Abstract
Background:
The dorsomedial prefrontal cortex (dmPFC) is considered a crucial node in emotional and cognitive processes. Voxel-mirrored homotopic connectivity (VMHC) is a validated methodology for investigating interhemispheric coordination. This study aims to elucidate the effects of electroconvulsive therapy (ECT) on the interhemispheric connectivity of the dmPFC in patients with depression, using VMHC as a measure of bilateral neural coordination.
Methods:
Thirty-three patients with depression, screened at the University of Science and Technology of China (USTC), and thirty-five patients with depression, screened at Anhui Medical University (AHMU), were selected as the subjects of this study. VMHC was employed to investigate the effects of ECT on bilateral hemispheric functional connectivity. The Hamilton Depression Rating Scale (HAMD) was used to assess depressive symptoms, and the relationships between changes in HAMD scores and VMHC values were examined.
Results:
Following ECT, the depressive symptoms of all participants decreased (p < 0.001). The VMHC values in the dmPFC were significantly increased in both groups after ECT (p < 0.01). No significant correlation was found between the increasing VMHC values in the dmPFC and the changes in HAMD scores in depressed patients (p > 0.05).
Conclusion:
These results show that ECT regulates interhemispheric functional connectivity in depressed patients, and significantly increases the VMHC values in the dmPFC. Our findings may provide a useful method for optimizing the treatment of depression.
Keywords: depression, dorsomedial prefrontal cortex, electroconvulsive therapy, voxel-mirrored homotopic connectivity
Introduction
Depression is characterized by feelings of sadness, low self-worth, hopelessness, and guilt [1]. Severe depression is correlated with an increased risk of morbidity and mortality [2]. Despite its high incidence, the exact pathogenesis of depression remains unclear.
Numerous brain imaging studies have accumulated evidence that depressive disorders are linked to interhemispheric imbalances in many brain regions [3, 4], especially the prefrontal cortex. Several mood disorders are attributed to abnormalities in the prefrontal cortex, a critical brain area for controlling emotion [5, 6]. For example, an electroencephalographic investigation revealed that the alpha waves between the bilateral frontal lobes are asymmetric in depressive patients [7], with the left prefrontal cortex being much less activated than the analogous region in the other hemisphere [8]. The dorsomedial prefrontal cortex (dmPFC) is a specific region of the prefrontal cortex. Research into psychopharmacological mechanisms has shown that first-line antidepressants currently used in clinical practice, although they immediately inhibit the uptake of central neurotransmitters [9], can reduce the sensitivity of neurotransmitter receptors with long-term use. This phenomenon not only reduces patient compliance with the treatment but also increases the risk of suicide among patients [10]. Therefore, finding safe, rapid-acting, and specific treatment methods for patients with depression has become a focal point of current clinical research.
Electroconvulsive therapy (ECT) is among the most efficient and extensively used therapies for treating patients with depression, particularly those with severe depression with acute suicidal thoughts and treatment-resistant depression [11, 12]. Additionally, ECT has provided new insights into the pathophysiology of depression and therapeutic methods. However, the rationale for ECT remains unclear. Recent research suggests that ECT may exert a rebalancing effect by modulating interhemispheric functional connectivity [13, 14].
Voxel-mirrored homotopic connectivity (VMHC) is a validated methodology for investigating interhemispheric coordination. It has been used to calculate the synchrony of spontaneous activities between geometrically homotopic regions in both brain hemispheres [15]. A neuroimaging study showed decreased VMHC values in the medial prefrontal cortex in individuals suffering from depression [16]. In an earlier study, we observed reduced VMHC in the middle frontal gyri and angular gyri in depressive patients compared to normal controls, and found that VMHC was normalized following ECT [17].
Due to the significance of unbalanced interhemispheric functional coordination in the pathophysiology of depression and the rapid anti-depressive effect of ECT, the current investigation sought to examine changes in interhemispheric functional connectivity before and after ECT. We hypothesized that the ability of ECT to regulate interhemispheric functional connectivity, as measured by VMHC, underlies its efficacy in treating depression. To determine the potential treatment mechanism, this study examined the effects of ECT on interhemispheric functional coordination in two distinct groups for the first time, using a large sample size.
