Abstract
Cytotoxic T lymphocytes (CTL) play a vital role in host defense against viral and intracellular bacterial infections. However, nonreplicating vaccines administered by intramuscular injection using a syringe and needle elicit predominantly humoral responses and not CTL responses. Here we report that epidermal powder immunization (EPI), a technology that delivers antigens on 1.5- to 2.5-μm gold particles to the epidermis using a needle-free powder delivery system, elicits CTL responses to nonreplicating antigens. Following EPI, a majority of the antigen-coated gold particles were found in the viable epidermis in the histological sections of the target skin. Further studies using transmission electron microscopy revealed the intracellular localization of the gold particles. Many Langerhans cells (LCs) at the vaccination site contained antigen-coated particles, as revealed by two-color immunofluorescence microscopy, and these cells were found in the draining lymph nodes 20 h later. Immune responses to several viral protein antigens after EPI were studied in mice. EPI with hepatitis B surface antigen (HBsAg) and a synthetic peptide of influenza virus nucleoprotein (NP peptide) elicited antigen-specific CTL responses as well as antibody responses. In an in vitro cell depletion experiment, we demonstrated that the CTL activity against HBsAg elicited by EPI was attributed to CD8+, not CD4+, T cells. As controls, needle injections of HBsAg or the NP peptide into deeper tissues elicited solely antibody, not CTL, responses. We further demonstrated that EPI with inactivated A/Aichi/68 (H3N2) or A/Sydney/97 (H3N2) influenza virus elicited complete protection against a mouse-adapted A/Aichi/68 virus. In summary, EPI directly delivers protein antigens to the cytosol of the LCs in the skin and elicits both cellular and antibody responses.
Humoral immunity is essential for the control of extracellular pathogens. Cell-mediated immunity, which is associated with the activation of CD8+ cytotoxic T lymphocytes (CTL), plays a vital role in host defense against intracellular pathogens, including viruses (22, 24–26, 51). CTL can lyse virus-infected cells (9) and secrete cytokines such as gamma interferon and tumor necrosis factor alpha that may contribute to the resolution of viral infections (21). In addition, memory CTL may provide long-lasting protection by allowing the host to mount rapid and heightened responses to reinfection with the same pathogen (1, 31).
While the important role of CTL in controlling virus infection has been widely recognized, most current nonreplicating vaccines, when administered by intramuscular (i.m.) injection, although effective in eliciting antibody responses, do not elicit CTL responses (41, 42). This is because needle injection delivers vaccines to the extracellular fluid, leading to antigen processing through the endosomal pathway and presentation in association with major histocompatibility complex (MHC) class II molecules (35, 38). In contrast, induction of CTL responses requires endogenous antigens that are processed in the proteosome and presented to the immune system under the restriction of MHC class I molecules (35, 36). Although an alternative MHC class I pathway may lead to a CTL response to exogenous antigens (3, 4, 39, 49), antigen presentation through the alternative pathway may be dependent on the nature and form of the antigen and possibly the involvement of a special subpopulation of antigen-presenting cells (APCs) (43, 46). In general, the most effective way of inducing a CTL response is to target vaccine antigens to the cytosol of the APCs.
Extensive research has been conducted to develop vaccines that induce both humoral and CTL responses. The live-attenuated virus and live-vector approaches have been pursued for several decades, but the success rate of developing these types of vaccines is low (13, 18). Increased concerns about the safety of live-attenuated vaccines have prompted researchers to seek alternative means of inducing CTL responses (18). DNA vaccination has been widely explored in the past few years (20). There are a large number of successful animal studies; however, to date there are few human studies showing the successful induction of humoral and cellular immune responses (45). Adjuvants have been used to induce CTL responses to subunit or inactivated viruses (28, 34, 50, 55). However, the discovery of adjuvants that are both safe and effective has proven to be a great challenge. Many adjuvants have been evaluated in preclinical studies, but very few meet the safety and efficacy requirements for human use.
We have recently demonstrated that epidermal powder immunization (EPI) with a split influenza virus vaccine embedded in water-soluble, sugar-based particles induces strong antibody responses and protection against experimental challenges in mice (10). Langerhans cells (LCs) in the viable epidermis of the skin have been shown to play an important role in antigen processing and presentation following skin immunizations (2, 5, 12, 14). We hypothesized that EPI with conventional subunit vaccines (peptide, protein, and inactivated pathogens) applied to the surfaces of gold microparticles may deliver vaccines to the cytosol of the LCs and induce CTL responses. To test this hypothesis, we first studied the tissue and subcellular localization of the antigen and carriers at the site of EPI and the draining lymph nodes. We then evaluated the immune responses to the hepatitis B virus surface antigen (HBsAg), a nucleoprotein peptide (NP peptide) from influenza virus (44), and inactivated influenza viruses following EPI. Evidence of intracellular delivery of antigens to the LCs and of induction of both CTL and antibody responses by EPI is presented.
