Abstract
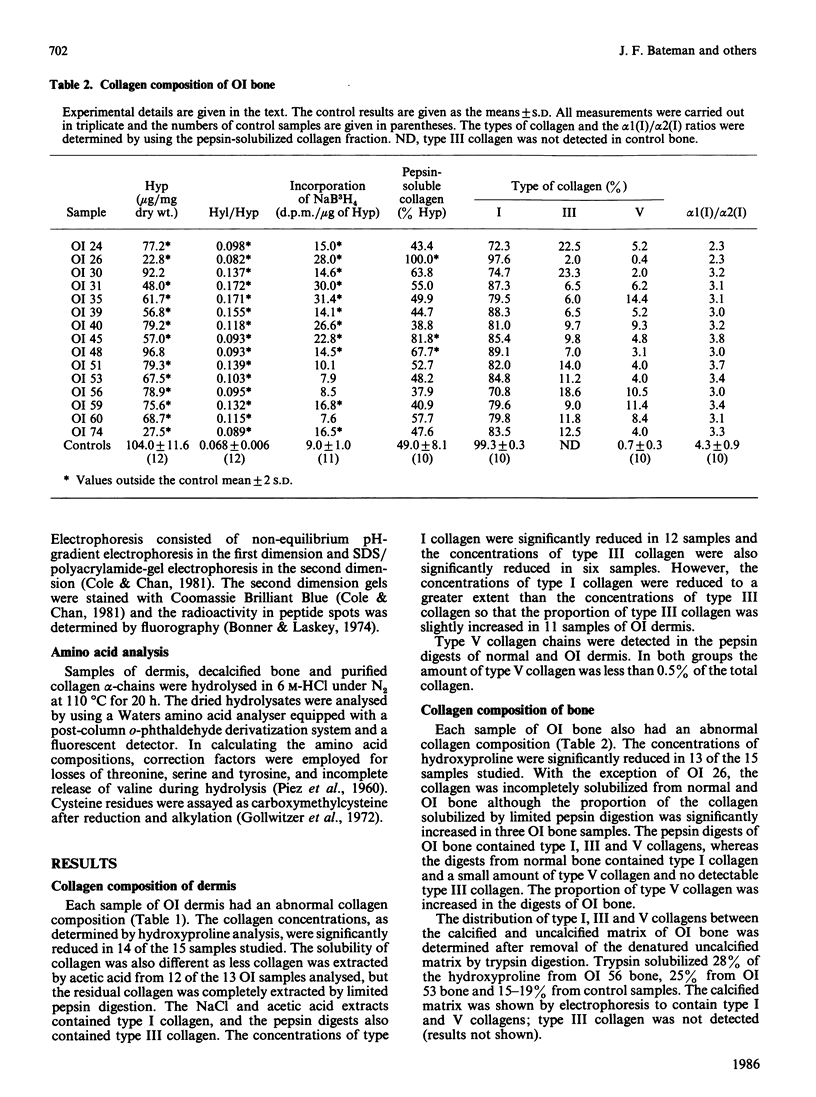
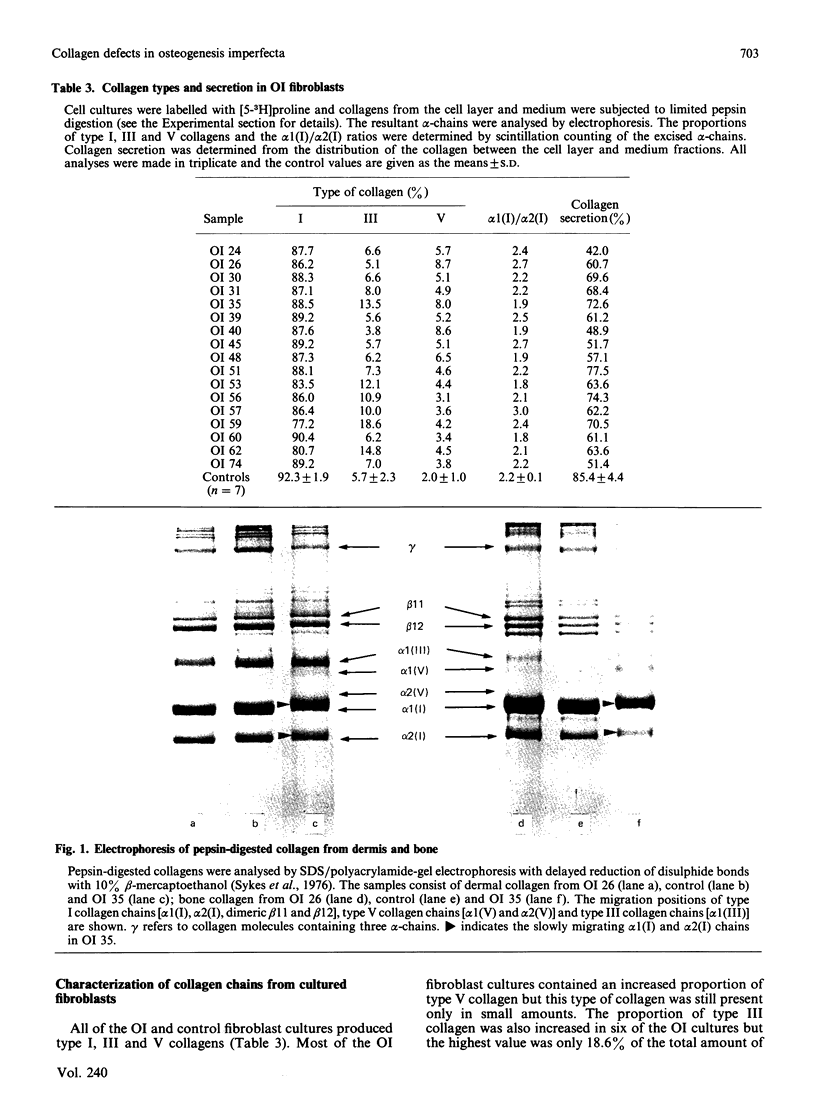
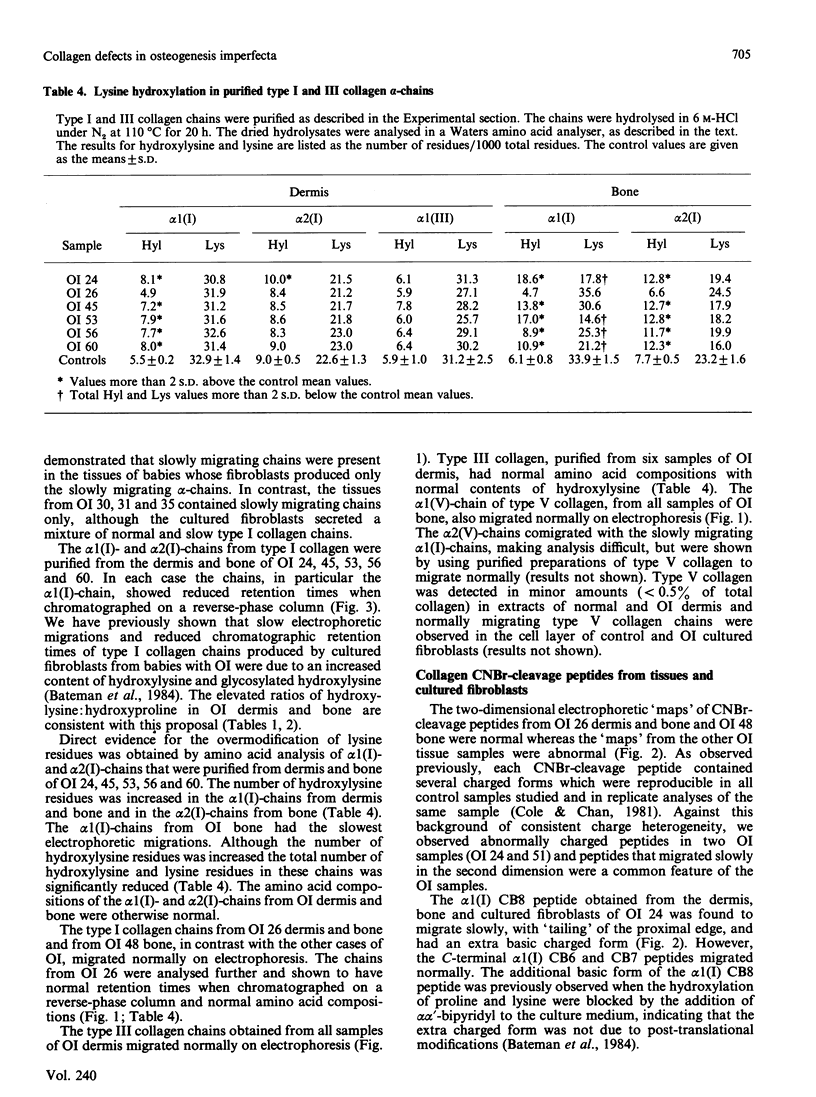
Quantitative and qualitative abnormalities of collagen were observed in tissues and fibroblast cultures from 17 consecutive cases of lethal perinatal osteogenesis imperfecta (OI). The content of type I collagen was reduced in OI dermis and bone and the content of type III collagen was also reduced in the dermis. Normal bone contained 99.3% type I and 0.7% type V collagen whereas OI bone contained a lower proportion of type I, a greater proportion of type V and a significant amount of type III collagen. The type III and V collagens appeared to be structurally normal. In contrast, abnormal type I collagen chains, which migrated slowly on electrophoresis, were observed in all babies with OI. Cultured fibroblasts from five babies produced a mixture of normal and abnormal type I collagens; the abnormal collagen was not secreted in two cases and was slowly secreted in the others. Fibroblasts from 12 babies produced only abnormal type I collagens and they were also secreted slowly. The slower electrophoretic migration of the abnormal chains was due to enzymic overmodification of the lysine residues. The distribution of the cyanogen bromide peptides containing the overmodified residues was used to localize the underlying structural abnormalities to three regions of the type I procollagen chains. These regions included the carboxy-propeptide of the pro alpha 1(I)-chain, the helical alpha 1(I) CB7 peptide and the helical alpha 1(I) CB8 and CB3 peptides. In one baby a basic charge mutation was observed in the alpha 1(I) CB7 peptide and in another baby a basic charge mutation was observed in the alpha 1(I) CB8 peptide. The primary defects in lethal perinatal OI appear to reside in the type I collagen chains. Type III and V collagens did not appear to compensate for the deficiency of type I collagen in the tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banes A. J., Yamauchi M., Mechanic G. L. Nonmineralized and mineralized compartments of bone: the role of pyridinoline in nonmineralized collagen. Biochem Biophys Res Commun. 