Abstract
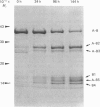
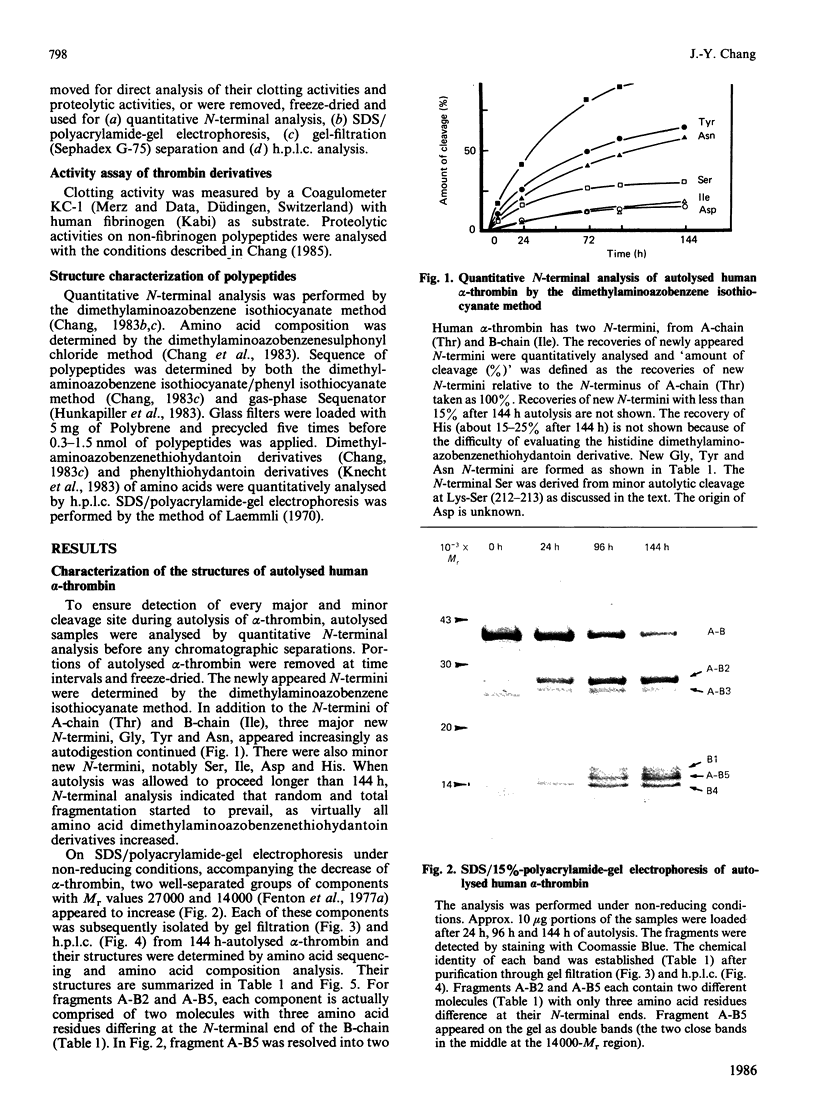
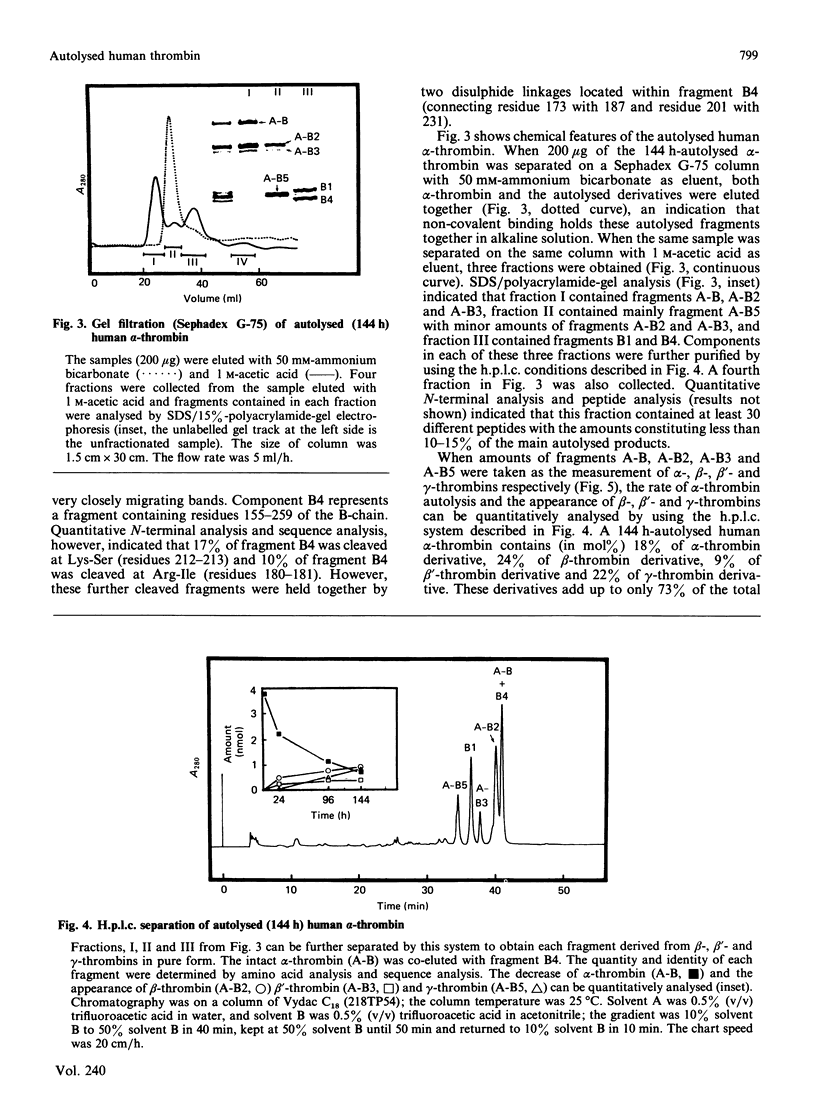
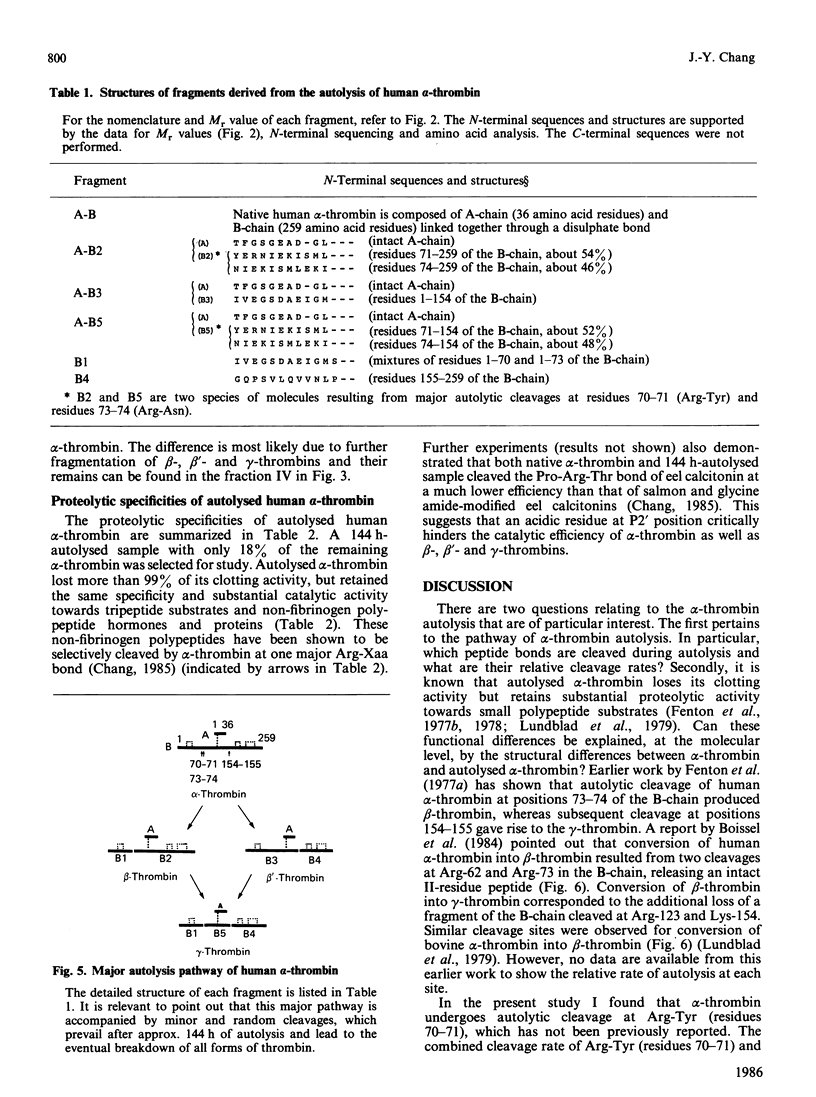
Three Arg/Lys-Xaa bonds in the B-chain of human alpha-thrombin were found to be the major autolytic sites. Under the conditions of 1 mg of alpha-thrombin/ml in 50 mM-ammonium bicarbonate solution at 25 degrees C, the 50% cleavage times of Lys-Gly (residues 154-155), Arg-Tyr (residues 70-71) and Arg-Asn (residues 73-74) were 32 h, 72 h and 96 h respectively. Fragments generated from these three major autolytic sites were purified and analysed. In addition, minor and random autolytic cleavages occurred simultaneously that eventually led to the complete breakdown of the enzyme. These results reveal several novel aspects about the process of autolysis and the structure of autolysed human thrombin. It identifies a major autolytic site at Arg-Tyr (residues 70-71) that has not been previously reported. It demonstrates that beta-thrombin is not an obligatory intermediate during the process of conversion of alpha-thrombin into gamma-thrombin. There exists a new form of autolysed thrombin, designated as beta'-thrombin (with cleavage at Lys-Gly only), which also serves as the intermediate in the conversion of alpha-thrombin into gamma-thrombin. It shows that autolysis of human alpha-thrombin does not proceed in an absolutely clear-cut manner. Numerous minor cleavages, which amount to approx. 20% of the three major autolytic sites, occur simultaneously. It is the first time that several autolytic sites of human alpha-thrombin have been quantitatively analysed, and that it has been shown that formation of beta-, beta'- and gamma-thrombins can be quantitatively followed by the h.p.l.c. method. Furthermore, the data demonstrate that alpha-thrombin and the autolysed thrombin (mixture of beta-, beta'- and gamma-thrombins) have comparable proteolytic activity and specificity towards various sizes of non-fibrinogen polypeptide substrates with relative molecular masses ranging from 3000 to 25,000.