MATERIALS AND METHODS
Antigens and formulations.
The H-2Kd-restricted influenza virus NP peptide TYQRTRALV (amino acids 147 to 155) (referred to below as the NP peptide) from A/PR/8/34 (H1N1) (44) was synthesized by Multiple Peptide Systems, Inc. (San Diego, Calif.). HBsAg was purchased from Rhein Biotech (Buenos Aires, Argentina). The A/Sydney/5/97 (H3N2) (referred to below as A/Sydney) and A/Beijing/262/95 (H1N1) (referred to below as A/Beijing) influenza viruses from the trivalent split influenza virus vaccine manufactured for the 1998-to-1999 season were purchased from the Swiss Serum and Vaccine Institute (Berne, Switzerland). The A/Aichi/68 (H3N2) virus (A/Aichi) was grown by culture in 10-day-old embryonated chicken eggs (48). Viruses were purified from the allantoic fluid by concentration with an Amicon hollow fiber system and two passages over continuous sucrose gradients by ultracentrifugation. The total protein concentration of the purified virus was determined by a microbicinchoninic acid assay (micro-BCA assay) (Pierce, Rockford, Ill.). The A/PR8/34 (H1N1) influenza virus (A/PR8) was purchased from Charles River Spafas (New Franklin, Conn.). Both the A/Aichi and A/PR8 viruses were inactivated with formalin (1:4,000 [vol/vol]) for 48 h at 4°C.
The NP peptide was precipitated onto 1.5- to 2.5-μm gold particles (Degussa, Plainfield, N.J.) using ethanol in the presence of 3 M sodium acetate. Briefly, the NP peptide and gold were combined at a ratio of 10 μg of peptide to 1 mg of gold and resuspended in 3 M sodium acetate. Prechilled (4°C) dehydrated ethanol was added drop by drop to precipitate the peptide onto the gold particles. The NP peptide-coated gold particles were collected and washed three times with dehydrated ethanol. The final preparation was resuspended in dehydrated ethanol for drying onto the interior surface of nylon tubing using a previously described method (32). The inactivated influenza virus and HBsAg were precipitated onto gold particles using 1% polyethylene glycol (molecular weight, 1,300 to 1,600) (Sigma Scientific Chemicals, St. Louis, Mo.) and ethanol by a similar procedure. The protein content in the final preparation was determined using the micro-BCA assay (Pierce).
The structures of DNA plasmids encoding the full-length NP protein of influenza virus and the HBsAg have been reported previously (16, 37). The previously described methods for preparation of DNA vaccines on 1.5- to 2.5-μm gold particles and immunization using the PowderJect XR-1 powder delivery device were used in this study (32).
Mice, immunization, and serum collection.
Female BALB/c mice (5 to 7 weeks old; Harlan-Sprague-Dawley, Indianapolis, Ind.) were used. Mice were fully anesthetized by an intraperitoneal (i.p.) injection of ketamine and xylazine, and their abdominal skin was shaved using a hair clipper prior to immunization. Antigen-coated gold particles dried onto the interior surface of a nylon tube (length, 0.5 in; diameter, 1/10 in) were administered to the shaved abdominal skin using the helium-powered PowderJect XR-1 powder delivery device (32).
In some studies, a DNA vaccine was used as a control. The DNA plasmid was precipitated onto 1.5- to 2.5-μm gold particles and administered to mice using the PowderJect XR-1 powder delivery device as previously reported (32). Other controls included mice that were immunized with the respective vaccines in saline by either i.p. or i.m. injections using a 26-gauge needle. Each injection delivered a 0.2-ml (i.p.) or 0.05-ml (i.m.) volume.
Blood was collected via retro-orbital bleeding under anesthesia prior to each vaccination and 2 weeks postboost.
Histology.
Localization of the antigen-coated gold particles in the target skin of mice was determined histologically. Immediately after administration, target skin was excised, fixed with 10% formalin, and embedded with paraffin. Sections (thickness, 6 μm) were cut, stained with hematoxylin and eosin (H&E), and visualized under a light microscope.
TEM.
Transmission electron microscopy (TEM) was performed to confirm the subcellular localization of the gold particles in the vaccination site. Target skin was excised immediately after EPI, fixed with 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.2) for 2 h at room temperature, and embedded in Spurr's low-viscosity resin. Ultrathin sections (thickness, 75 to 90 nm) were collected, stained with uranyl acetate and lead citrate, and observed under a Philips (Einhoven, The Netherlands) CM120 transmission electron microscope at 80 kV. Photographic images were produced using a Kodak MegaPlus 1.4 camera (AMT, Danvers, Mass.).
Immunofluorescence staining.