Methods
Participants
The study comprised 68 participants diagnosed with depression who underwent ECT at the Anhui Mental Health Center in China. Participants were divided into two groups. The first group, consisting of 33 depressive patients aged between 17 and 52, was screened between February 2017 and November 2018 at the University of Science and Technology of China (USTC). The second group, comprising 35 depressive patients aged between 18 and 51, was screened between January 2013 and May 2017 at the Anhui Medical University (AHMU). All participants were professionally diagnosed with depression by a psychiatrist and met the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders–IV (DSM-IV). ECT was prescribed by the psychiatrist due to severe suicidal tendencies or resistance to pharmacotherapy. Participants were excluded from the study if they met any of the following criteria: (1) pregnancy; (2) comorbid psychiatric disorders or other serious medical illnesses; (3) prior ECT treatments; (4) alcohol or substance abuse; and/or (5) Magnetic Resonance Imaging (MRI) scanning limitations. Each participant’s right-handedness was assessed using the Edinburgh Handedness Scale [18].
Evaluation of Clinical Symptoms
Depressive symptoms were evaluated using the 17-item Hamilton Depression Rating Scale (HAMD) [19]. Patient symptoms were assessed approximately 12–24 hours before the first ECT session and 1–3 days following the final ECT session. Throughout the trial, patients in both groups adhered to a consistent antidepressant regimen. The specific medications used are outlined in Table 1.
Table 1.
The categories of medicine.
| Medicine category | USTC (n = 33) | AHMU (n = 35) |
| SSRIs | 22 | 19 |
| SNRIs | 10 | 12 |
| SARIs | 1 | 2 |
| NaSSAs | 8 | 7 |
| NRIs | 0 | 1 |
| Antipsychotics | 16 | 16 |
| Anti-convulsantsa | 1 | 12 |
| Non-benzodiazepine hypnotic | 9 | 4 |
| Anti-anxietyb | 1 | 4 |
| χ2 | 14.33 | |
| p | 0.074 | |
Note:
aAnticonvulsants mainly include valproate and benzodiazepines, used for bipolar disorder or insomnia, and discontinued during electroconvulsive therapy (ECT).
bThe anti-anxiety category includes buspirone and tandospirone.
Abbreviations: USTC, University of Science and Technology of China; AHMU, Anhui Medical University; SSRIs, selective serotonin reuptake inhibitors; SNRIs, serotonin–norepinephrine reuptake inhibitors; SARIs, serotonin antagonist/reuptake inhibitors; NaSSAs, norepinephrine and specificity serotonergic antidepressants; NRIs, selective norepinephrine reuptake inhibitors.
ECT Procedures
The ECT procedures followed previously described protocols [20]. A Thymatron System IV Integrated ECT System (Somatics, Inc., Lake Bluff, IL, USA) administered stimuli with a constant current (0.9 A) and a brief pulse (1 ms) at a frequency of 50 Hz, following intravenous induction of anesthesia with propofol (1.4 mg/kg body mass) and muscle paralysis with succinylcholine (0.5 mg/kg body mass). Patients underwent three ECT sessions per week. The initial three sessions occurred on consecutive days until symptom relief was achieved, followed by subsequent sessions every other day with weekends off. Individual patient ages were taken into account when determining the initial stimulation dose.
Acquisition of MRI Data
All patients underwent structural and functional MRI scans at either AHMU or USTC. Scans were conducted on patients with depression 12–24 hours before the initial ECT session and 1–3 days after the final ECT session. During scanning, patients were instructed to keep their eyes closed, relax, avoid dozing off, and refrain from focusing on specific thoughts. Imaging was performed using a Signa HDxt 3.0 T whole-body MRI scanner (GE Healthcare, Amersham, UK). The standard echo planar imaging procedure was employed to acquire resting-state functional images with the following parameters: repetition time = 2000 ms, echo time = 22.5 ms, 33 continuous slices, slice thickness = 4.0 mm, flip angle = 30°, voxel size = 3.4 3.4 4.6 mm3, matrix size = 64 64, and field of view = 220 220 mm2. Additionally, a 3-dimensional inversion recovery prepared rapid spoiled gradient recalled sequence was used to obtain 188 slices of T1-weighted anatomical images in the sagittal orientation, with parameters: voxel size = 1 1 1 mm3, slice thickness = 1 mm, matrix size = 256 256, field of view = 256 256 mm2, flip angle = 8°, inversion time = 800 ms, echo time = 3.184 ms, and repetition time = 8.676 ms.