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan S. S., Ball R. K., Chang J. Y., Braun D. G. Heterogeneity of cross-reactive idiotypes. Serological and structural analysis of monoclonal anti-p-azobenzene-arsonate antibodies expressing major and minor idiotypes. Mol Immunol. 1983 Feb;20(2):203–211. doi: 10.1016/0161-5890(83)90132-3. [DOI] [PubMed] [Google Scholar]
- Berliner L. J., Shen Y. Y. Physical evidence for an apolar binding site near the catalytic center of human alpha-thrombin. Biochemistry. 1977 Oct 18;16(21):4622–4626. doi: 10.1021/bi00640a015. [DOI] [PubMed] [Google Scholar]
- Berliner L. J., Sugawara Y., Fenton J. W., 2nd Human alpha-thrombin binding to nonpolymerized fibrin-Sepharose: evidence for an anionic binding region. Biochemistry. 1985 Nov 19;24(24):7005–7009. doi: 10.1021/bi00345a038. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Blombäck M., Hessel B., Iwanaga S. Structure of N-terminal fragments of fibrinogen and specificity of thrombin. Nature. 1967 Sep 30;215(5109):1445–1448. doi: 10.1038/2151445a0. [DOI] [PubMed] [Google Scholar]
- Boissel J. P., Le Bonniec B., Rabiet M. J., Labie D., Elion J. Covalent structures of beta and gamma autolytic derivatives of human alpha-thrombin. J Biol Chem. 1984 May 10;259(9):5691–5697. [PubMed] [Google Scholar]
- Chang J. Y., Alkan S. S., Hilschmann N., Braun D. G. Thrombin specificity. Selective cleavage of antibody light chains at the joints of variable with joining regions and joining with constant regions. Eur J Biochem. 1985 Sep 2;151(2):225–230. doi: 10.1111/j.1432-1033.1985.tb09092.x. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. Amino-terminal analysis with dimethylaminoazobenzene isothiocyanate. Methods Enzymol. 1983;91:79–84. doi: 10.1016/s0076-6879(83)91012-1. [DOI] [PubMed] [Google Scholar]