Texas Red-labeled bovine serum albumin (BSA; Sigma) was used as a model antigen to monitor the fate of antigen after EPI. BSA was labeled with Texas Red (Molecular Probes, Eugene, Oreg.) according to the manufacturer's instructions prior to precipitation onto gold particles. EPI was performed on the mouse ear using 0.5 mg of gold coated with 5 μg of Texas Red-labeled BSA. Immediately after EPI, target skin was collected and the epidermal sheet was prepared by treatment with 1 M sodium bromide as previously described (30). The epidermal sheet was fixed in ice-cold acetone for 10 min and washed three times with phosphate-buffered saline (PBS) prior to immunofluorescence staining.
LCs in the epidermal sheet were stained in an indirect immunofluorescence assay. Briefly, the epidermal sheet was placed in a well of a 48-well tissue culture plate (Costar) and incubated overnight at 4°C with a monoclonal antibody (MAb) to I-Ad antigen (PharMingen) in PBS containing 0.5% ovalbumin. The epidermal sheet was then washed with PBS and sequentially incubated with biotin-labeled goat anti-mouse immunoglobulin G (IgG) (heavy plus light chains) (Southern Biotechnology Associates, Birmingham, Ala.) and a fluorescein isothiocyanate (FITC)-streptavidin conjugate (PharMingen). After three washes with PBS, the epidermal sheet was mounted on a slide and examined using a fluorescent microscope with a dual-wavelength filter (red and green) and a camera (Nikon, Melville, N.Y.).
Migration of LCs.
The migration of LCs from the site of EPI to the draining lymph nodes was studied to determine the roles of LCs in the subsequent immune responses. Approximately 500 μl of FITC (Molecular Probes) dissolved in N,N-dimethylformamide (DMF) (5 mg/ml) was applied to the shaved abdominal skin of an anesthetized mouse. EPI was performed 4 h later by administering 0.5 mg of gold coated with 5 μg of Texas Red-labeled BSA. Inguinal lymph nodes were collected 20 h later, and single-cell preparations were made using a cell strainer (Fisher Scientific Products, Pittsburgh, Pa.). The cells were applied to a glass slide using a Shandon (Pittsburgh, Pa.) cytospin centrifuge and were examined under a fluorescent microscope with a dual-wavelength filter (red and green) (Nikon).
Controls included mice treated with FITC alone and mice receiving EPI with 0.5 mg of gold coated with Texas Red-labeled BSA without prior treatment with FITC.
Mouse challenge.
A mouse-adapted A/Aichi virus was generated by several passages in BALB/c mice, and the 50% lethal dose (LD50) was experimentally determined to be 104 PFU. Mice were first fully anesthetized by i.p. injection of a mixture of ketamine and xylazine and then intranasally instilled with 105 PFU (10 LD50s) of the mouse-adapted A/Aichi virus in 50 μl of PBS. Mortality and changes in body weight were monitored daily for 16 days.
ELISA.
Titers of antibody to the NP peptide, HBsAg, and influenza viruses were determined using a modified enzyme-linked immunosorbent assay (ELISA) (10). Briefly, a 96-well Costar plate (Fisher Scientific Products) was coated with the appropriately diluted antigen in 30 mM PBS (pH 7.4) overnight at 4°C. Plates were washed and incubated with test sera diluted in PBS containing 5% dry milk for 1.5 h at room temperature, followed by three washes and a 1-h incubation with biotin-labeled goat anti-mouse IgG (Southern Biotechnology Associates) at room temperature. Following three additional washes, plates were incubated with streptavidin-horseradish peroxidase conjugates (Southern Biotechnology Associates) for 1 h at room temperature. Finally, plates were washed and developed with the substrate 3,3′,5,5′-tetramethylbenzidine (Bio-Rad Laboratories, Melville, N.Y.). After plates were developed for 15 min, the reaction was stopped by addition of 1 N sulfuric acid. Plates were read at a wavelength of 450 nm using an Emax plate reader (Molecular Devices, Sunnyvale, Calif.). The end point titers of the sera were determined by 4-parameter analysis using the Softmax Pro 4.1 program (Molecular Devices) and normalized to a reference serum of known titer.
HI assay.
Hemagglutination inhibition (HI) titers were determined using a previously described method (32). Receptor-destroying enzymes from Vibrio cholerae were used to remove the nonspecific inhibitory activity of the mouse sera.
CTL assay.
CTL responses were measured as previously described (16, 37). Briefly, BALB/c mice were sacrificed following a prime-boost immunization on days 0 and 28. In some studies, each group was divided into two subgroups and splenocytes pooled from two to four mice were assayed on separate days to demonstrate consistency. Splenocytes (6.3 × 106 cells) were stimulated for 6 days with stimulator cells (2.4 × 106 cells) at 37°C in a 5% CO2 incubator prior to measurement of lytic activities in a 4-h standard chromium release assay. The influenza virus NP peptide CTL assay used naïve mouse splenocytes that had been treated with mitomycin C and pulsed with the synthetic NP peptide as stimulator cells, and it used peptide-pulsed P815 cells (American Type Culture Collection, Manassas, Va.) as target cells. The HBsAg CTL assay used P815 cells transformed with the full-length HBsAg gene both as stimulator cells and as target cells.