Data Preprocessing
The statistical Parametric Mapping software (version 12, Wellcome Centre for Human Neuroimaging, University College London, London, United Kingdom; https://www.fil.ion.ucl.ac.uk/spm/), Resting-State fMRI Toolkit (REST; http://www.restfmri.net), and Data Processing Assistant for Resting-State fMRI Toolkit (DPARSF, http://rfmri.org/dpabi) were utilized for preprocessing the functional imaging data. To ensure steady-state longitudinal magnetization and allow patients time to adjust to the magnetic field, the initial 10 volumes of the time series were discarded. Subsequently, slice timing correction, realignment, and head-motion correction were performed. Participants with maximum head movements exceeding 3.0 mm in any direction (x, y, or z) or exceeding 3° in angular motion were excluded from the analysis. Functional MRI images were resampled to a resolution of 3 3 3 mm3 and co-registered to the Montreal Neurological Institute (MNI) space. Individual T1-weighted images were reoriented and co-registered with the functional images. The co-registered T1-weighted images were segmented into grey matter, white matter, and cerebrospinal fluid for spatial normalization, followed by normalization to the MNI space using a 12-parameter nonlinear transformation. Functional images were transformed using these parameters and then smoothed with a 6-mm-wide, half-maximum Gaussian kernel. Data were linearly detrended and band-pass filtered (0.01–0.08 Hz) to remove low-frequency drift and high-frequency noise. Potential sources of spurious variables, including signals from white matter and cerebrospinal fluid, as well as 24 Friston motion parameters acquired through rigid body correction, were regressed out from the data.
The normalized functional images were converted into a symmetrical space following a prescribed method to derive the VMHC values. Initially, a mean image was generated by averaging each normalized grey matter image. To create a symmetrical template and mask for VMHC, this mean image was combined with its bilateral mirrored counterpart. Subsequently, the individual normalized grey matter images were registered to the created symmetric template, and the nonlinear transformation was applied to the normalized functional images.
Voxel-Mirrored Homotopic Connectivity
VMHC processing was additionally conducted using the DPARSF software (version 2.0, Institute of Psychology, Chinese Academy of Sciences, Hefei, Anhui, China). To generate VMHC maps, Pearson correlations were computed between the preprocessed time series of each voxel and its mirrored counterpart in the opposite hemisphere for each patient. Fisher’s r-to-z transformation was then applied to the correlation maps to produce normalized z-map data. For statistical evaluations of VMHC, a unilateral hemispheric mask derived from the resulting symmetric template was utilized.
Statistical Analysis
Using Statistical Product and Service Solutions (SPSS) software (v.23.0, SPSS, Chicago, IL, USA), the clinical features and demographic information of the patients were examined. Paired 2-sample t-tests, conducted with REST software (version 1.8, Laboratory of Cognitive Neuroscience, Beijing Normal University, Beijing, China), were employed to assess pre- and post-ECT differences in VMHC. Additionally, each participant’s framewise displacement (FD) values were determined using the method outlined by Jenkinson et al. [21], as micromovements can influence resting-state functional connectivity results from volume to volume [22]. Group comparisons of VMHC were analyzed using mean FD as a nuisance covariate. Multiple connectivity data comparisons within the unilateral hemisphere of the symmetric template were adjusted using Gaussian Random Field (GRF) analysis at a threshold of voxel p value 0.001 and cluster p value 0.05. Using DPARSF software, altered brain regions were preserved as masks to extract VMHC data, and paired t-tests were used to compare altered VMHC values between patients before and after ECT.
The Shapiro-Wilk test was employed to assess the normality of the data. VMHC changes in altered brain areas, the number of ECT sessions, and the reduction in HAMD scores before and after ECT were investigated using Pearson (for normal distribution) or Spearman (for non-normal distribution) correlation analysis. Measurement data with a normal distribution were expressed as mean standard deviation, and comparison between two groups was analyzed using independent/paired sample t-tests. Measurement data that did not meet normal distribution were expressed as [median (M25, M75)], and comparison between two groups was performed using Wilcoxon test (paired samples) or Mann-Whitney rank sum test (independent samples). Count data were analyzed using chi-square test. A p value 0.05 was considered statistically significant.
Results
Demographic and Clinical Features
A total of 68 depressed patients were included in the final analyses. There were no statistically significant differences in age (t = 0.261, p = 0.7950), gender (2 = 3.683, p = 0.055), education level (Z = 1.722, p = 0.085), and number of episodes (2 = 1.414, p = 0.2344) between patients from USTC and AHMU (Table 2).