1983 Jun 29;113(3):975–981. doi: 10.1016/0006-291x(83)91094-x. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., David K. E., Byers P. H. Type I osteogenesis imperfecta: a nonfunctional allele for pro alpha 1 (I) chains of type I procollagen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3838–3842. doi: 10.1073/pnas.79.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Rapid fractionation of collagen chains and peptides by high-performance liquid chromatography. Anal Biochem. 1986 Apr;154(1):338–344. doi: 10.1016/0003-2697(86)90534-8. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Holbrook K. A., Gelinas R. E., Jacob J., Byers P. H. Altered triple helical structure of type I procollagen in lethal perinatal osteogenesis imperfecta. J Biol Chem. 1985 Feb 10;260(3):1734–1742. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burgeson R. E., El Adli F. A., Kaitila I. I., Hollister D. W. Fetal membrane collagens: identification of two new collagen alpha chains. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2579–2583. doi: 10.1073/pnas.73.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D., Cole W. G. Quantitation of type I and III collagens using electrophoresis of alpha chains and cyanogen bromide peptides. Anal Biochem. 1984 Jun;139(2):322–328. doi: 10.1016/0003-2697(84)90012-5. [DOI] [PubMed] [Google Scholar]
- ChandraRajan J. Separation of type III collagen from type I collagen and pepsin by differential denaturation and renaturation. Biochem Biophys Res Commun. 1978 Jul 14;83(1):180–186. doi: 10.1016/0006-291x(78)90414-x. [DOI] [PubMed] [Google Scholar]
- Cheung D. T., Benya P. D., Hofer D. P., Nimni M. E. The use of 2-dimensional CNBr peptide maps for the analysis of crosslinked peptides in bone collagen. Coll Relat Res. 1981 Apr;1(3):247–256. doi: 10.1016/s0174-173x(81)80002-7. [DOI] [PubMed] [Google Scholar]
- Cheung D. T., DiCesare P., Benya P. D., Libaw E., Nimni M. E. The presence of intermolecular disulfide cross-links in type III collagen. J Biol Chem. 1983 Jun 25;258(12):7774–7778. [PubMed] [Google Scholar]