- Chang J. Y., Knecht R., Braun D. G. Amino acid analysis in the picomole range by precolumn derivatization and high-performance liquid chromatography. Methods Enzymol. 1983;91:41–48. doi: 10.1016/s0076-6879(83)91009-1. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. Manual micro-sequence analysis of polypeptides using dimethylaminoazobenzene isothiocyanate. Methods Enzymol. 1983;91:455–466. doi: 10.1016/s0076-6879(83)91043-1. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. The functional domain of hirudin, a thrombin-specific inhibitor. FEBS Lett. 1983 Dec 12;164(2):307–313. doi: 10.1016/0014-5793(83)80307-x. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. Thrombin specificity. Requirement for apolar amino acids adjacent to the thrombin cleavage site of polypeptide substrate. Eur J Biochem. 1985 Sep 2;151(2):217–224. doi: 10.1111/j.1432-1033.1985.tb09091.x. [DOI] [PubMed] [Google Scholar]
- Dodt J., Seemüller U., Maschler R., Fritz H. The complete covalent structure of hirudin. Localization of the disulfide bonds. Biol Chem Hoppe Seyler. 1985 Apr;366(4):379–385. doi: 10.1515/bchm3.1985.366.1.379. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Fenton J. W., 2nd Thrombin specificity. Ann N Y Acad Sci. 1981;370:468–495. doi: 10.1111/j.1749-6632.1981.tb29757.x. [DOI] [PubMed] [Google Scholar]
- Gorman J. J., Castaldi P. A., Shaw D. C. The structure of human thrombin in relation to autolytic degradation. Biochim Biophys Acta. 1976 Jul 19;439(1):1–16. doi: 10.1016/0005-2795(76)90154-9. [DOI] [PubMed] [Google Scholar]
- Hanna L. S., Scheraga H. A., Francis C. W., Marder V. J. Comparison of structures of various human fibrinogens and a derivative thereof by a study of the kinetics of release of fibrinopeptides. Biochemistry. 1984 Sep 25;23(20):4681–4687. doi: 10.1021/bi00315a025. [DOI] [PubMed] [Google Scholar]