Table 2.
Demographic and clinical variables.
| Variable | USTC (n = 33) | AHMU (n = 35) | t/Z/χ2 | p |
| Age (years) | 40.64 12.78 | 39.86 11.87 | 0.261 | 0.795 |
| Gender (male/female) | 4/29 | 11/24 | 3.683 | 0.055 |
| Education level (years) | 9 [6, 15] | 8 [5, 11] | 1.722 | 0.085 |
| Episodes (first/recurrence, n) | 16/17 | 12/23 | 1.414 | 0.234 |
| Number of ECT sessions | 8 [8, 8.5] | 8 [6, 8] | 2.466 | 0.014 |
| Interval of 2 scans (days) | 21.64 8.17 | 17.34 5.27 | 2.594 | 0.002 |
| HAMD pre-ECT | 25.42 5.12 | 22.80 4.29 | 2.292 | 0.025 |
| HAMD post-ECT | 6 [3, 11] *** | 4 [2, 6] *** | –2.327 | 0.020 |
Note: *** indicates p 0.001 compared with HAMD pre-ECT within the group.
Abbreviations: USTC, University of Science and Technology of China; AHMU, Anhui Medical University; ECT, electroconvulsive therapy; HAMD, Hamilton Depression Rating Scale.
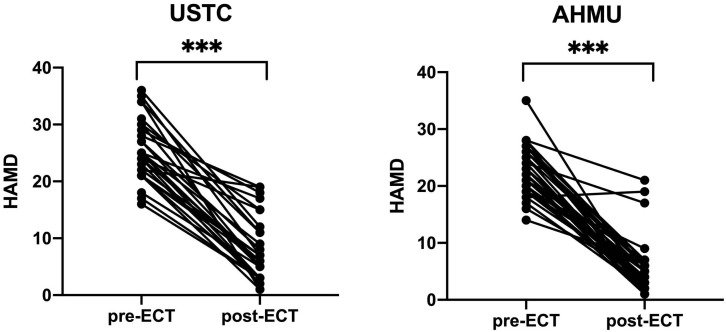
Patients at both AHMU (t = 16.178, p 0.001) and USTC (t = 16.516, p 0.001) experienced a significant reduction in their HAMD scores after receiving ECT (Table 2, Fig. 1).
Fig. 1.
The HAMD scores of patients before and after ECT. *** p 0.001. Abbreviations: USTC, University of Science and Technology of China; AHMU, Anhui Medical University; ECT, electroconvulsive therapy; HAMD, Hamilton Depression Rating Scale.
Effectiveness of ECT on VMHC in Depressed Patients
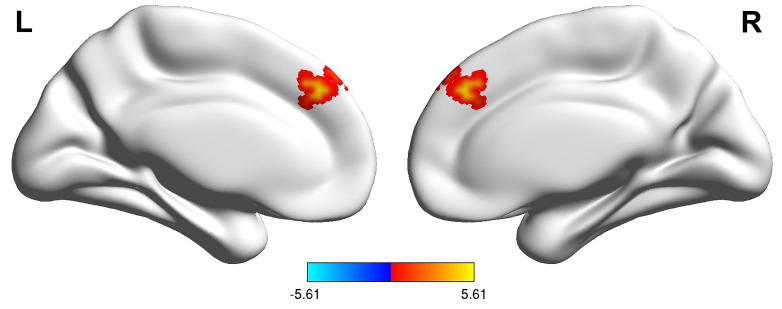
To examine potentially significant variations in VMHC between depressive patients before and after ECT, the analysis of grey matter was adjusted to a significance level of p 0.001 for each voxel and p 0.05 for each cluster as established by GRF. At USTC, patients exhibited increased activity in the dmPFC after ECT (Fig. 2). Similar VMHC values were observed in the same brain areas of AHMU patients between the pre- and post-ECT groups. However, these differences were significantly less pronounced than those observed in USTC patients and did not reach statistical significance after multiple comparison corrections.
Fig. 2.
Comparison of voxel t-value mapping of VMHC in the dmPFC before and after ECT in USTC group patients. The bar graph below the figure represents t-values. Abbreviations: VMHC, voxel-mirrored homotopic connectivity; dmPFC, dorsomedial prefrontal cortex; L, Left; R, Right.