Splenocytes depleted of CD4+ or CD8+ T cells were used in certain CTL assays. Mouse spleens from eight animals were pooled, and splenocyte suspensions were prepared and divided into three equal fractions. Two of the fractions were treated with Dynabeads tagged with an anti-CD4 (P/N114.05) or anti-CD8 (P/N114.07) MAb according to the manufacturer's instructions (Dynal AS & Nordic, Oslo, Norway). Complete depletion of the T-cell subpopulations was confirmed by labeling with an anti-CD4 or anti-CD8 MAb and flow cytometry analysis. The remaining cells were stimulated, and CTL activity was determined as described above and compared to that in untreated bulk splenocytes.
RESULTS
EPI targets epidermis.
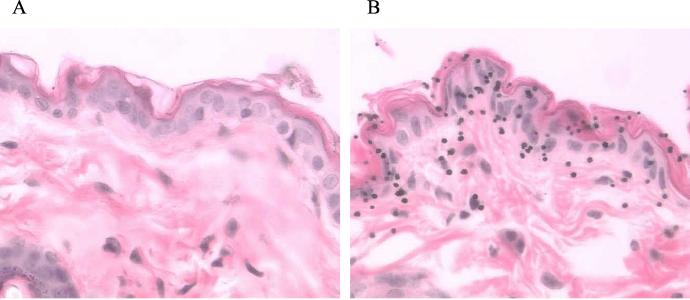
Examination of the H&E-stained sections of a normal mouse skin under a light microscope revealed three layers of structures: stratum corneum, viable epidermis, and dermis (Fig. 1A). The viable epidermis, a tissue in which LCs are located, contained one to three cell layers. Gold particles were found mainly in the stratum corneum, the viable epidermis, and the epidermis-dermis junction (Fig. 1B). Some gold particles could be seen in the upper dermis. There was no apparent tissue damage and no clearly detectable extracellular spaces around the gold particles. Presumably, the micrometer-size entry portals of the gold particles were closed shortly after delivery.
FIG. 1.
Localization of gold particles at the EPI site by light microscopy. Light-microscopic images of H&E-stained histological sections from a normal mouse skin (A) and a target skin (B) are shown. Gold particles appear as dark spheres (magnification, ×240).
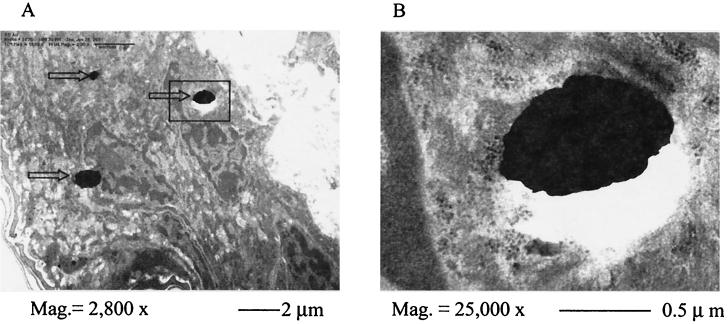
TEM photographic images of the vaccination site revealed very tight junctions between the cells in the viable epidermis. There were no clearly discernible extracellular spaces, and the gold particles were much smaller than the epidermal cells; all gold particles were deposited inside the cells. Most gold particles were in the cytoplasm, as shown in Fig. 2A, but occasionally gold particles could be seen inside the nuclei (data not shown). Close examination at a higher magnification showed that there were no membrane-like structures surrounding the gold particles, ruling out the possibility that gold particles entered cells via endocytosis (Fig. 2B). The major types of cells in the viable epidermis, LCs and keratinocytes, could not be clearly differentiated under TEM.
FIG. 2.
Subcellular localization by TEM of gold particles in the target skin following EPI. (A) TEM images of target skin sections (magnification, ×2,800) show gold particles (indicated by arrows) in the cytosol of viable epidermal cells. (B) Enlarged view (magnification, ×25,000) of the boxed area in panel A shows no membrane-like structures around the gold particles.
EPI targets LCs.