- Hogg D. H., Blombäck B. The mechanism of the fibrinogen-thrombin reaction. Thromb Res. 1978 Jun;12(6):953–964. doi: 10.1016/0049-3848(78)90051-8. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Knecht R., Seemüller U., Liersch M., Fritz H., Braun D. G., Chang J. Y. Sequence determination of eglin C using combined microtechniques of amino acid analysis, peptide isolation, and automatic Edman degradation. Anal Biochem. 1983 Apr 1;130(1):65–71. doi: 10.1016/0003-2697(83)90650-4. [DOI] [PubMed] [Google Scholar]
- LANDABURU R. H., SEEGERS W. H. The acetylation of prothrombin. Can J Biochem Physiol. 1960 Jun;38:613–620. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundblad R. L., Kingdon H. S., Mann K. G. Thrombin. Methods Enzymol. 1976;45:156–176. doi: 10.1016/s0076-6879(76)45017-6. [DOI] [PubMed] [Google Scholar]
- Lundblad R. L., Nesheim M. E., Straight D. L., Sailor S., Bowie J., Jenzano J. W., Roberts J. D., Mann K. G. Bovine alpha- and beta-thrombin. Reduced fibrinogen-clotting activity of beta-thrombin is not a consequence of reduced affinity for fibrinogen. J Biol Chem. 1984 Jun 10;259(11):6991–6995. [PubMed] [Google Scholar]
- Lundblad R. L., Noyes C. M., Mann K. G., Kingdon H. S. The covalent differences between bovine alpha- and beta-thrombin. A structural explanation for the changes in catalytic activity. J Biol Chem. 1979 Sep 10;254(17):8524–8528. [PubMed] [Google Scholar]
- Marsh H. C., Jr, Meinwald Y. C., Thannhauser T. W., Scheraga H. A. Mechanism of action of thrombin on fibrinogen. Kinetic evidence for involvement of aspartic acid at position P10. Biochemistry. 1983 Aug 30;22(18):4170–4174. doi: 10.1021/bi00287a002. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Waugh D. F. Multiple bovine thrombin components. J Biol Chem. 1970 Oct 10;245(19):5049–5056. [PubMed] [Google Scholar]
- Sonder S. A., Fenton J. W., 2nd Proflavin binding within the fibrinopeptide groove adjacent to the catalytic site of human alpha-thrombin. Biochemistry. 1984 Apr 10;23(8):1818–1823. doi: 10.1021/bi00303a037. [DOI] [PubMed] [Google Scholar]