Abstract
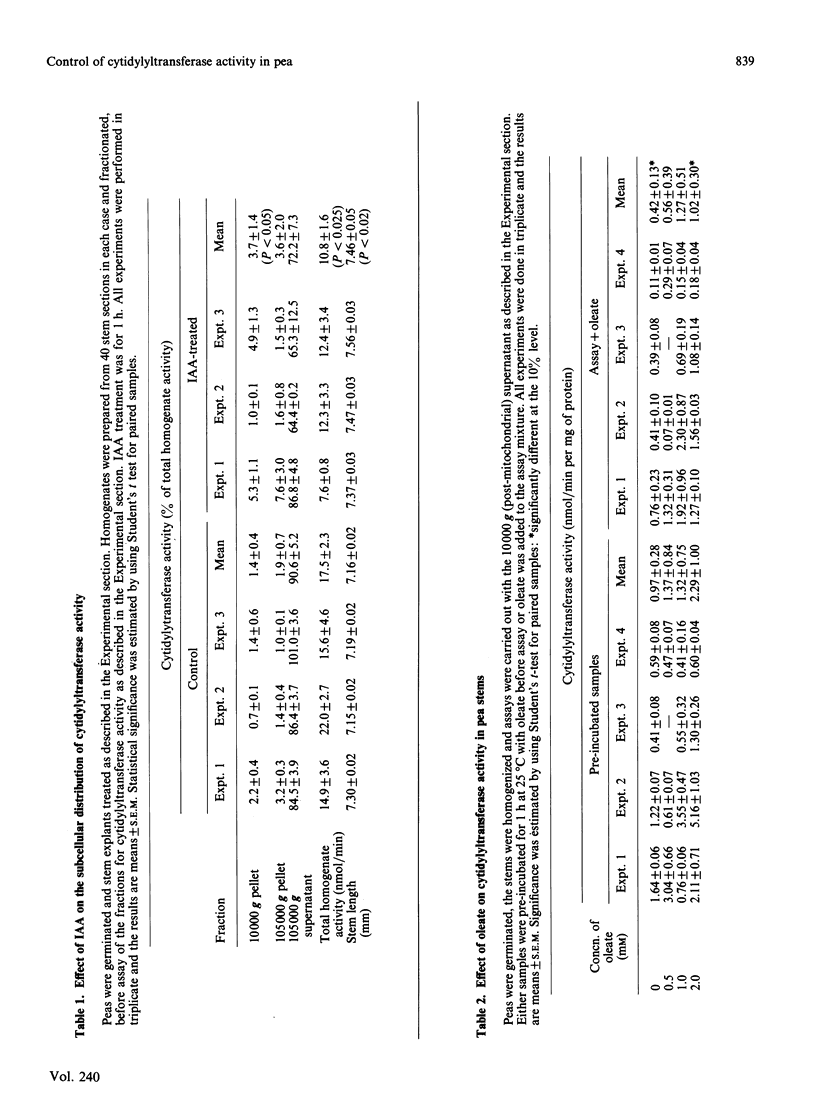
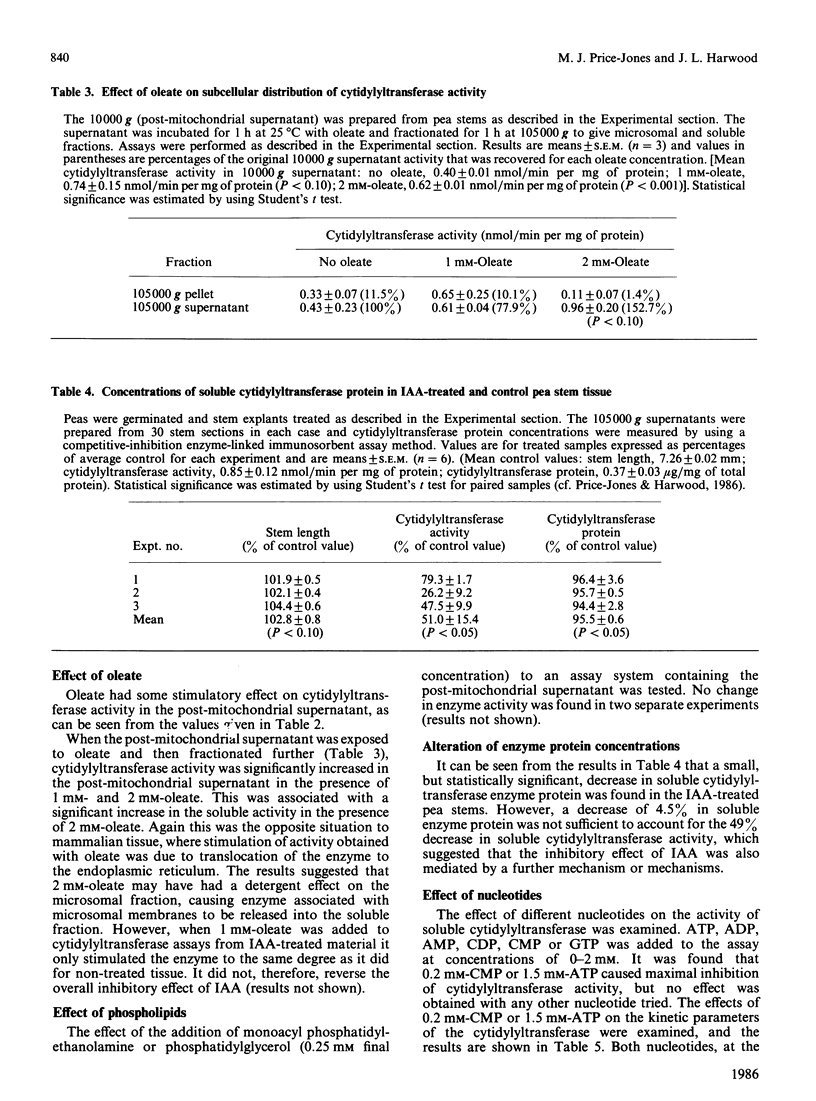
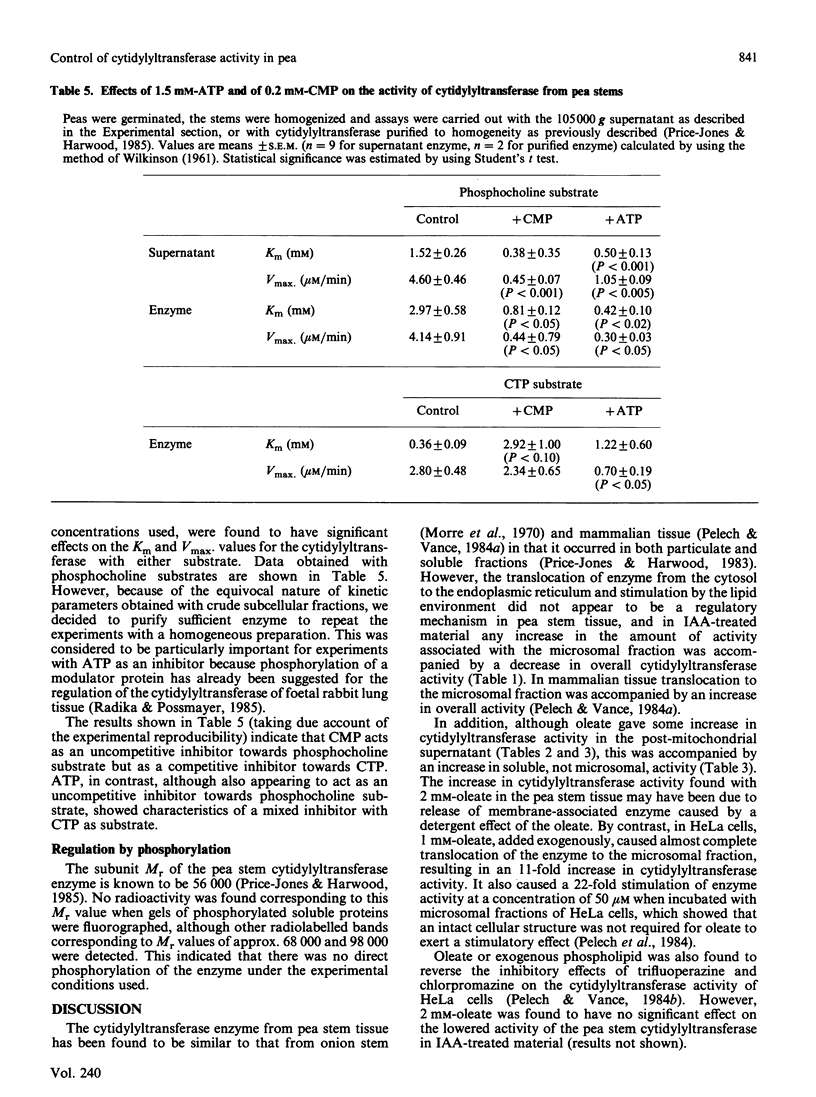
Several possible control mechanisms for CTP:choline-phosphate cytidylyltransferase (EC 2.7.7.15) activity in pea (Pisum sativum L.) stems were investigated. Indol-3-ylacetic acid (IAA) treatment of the pea stems decreased total cytidylyltransferase activity but did not affect its subcellular distribution. Oleate (2 mM) caused some stimulation of enzyme activity by release of activity from the microsomal fraction into the cytosol, but neither phosphatidylglycerol nor monoacyl phosphatidylethanolamine had an effect on activity or subcellular distribution. A decrease in soluble cytidylyltransferase protein concentrations was found in IAA-treated pea stems, but this was not sufficient to account for all of the decrease in cytidylyltransferase activity. A 50% inhibition of enzyme activity could be obtained with 0.2 mM-CMP, which indicated possible allosteric regulation. Similar inhibition was obtained with 1.5 mM-ATP, but other nucleotides had no effect. The cytidylyltransferase enzyme protein was not directly phosphorylated, and the inhibition with 1.5 mM-ATP occurred with the purified enzyme, thus excluding an obligatory mediation via a modulator protein. The results indicate that the cytosolic form of cytidylyltransferase is the most important in pea stem tissue and that the decrease in cytidylyltransferase activity in IAA-treated material appears to be brought about by several methods.