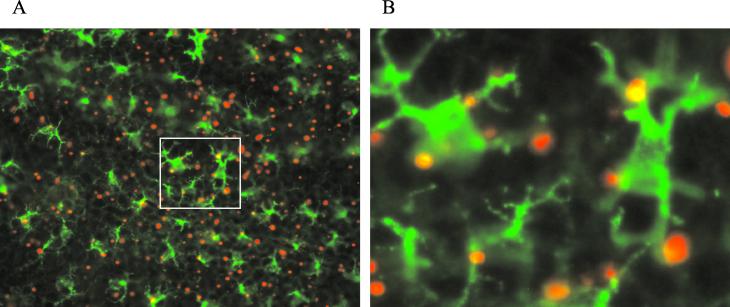
Immediately after EPI with gold particles coated with Texas Red-labeled BSA, the target skin was excised. The epidermal sheet was prepared, stained by indirect immunofluorescence using an FITC conjugate, and visualized under a fluorescence microscope with a two-color filter. The LCs, the only type of cells in the epidermis expressing class II antigens, were stained green. The gold particles coated with Texas Red-labeled BSA appeared red if they were inside nonfluorescent cells, e.g., keratinocytes, and yellow if they were inside the green-colored LCs. Figure 3A is a representative photographic image of an epidermal sheet from the vaccination site. The green-colored LCs with extruding dendrites are clearly visible in the epidermal sheet. These cells formed a dense network covering a large proportion of the surface area. A large number of red- and yellow-colored gold particles were clearly visible, confirming the epidermal localization of the gold-particles following EPI as revealed by the H&E-stained histological sections. Most of the gold particles appeared red, indicating keratinocyte localization, but a significant number appeared yellow, indicating LC localization. Approximately 50% of the LCs contained antigen-coated particles after administration of 0.5 mg of antigen-coated gold particles to a 113-mm2 target area. Many LCs contained multiple gold particles (Fig. 3B).
FIG. 3.
Localization of antigen-coated gold particles in the LCs by immunofluorescence staining. Immediately after EPI using Texas Red-labeled BSA-coated gold particles (red), target skin was excised, an epidermal sheet was prepared, LCs were labeled with FITC (green), and the epidermal sheet was examined by fluorescence microscopy. (A) Representative light microscopic image of the stained epidermal sheet (magnification, ×60). (B) Closer view (magnification, ×240) of the boxed area in panel A. The gold particles inside the LCs appear yellow, whereas those in nonfluorescent cells appear red.
Migration of LCs from the site of EPI.
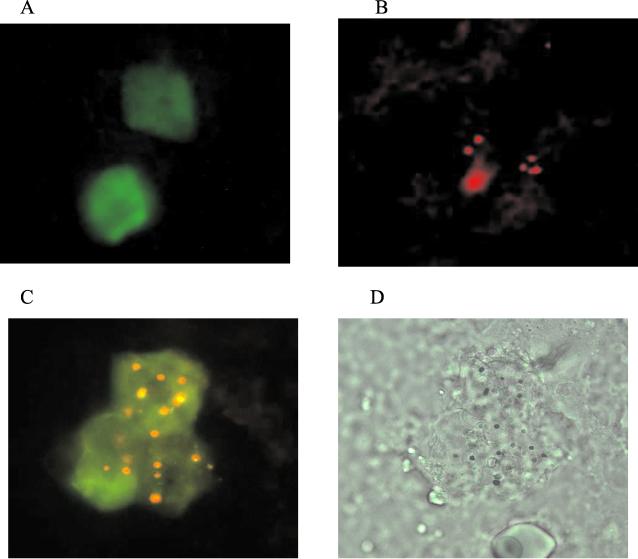
The migration of LCs from the vaccination site into the draining lymph nodes was studied in order to determine the role of LCs in the immune responses following EPI. In a typical study, three groups of mice were used; the abdominal skin of mice in groups 1 and 3 was painted with FITC dissolved in DMF, while mice in group 2 were treated with DMF only. Four hours later, EPI using gold particles coated with Texas Red-labeled BSA was administered to the painted skin of mice in groups 2 and 3. Fluorescent cells in the draining lymph nodes were examined 20 h later. Four hours after application of FITC to the abdominal skin of mice in group 1, an epidermal sheet was made and all cells in the viable epidermis were stained with FITC (data not shown). Green fluorescent LCs could be detected in the inguinal lymph nodes 20 h later (Fig. 4A). Mice painted with DMF followed by EPI (group 2) contained only red fluorescent cells in their draining lymph nodes (Fig. 4B). Mice treated with both FITC and EPI had green fluorescent cells containing yellow-colored gold particles in their draining lymph nodes. Figure 4C is a representative image showing three green LCs, each containing several yellow-colored gold particles. Figure 4D is the bright-field image of the same cells. These cells are believed to be LCs originating from the site that had been directly targeted by EPI.
FIG. 4.
Migration of LCs from the vaccination site to the draining lymph node. Vaccination sites were treated with FITC (dissolved in DMF) by topical application prior to EPI with gold particles coated with Texas Red-labeled BSA. (A through C) Mice were treated either with FITC without EPI (A), with DMF followed by EPI (B), or with both FITC and EPI (C). Fluorescent cells in the draining lymph nodes were examined 20 h later by fluorescent microscopy under UV light. (D) Bright-field image of the same cells shown in panel C. Magnification, ×240.