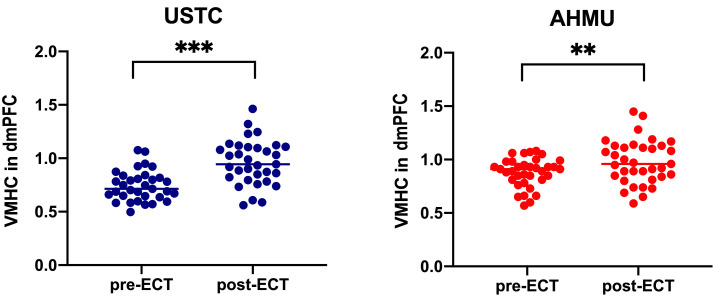
The altered cluster (dmPFC) in the USTC sample was then preserved as a mask, and the mean VMHC values were retrieved and compared across patients before and after ECT. Compared to the pre-ECT groups, VMHC was significantly increased in the dmPFC of both the USTC (t = –5.72, p 0.001) and AHMU patients in the post-ECT groups (t = –3.07, p = 0.004) (Fig. 3).
Fig. 3.
Changes in the VMHC scores in the dmPFC of the depression patients before and after ECT. ** p 0.01; *** p 0.001. Abbreviations: USTC, University of Science and Technology of China; AHMU, Anhui Medical University; ECT, electroconvulsive therapy; HAMD, Hamilton Depression Rating Scale; VMHC, voxel-mirrored homotopic connectivity; dmPFC, dorsomedial prefrontal cortex.
Relationships between Altered VMHC and Altered HAMD Scores
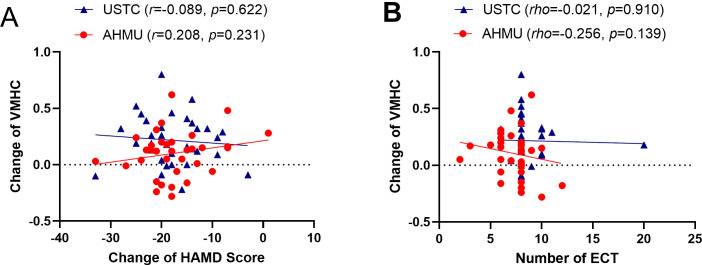
Pearson correlation analysis was conducted on the change of VMHC values in the dmPFC (VMHC post-ECT – VMHC pre-ECT) and the change of HAMD score (post-ECT HAMD scores – pre-ECT HAMD scores). The correlation was not significant between the VMHC changes in the dmPFC or the alterations in the HAMD scores for the datasets from USTC (r = –0.089, p = 0.622) or AHMU (r = 0.208, p = 0.231) (Fig. 4A).
Fig. 4.
Analysis of correlation. (A) The correlation between the change of VMHC values in the dmPFC and the change of HAMD score was examined using Pearson correlation analysis. (B) The correlation between the changes of VMHC values in the dmPFC and the number of ECT sessions was assessed using Spearman correlation analysis. Abbreviations: USTC, University of Science and Technology of China; AHMU, Anhui Medical University; ECT, electroconvulsive therapy; HAMD, Hamilton Depression Rating Scale; VMHC, voxel-mirrored homotopic connectivity.
Correlations between the Number of ECT Sessions and the Altered VMHC
To examine the dosage effect of ECT, a Spearman correlation analysis was conducted to investigate the relationship between the changes in VMHC and the number of ECT sessions. An insignificant association was observed between the number of ECT sessions and the changes in VMHC in USTC (rho = –0.021, p = 0.910) or AHMU (rho = –0.256, p = 0.139) (Fig. 4B).
Discussion
Our previous research suggests that the functional plasticity of the dmPFC may underlie the antidepressant effects of ECT [23]. The dmPFC is a critical component of the central executive network (CEN), involved in various cognitive processes such as working memory, executive control, and attention. It is considered a crucial node in emotional and cognitive processes, essential for controlling emotion in adults [24]. Depressed patients often exhibit aberrant dmPFC activity, reflecting self-monitoring mechanisms and emotional awareness during emotional processing [25]. Previous rs-fMRI study has highlighted dysfunction of the CEN in depressive patients, associated with difficulties in emotion recognition and insufficient cognitive control [26]. Zhang et al. [27] found that functional alteration was more significant in the left dmPFC than bilateral alteration in depressive patients, suggesting an imbalance between left and right dmPFC activity. Additionally, research has shown increased activation in the dmPFC of depressed patients following cognitive-behavioral therapy or pharmacotherapy, which regulates the bilateral imbalance in the dmPFC, accompanied by an improvement in depressive symptoms [28, 29]. Intriguingly, further study using VMHC analysis indicate that the reduction in homotopic interhemispheric functional connectivity primarily occurs in the CEN [30].