- Cole W. G., Chan D. Analysis of the heterogeneity of human collagens by two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1981 Aug 1;197(2):377–383. doi: 10.1042/bj1970377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole W. G., Chan D., Hickey A. J., Wilcken D. E. Collagen composition of normal and myxomatous human mitral heart valves. Biochem J. 1984 Apr 15;219(2):451–460. doi: 10.1042/bj2190451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Fujii K., Tanzer M. L. Osteogenesis imperfecta: biochemical studies of bone collagen. Clin Orthop Relat Res. 1977 May;(124):271–277. [PubMed] [Google Scholar]
- Gollwitzer R., Timpl R., Becker U., Furthmayr H. Chemical and immunological properties of reduced and alkylated polypeptide chains of bovine fibrinogen. Eur J Biochem. 1972 Aug 4;28(4):497–506. doi: 10.1111/j.1432-1033.1972.tb01937.x. [DOI] [PubMed] [Google Scholar]
- JACKSON D. S., BENTLEY J. P. On the significance of the extractable collagens. J Biophys Biochem Cytol. 1960 Feb;7:37–42. doi: 10.1083/jcb.7.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamall I. S., Finelli V. N., Que Hee S. S. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981 Mar 15;112(1):70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- Kirsch E., Glanville R. W., Krieg T., Müller P. Analysis of cyanogen bromide peptides of type I collagen from a patient with lethal osteogenesis imperfecta. Biochem J. 1983 Jun 1;211(3):599–603. doi: 10.1042/bj2110599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboki Y., Tsuzaki M., Sasaki S., Liu C. F., Mechanic G. L. Location of the intermolecular cross-links in bovine dentin collagen, solubilization with trypsin and isolation of cross-link peptides containing dihydroxylysinonorleucine and pyridinoline. Biochem Biophys Res Commun. 1981 Sep 16;102(1):119–126. doi: 10.1016/0006-291x(81)91497-2. [DOI] [PubMed] [Google Scholar]
- Light N. D., Bailey A. J. Changes in crosslinking during aging in bovine tendon collagen. FEBS Lett. 1979 Jan 1;97(1):183–188. doi: 10.1016/0014-5793(79)80080-0. [DOI] [PubMed] [Google Scholar]