CTL and antibody responses to HBsAg elicited by EPI.
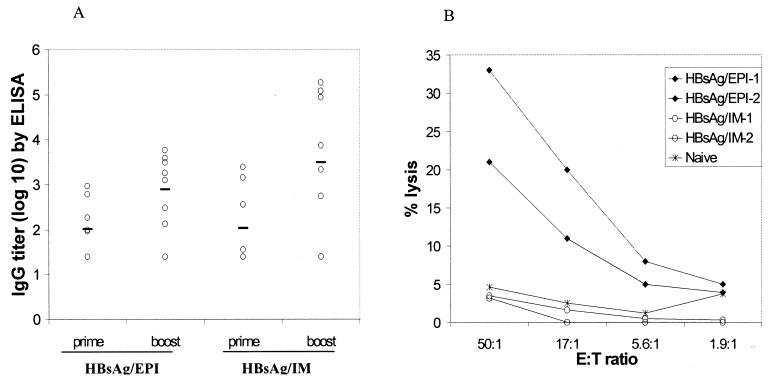
In the first study, BALB/c mice (eight mice per group) were immunized with 2 μg of HBsAg to determine whether EPI induced a CTL response as well as a humoral response. Control mice were immunized with 2 μg of HBsAg in PBS by i.m. needle injection. Antibody responses were determined by an ELISA with blood samples collected prior to each immunization (days 0 and 28) and 14 days after booster immunization. HBsAg-specific CTL responses were determined on day 42 using four animals from each group.
EPI of mice induced both a primary and a secondary antibody response to HBsAg, and these titers were comparable to that elicited by i.m. injection (Fig. 5A). Mice receiving EPI exhibited HBsAg-specific CTL responses. In contrast, the i.m.-immunized control had no detectable CTL activities (Fig. 5B). This indicated that EPI elicited a qualitatively different immune response to HBsAg than the conventional immunization method.
FIG. 5.
Immune responses to HBsAg following EPI compared to i.m. injection. Mice were immunized on days 0 and 28 by either EPI or i.m. injection. (A) Antibody responses were determined by ELISA with sera collected on day 42. Data are individual titers; solid lines indicate geometric mean titers. (B) CTL responses from four mice were determined after in vitro stimulation using a standard chromium release assay. Each line represents the CTL activities using pooled splenocytes from two animals.
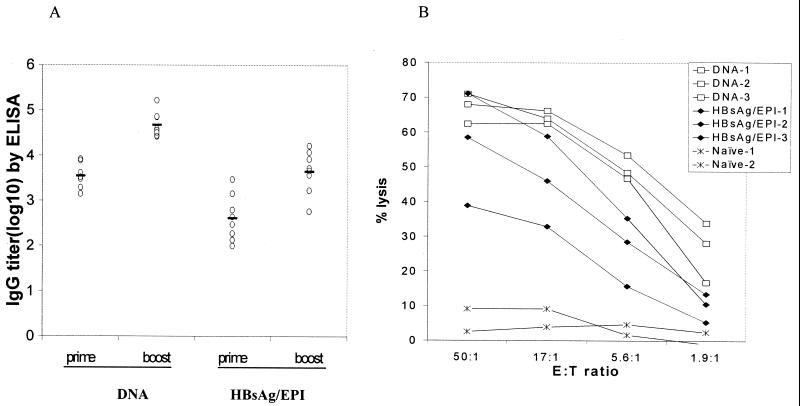
To compare the immune responses elicited by EPI to that elicited by particle-mediated DNA immunization, mice (eight per group) were immunized either with 2 μg of HBsAg via EPI or with 2 μg of DNA plasmid encoding HBsAg. Post-prime immunization (day 28) and post-boost immunization (day 42) antibody responses to HBsAg were determined by ELISA. CTL responses were determined on days 42 and 49 using spleens pooled from two to four animals from each group.
Both EPI with HBsAg and DNA immunization elicited a primary and a secondary antibody response (Fig. 6A). Antibody titers were comparable when the postprime or postboost titers were compared for the two different vaccines. Furthermore, both EPI and DNA immunization elicited CTL responses (Fig. 6B). The naïve control animal had no CTL response. This suggests that EPI with HBsAg elicits an immune response that is qualitatively similar to that elicited by DNA immunization.
FIG. 6.
Immune responses to HBsAg elicited by EPI compared to DNA vaccine. Mice were immunized on days 0 and 28 with 2 μg of HBsAg or 1 μg of DNA plasmid encoding HBsAg. (A) Antibody responses from individual sera collected on days 28 and 42 were analyzed by ELISA. Solid lines, geometric mean titers. (B) CTL responses were determined on days 42 and 49 using pooled spleens (n = 2 to 4).