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Choy P. C., Vance D. E. Lipid requirements for activation of CTP: phosphocholine cytidylyltransferase from rat liver. J Biol Chem. 1978 Jul 25;253(14):5163–5167. [PubMed] [Google Scholar]
- Feldman D. A., Kovac C. R., Dranginis P. L., Weinhold P. A. The role of phosphatidylglycerol in the activation of CTP:phosphocholine cytidylyltransferase from rat lung. J Biol Chem. 1978 Jul 25;253(14):4980–4986. [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante J. P., Kinsella J. E. A novel method for determining equilibrium constants. CTP:phosphorylcholine cytidyltransferase. Biochim Biophys Acta. 1978 Oct 12;526(2):440–449. doi: 10.1016/0005-2744(78)90135-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Nyquist S., Rivera E. Lecithin Biosynthetic Enzymes of Onion Stem and the Distribution of Phosphorylcholine-Cytidyl Transferase among Cell Fractions. Plant Physiol. 1970 Jun;45(6):800–804. doi: 10.1104/pp.45.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Cook H. W., Paddon H. B., Vance D. E. Membrane-bound CTP:phosphocholine cytidylyltransferase regulates the rate of phosphatidylcholine synthesis in HeLa cells treated with unsaturated fatty acids. Biochim Biophys Acta. 1984 Oct 4;795(3):433–440. doi: 10.1016/0005-2760(84)90169-3. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Vance D. E. Regulation of phosphatidylcholine biosynthesis. Biochim Biophys Acta. 1984 Jun 25;779(2):217–251. doi: 10.1016/0304-4157(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Vance D. E. Trifluoperazine and chlorpromazine inhibit phosphatidylcholine biosynthesis and CTP:phosphocholine cytidylyltransferase in HeLa cells. Biochim Biophys Acta. 1984 Oct 4;795(3):441–446. doi: 10.1016/0005-2760(84)90170-x. [DOI] [PubMed] [Google Scholar]
- Price-Jones M. J., Harwood J. L. Hormonal regulation of phosphatidylcholine synthesis in plants. The inhibition of cytidylyltransferase activity by indol-3-ylacetic acid. Biochem J. 1983 Dec 15;216(3):627–631. doi: 10.1042/bj2160627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radika K., Possmayer F. Inhibition of foetal pulmonary choline-phosphate cytidylyltransferase under conditions favouring protein phosphorylation. Biochem J. 1985 Dec 15;232(3):833–840. doi: 10.1042/bj2320833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium- and calmodulin-regulated phosphorylation of soluble and membrane proteins from corn coleoptiles. Plant Physiol. 1984 Oct;76(2):359–365. doi: 10.1104/pp.76.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Brosom C. O., Ho E. S., Kreb E. G. Catlysis of the phosphrylaseinase actition reaction. J Biol Chem. 1971 Apr 10;246(7):1968–1976. [PubMed] [Google Scholar]