- Ornoy A., Kim O. J. Scanning electron microscopy findings in osteogenesis imperfecta fetalis. Isr J Med Sci. 1977 Jan;13(1):26–32. [PubMed] [Google Scholar]
- PIEZ K. A., WEISS E., LEWIS M. S. The separation and characterization of the alpha- and beta-components of calf skin collagen. J Biol Chem. 1960 Jul;235:1987–1991. [PubMed] [Google Scholar]
- Petrovic O. M., Miller E. J. An unusual pattern of peptide-bound lysine metabolism in collagen from an infant with lethal perinatal osteogenesis imperfecta. J Clin Invest. 1984 Jun;73(6):1569–1575. doi: 10.1172/JCI111363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope F. M., Nicholls A. C., Eggleton C., Narcissi P., Hey E. N., Parkin J. M. Osteogenesis imperfecta (lethal) bones contain types III and V collagens. J Clin Pathol. 1980 Jun;33(6):534–538. doi: 10.1136/jcp.33.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Prockop D. J. Osteogenesis imperfecta: phenotypic heterogeneity, protein suicide, short and long collagen. Am J Hum Genet. 1984 May;36(3):499–505. [PMC free article] [PubMed] [Google Scholar]
- Rowe D. W., Shapiro J. R., Poirier M., Schlesinger S. Diminished type I collagen synthesis and reduced alpha 1(I) collagen messenger RNA in cultured fibroblasts from patients with dominantly inherited (type I) osteogenesis imperfecta. J Clin Invest. 1985 Aug;76(2):604–611. doi: 10.1172/JCI112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. G., Veis A. The cyanogen bromide peptides of bovine soluble and insoluble collagens. I. Characterization of peptides from soluble type I collagen by sodium dodecylsulphate polyacrylamide gel electrophoresis. Connect Tissue Res. 1976;4(2):107–116. doi: 10.3109/03008207609152206. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Rubin D., Gross J. Osteogenesis imperfecta congenita: evidence for a generalized molecular disorder of collagen. Lab Invest. 1977 May;36(5):501–508. [PubMed] [Google Scholar]
- van der Rest M., Fietzek P. P. A comprehensive approach to the study of collagen primary structure based on high-performance liquid chromatography. Eur J Biochem. 1982 Jul;125(3):491–496. doi: 10.1111/j.1432-1033.1982.tb06709.x. [DOI] [PubMed] [Google Scholar]