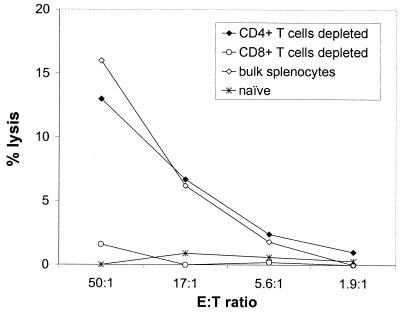
CTL response to HBsAg is CD8+ T-cell dependent.
The association between CTL activity and CD8+ T cells was determined by a CTL assay using bulk splenocytes and splenocytes depleted of either CD4+ T cells or CD8+ T cells (Fig. 7). Mice received 2 μg of HBsAg via EPI as described above. Depletion of CD4+ T cells had no impact on CTL activity compared to that for bulk splenocytes. In contrast, depletion of CD8+ T cells resulted in a complete loss of CTL activity. This suggests that EPI induces CTL responses that are CD8+ T-cell dependent.
FIG. 7.
CTL responses induced by EPI are CD8+ T-cell dependent. Mice were immunized on days 0 and 28 with 2 μg of HBsAg via EPI. CTL activity was measured in splenocytes pooled from eight mice; either bulk splenocytes or fractions depleted of CD4+ or CD8+ T cells were used.
CTL response to the NP peptide of influenza virus.
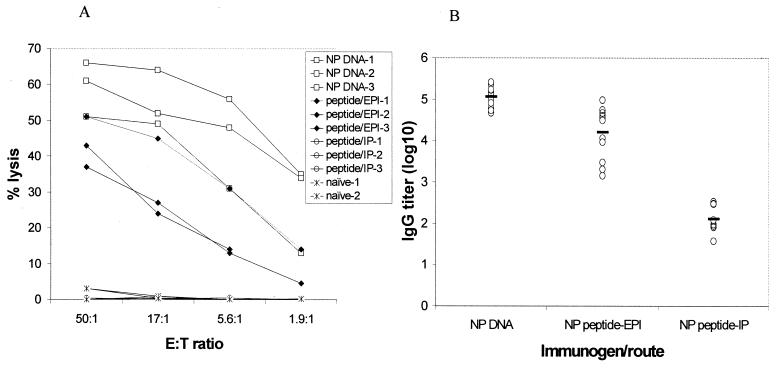
This study was designed to determine if EPI was suitable for the delivery of peptide antigens to induce a CTL response. The influenza virus NP peptide TYQRTRALV (amino acids 147 to 155) from the PR8 virus is a known CTL epitope (44) and has previously been used to generate target cells to assess the CTL response (37). We are not aware of any other studies using this peptide as an immunogen to induce CTL responses.
BALB/c mice (12 animals per group) were immunized with 5 μg of the NP peptide by either EPI or i.p. injection on days 0 and 28. Control mice were immunized with a DNA plasmid encoding the full-length NP protein. As expected, the DNA-immunized control had a CTL response. CTL responses were also detected in mice receiving EPI (Fig. 8A). i.p. injection of the peptide did not result in a detectable CTL response.
FIG. 8.
Immune responses to an influenza virus NP peptide following EPI. Mice were immunized by either EPI or i.p. injection with 5 μg of the A/PR8 NP peptide on days 0 and 28. Control mice were vaccinated with 2.5 μg of DNA plasmid encoding a full-length NP. (A) CTL responses were determined on days 37 and 39. Each data point represents CTL activities in pooled splenocytes (n = 4). (B) Postboost antibody responses were determined by an ELISA using inactivated A/PR8 as the detection antigen. Data are individual serum IgG titers; solid lines indicate mean titers.
Because the NP peptide is a CTL epitope and is smaller than a typical B-cell epitope, we did not expect to see an antibody response in NP peptide-immunized mice. However, when postboost sera were examined by an ELISA using inactivated A/PR8 influenza virus as the detection antigen, high IgG titers were seen in mice immunized with the NP peptide by EPI (Fig. 8B). Antibody titers elicited by the i.p.-injected peptide were significantly lower (P < 0.01). Immunization with the DNA plasmid encoding the full-length NP protein also elicited an antibody response. These results indicate that EPI with the NP peptide, like the DNA vaccine, elicits both antibody and CTL responses.
Immune responses to inactivated influenza virus and protection.