The present study aimed to investigate changes in homotopic functional connectivity in depressed individuals before and after ECT using VMHC. Patients at both AHMU and USTC exhibited significantly greater VMHC values in the dmPFC after ECT, along with lower HAMD scores compared to those before ECT. These findings indicate that ECT can regulate interhemispheric functional coordination in patients with depression, thereby alleviating their depressive symptoms. Our results support the notion that modulation of functional coordination between hemispheres is an integral part of the therapeutic mechanism by which ECT effectively treats depression.
Notably, there were no significant differences in voxel t-value mapping of dmPFC VMHC before and after ECT in AHMU patients. However, significant differences were observed in changes in VMHC values before and after treatment. We hypothesize that VMHC analysis may be more sensitive to detecting small or localized changes, while 3D brain mapping focuses more on broader or global changes. This suggests that even if global changes are not significant, localized changes in VMHC values could still be statistically significant.
Compared to previous studies, the most distinctive aspect of our study was validation with two independent groups of patients. Given the growing concern about replicability in psychological science, the inclusion of two independent samples increased the validity and reliability of our results. The dmPFC was closely examined in this study, and the accuracy of the data was improved by using a more stringent correction technique, such as GRF correction, along with a larger sample size.
Yang et al.’s [31] study suggested that VMHC values of frontal white matter in patients with depression were negatively correlated with HAMD scores, though the underlying mechanisms remained unclear. In contrast, our investigation found no significant correlation between altered VMHC levels in the bilateral dmPFC and lower HAMD scores post-ECT. Similarly, no dosage effect between altered VMHC values and the number of ECT sessions was observed in either sample. We hypothesize that the small sample size of this study may have limited our ability to accurately detect the true relationship between these variables. The neural mechanisms underlying the improvement of depressive symptoms by ECT are complex, and VMHC may be part of these mechanisms. Future studies plan to increase the sample size and conduct more comprehensive neuroimaging analyses to elucidate the neural mechanisms of depression improvement. Given that the antidepressant effects of ECT are primarily attributed to changes in VMHC, further research into potential factors affecting VMHC values is warranted.
This study had several limitations. Firstly, it only examined patients before and after ECT to investigate whether increased VMHC in the dmPFC may account for the effectiveness of ECT in depression. Further research is required to confirm the correlation between VMHC values and the pathogenesis of depression, as a healthy control group was not available for comparison. Secondly, the calculation of VMHC adheres to a symmetrical standard template; however, the human brain is not perfectly symmetrical. While morphological asymmetry is unlikely to cause changes in VMHC values, the bias introduced by this asymmetry should not be dismissed. Thirdly, all the patients included in the current study used antidepressants during ECT, and the potential effect of the medication taken by the patients on interhemispheric functional coordination cannot be ignored. Thus, it may be challenging to observe a significant correlation between the number of ECT sessions and VMHC.
The study demonstrated that ECT regulates hemispheric functional connectivity in depression patients, but the correlation shown in this study may indicate a link between ECT and VMHC. However, the neural mechanism of ECT to improve depression is complex, and VMHC may be only one part of its mechanism. Comparing an ECT group with an antidepressant control group in our upcoming study will help further investigate the effects of ECT on the normalization of abnormal bilateral interhemispheric functional coordination. Additionally, VMHC has methodological constraints, and it could not be used to determine which hemisphere was affected or to reveal intra-hemispheric connectivity.
Conclusion
The current study demonstrated that patients with depression exhibit abnormal bilateral interhemispheric functional coordination in the dmPFC, and this coordination improves after ECT. These findings support our hypothesis that bilateral hemispheric balancing and the regulation of homotopic interhemispheric functional connectivity play a role in the antidepressant effect of ECT.
Availability of Data and Materials
All experimental data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgment
Not applicable.
