI] [PubMed] [Google Scholar]
- Schieven G., Thorner J., Martin G. S. Protein-tyrosine kinase activity in Saccharomyces cerevisiae. Science. 1986 Jan 24;231(4736):390–393. doi: 10.1126/science.2417318. [DOI] [PubMed] [Google Scholar]
- Walter R. D., Opperdoes F. R. Subcellular distribution of adenylate cyclase, cyclic-AMP phosphodiesterase, protein kinases and phosphoprotein phosphatase in Trypanosoma brucei. Mol Biochem Parasitol. 1982 Nov;6(5):287–295. doi: 10.1016/0166-6851(82)90061-5. [DOI] [PubMed] [Google Scholar]
- Willadsen P. Protein phosphorylation by intact Babesia bovis. Mol Biochem Parasitol. 1984 Jun;12(2):195–205. doi: 10.1016/0166-6851(84)90135-x. [DOI] [PubMed] [Google Scholar]