Author Contributions
LZ and YG designed the research study; MZ, QW, YT and GJ collected the data; TB, HL, WX, YW, KW and JS analyzed the data. All authors contributed to the drafting or important editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Fourth People’s Hospital of Hefei (NO. HSY-IRB-YJ-LWTG-ZLF001) and informed consent was taken from all the patients or their guardian.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 32071054) [to YT]; Research Fund of Anhui Institute of Translational Medicine (Grant No. 2021zhyx-B10) [to YT]; Anhui Province Clinical Medical Research Transformation Special Project (Grant No. 202204295107020006) [to KW].
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].McCarron RM, Shapiro B, Rawles J, Luo J. Depression. Annals of Internal Medicine . 2021;174:ITC65–ITC80. doi: 10.7326/AITC202105180. ITC. [DOI] [PubMed] [Google Scholar]
- [2].GBD 2019 Mental Disorders Collaborators Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. Psychiatry . 2022;9:137–150. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hui J, Zomorrodi R, Lioumis P, Ensafi E, Voineskos D, Voineskos A, et al. Altered interhemispheric signal propagation in schizophrenia and depression. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology . 2021;132:1604–1611. doi: 10.1016/j.clinph.2021.03.039. [DOI] [PubMed] [Google Scholar]
- [4].Gray JP, Müller VI, Eickhoff SB, Fox PT. Multimodal Abnormalities of Brain Structure and Function in Major Depressive Disorder: A Meta-Analysis of Neuroimaging Studies. American Journal of Psychiatry . 2020;177:422–434. doi: 10.1176/appi.ajp.2019.19050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pizzagalli DA, Roberts AC. Prefrontal cortex and depression. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology . 2022;47:225–246. doi: 10.1038/s41386-021-01101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cabello-Arreola A, Ho AMC, Ozerdem A, Cuellar-Barboza AB, Kucuker MU, Heppelmann CJ, et al. Differential Dorsolateral Prefrontal Cortex Proteomic Profiles of Suicide Victims with Mood Disorders. Genes . 2020;11:256. doi: 10.3390/genes11030256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin IM, Chen TC, Lin HY, Wang SY, Sung JL, Yen CW. Electroencephalogram patterns in patients comorbid with major depressive disorder and anxiety symptoms: Proposing a hypothesis based on hypercortical arousal and not frontal or parietal alpha asymmetry. Journal of Affective Disorders . 2021;282:945–952. doi: 10.1016/j.jad.2021.01.001. [DOI] [PubMed] [Google Scholar]
- [8].Trinkl M, Greimel E, Bartling J, Grünewald B, Schulte-Körne G, Grossheinrich N. Right-lateralization of N2-amplitudes in depressive adolescents: an emotional go/no-go study. Journal of Child Psychology and Psychiatry, and Allied Disciplines . 2015;56:76–86. doi: 10.1111/jcpp.12282. [DOI] [PubMed] [Google Scholar]
- [9].Lin CC, Yang CP, Cheng PY, Hsiao M, Liu YP. Escitalopram reversibility of the impacts following chronic stress on central 5-HT profiles - Implications to depression and anxiety. Behavioural Brain Research . 2023;453:114613. doi: 10.1016/j.bbr.2023.114613. [DOI] [PubMed] [Google Scholar]
- [10].Bailly F, Belaid H. Suicidal ideation and suicide attempt associated with antidepressant and antiepileptic drugs: Implications for treatment of chronic pain. Joint Bone Spine . 2021;88:105005. doi: 10.1016/j.jbspin.2020.04.016. [DOI] [PubMed] [Google Scholar]
- [11].Rhee TG, Shim SR, Forester BP, Nierenberg AA, McIntyre RS, Papakostas GI, et al. Efficacy and Safety of Ketamine vs Electroconvulsive Therapy Among Patients with Major Depressive Episode: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2022;79:1162–1172. doi: 10.1001/jamapsychiatry.2022.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Han KY, Wang CM, Du CB, Qiao J, Wang YL, Lv LZ. Treatment outcomes and cognitive function following electroconvulsive therapy in patients with severe depression. World Journal of Psychiatry . 2023;13:949–957. doi: 10.5498/wjp.v13.i11.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li X, Guo J, Chen X, Yu R, Chen W, Zheng A, et al. Predicting Responses to Electroconvulsive Therapy in Adolescents with Treatment-Refractory Depression Based on Resting-State fMRI. Journal of Clinical Medicine . 2023;12:3556. doi: 10.3390/jcm12103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Takamiya A, Kishimoto T, Hirano J, Nishikata S, Sawada K, Kurokawa S, et al. Neuronal network mechanisms associated with depressive symptom improvement following electroconvulsive therapy. Psychological Medicine . 2021;51:2856–2863. doi: 10.1017/S0033291720001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chu Y, Wu J, Wang D, Huang J, Li W, Zhang S, et al. Altered voxel-mirrored homotopic connectivity in right temporal lobe epilepsy as measured using resting-state fMRI and support vector machine analyses. Frontiers in Psychiatry . 2022;13:958294. doi: 10.3389/fpsyt.2022.958294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zheng G, Yingli Z, Shengli C, Zhifeng Z, Bo P, Gangqiang H, et al. Aberrant Inter-hemispheric Connectivity in Patients with Recurrent Major Depressive Disorder: A Multimodal MRI Study. Frontiers in Neurology . 2022;13:852330. doi: 10.3389/fneur.2022.852330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wei Q, Tian Y, Yu Y, Zhang F, Hu X, Dong Y, et al. Modulation of interhemispheric functional coordination in electroconvulsive therapy for depression. Translational Psychiatry . 2014;4:e453. doi: 10.1038/tp.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gori B, Grippo A, Focardi M, Lolli F. The Italian version of Edinburgh Handedness Inventory: Translation, transcultural adaptation, and validation in healthy subjects. Laterality . 2024;29:151–168. doi: 10.1080/1357650X.2024.2315851. [DOI] [PubMed] [Google Scholar]
- [19].Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry . 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang D, Tian Y, Li M, Dahmani L, Wei Q, Bai T, et al. Functional connectivity underpinnings of electroconvulsive therapy-induced memory impairments in patients with depression. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology . 2020;45:1579–1587. doi: 10.1038/s41386-020-0711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage . 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- [22].Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage . 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bai T, Wei Q, Zu M, Xie W, Wang J, Gong-Jun J, et al. Functional plasticity of the dorsomedial prefrontal cortex in depression reorganized by electroconvulsive therapy: Validation in two independent samples. Human Brain Mapping . 2019;40:465–473. doi: 10.1002/hbm.24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Clairis N, Lopez-Persem A. Debates on the dorsomedial prefrontal/dorsal anterior cingulate cortex: insights for future research. Brain: a Journal of Neurology . 2023;146:4826–4844. doi: 10.1093/brain/awad263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yin JB, Liang SH, Li F, Zhao WJ, Bai Y, Sun Y, et al. dmPFC-vlPAG projection neurons contribute to pain threshold maintenance and antianxiety behaviors. The Journal of Clinical Investigation . 2020;130:6555–6570. doi: 10.1172/JCI127607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stange JP, Bessette KL, Jenkins LM, Peters AT, Feldhaus C, Crane NA, et al. Attenuated intrinsic connectivity within cognitive control network among individuals with remitted depression: Temporal stability and association with negative cognitive styles. Human Brain Mapping . 2017;38:2939–2954. doi: 10.1002/hbm.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang L, Shi L, Zhang B, Zhao L, Dong Y, Liu J, et al. Probabilistic Entity-Relationship Diagram: A correlation between functional connectivity and spontaneous brain activity during resting state in major depressive disorder. PloS One . 2017;12:e0178386. doi: 10.1371/journal.pone.0178386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shiota S, Okamoto Y, Okada G, Takagaki K, Takamura M, Mori A, et al. Effects of behavioural activation on the neural basis of other perspective self-referential processing in subthreshold depression: a functional magnetic resonance imaging study. Psychological Medicine . 2017;47:877–888. doi: 10.1017/S0033291716002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, et al. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology . 2017;42:1210–1219. doi: 10.1038/npp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Qin Y, Sun B, Zhang H, Li Y, Zhang T, Luo C, et al. Aberrant Interhemispheric Functional Organization in Children with Dyskinetic Cerebral Palsy. BioMed Research International . 2019;2019:4362539. doi: 10.1155/2019/4362539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang H, Wang C, Ji G, Feng Z, Duan J, Chen F, et al. Aberrant interhemispheric functional connectivity in first-episode, drug-naïve major depressive disorder. Brain Imaging and Behavior . 2019;13:1302–1310. doi: 10.1007/s11682-018-9917-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All experimental data used to support the findings of this study are available from the corresponding author upon request.