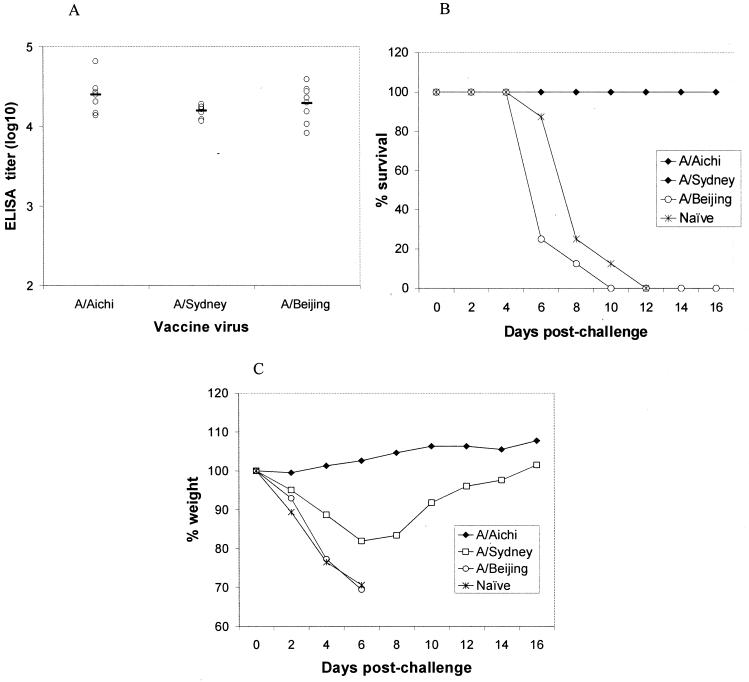
To determine if EPI can elicit immune responses to inactivated whole virus and protection against lethal challenge, we separately immunized three groups of mice (eight animals per group) with one of the following influenza viruses: A/Aichi/, A/Sydney, and A/Beijing, respectively. Each animal received a prime and a booster immunization on days 0 and 28, respectively, with 2.5 μg of total viral protein. Serum antibody responses were determined on day 42 by a standard ELISA using a detection antigen that was the same as the immunogen for the virus. Mice were challenged with 10 LD50s of a mouse-adapted A/Aichi virus on day 44. Weight loss and survival were monitored for 16 days postchallenge.
EPI immunization elicited antibody responses to all three influenza viruses, and the ELISA titers were indistinguishable (Fig. 9A). Following a lethal challenge with the mouse-adapted A/Aichi virus, all mice that had been immunized with the A/Aichi and A/Sydney viruses via EPI survived (Fig. 9B). All naïve mice and mice immunized with the A/Beijing virus died within 10 days postchallenge. CTL responses for these mice could not be determined due to the lack of an appropriate CTL assay for each influenza virus.
FIG. 9.
Immune responses to inactivated influenza viruses and protection against challenge following EPI. Mice were immunized with 2.5 μg of the indicated inactivated or split influenza viruses on days 0 and 28. Mice were challenged intranasally with 10 LD50s of a mouse-adapted A/Aichi virus on day 44. (A) Antibody titers were determined by a standard ELISA; solid lines indicate geometric mean titers. (B and C) Survival (B) and changes in body weight (C) were monitored for 16 days.
Naïve mice lost an average of 30% of their body weight by day 6 prior to succumbing to the influenza virus infection between days 8 and 10 (Fig. 9C). Mice immunized with the A/Aichi virus did not experience appreciable weight loss, whereas mice immunized with the A/Sydney virus had an average weight loss of approximately 20% 6 days postchallenge. At the end of the 16-day monitoring period, these mice had nearly recovered their original weight. Immunization with the A/Beijing virus offered no protection against weight loss.
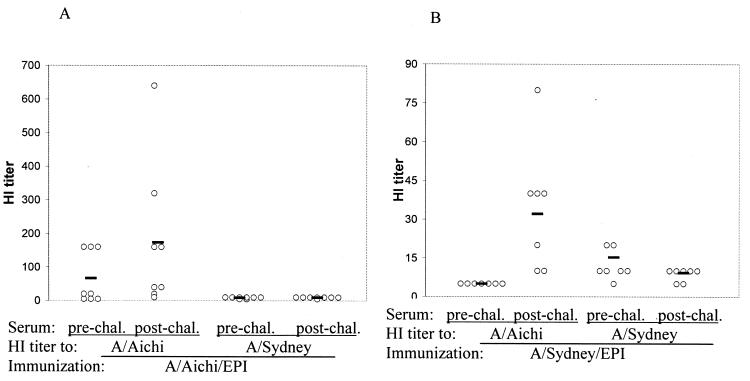
To determine if protection was attributed to HI antibodies, HI titers of prechallenge sera (day 42) and postchallenge sera (day 61) from mice immunized with the A/Aichi or A/Sydney virus were determined. Prior to challenge, mice immunized with the A/Aichi virus had HI titers to the A/Aichi virus only, not to the A/Sydney virus (Fig. 10A), and A/Sydney virus-immunized mice had HI titers to the A/Sydney virus only, not to the A/Aichi virus (Fig. 10B). This indicated that there were no cross-reacting HI antibodies between the A/Aichi and A/Sydney viruses. Compared to those in prechallenge sera, HI titers to A/Aichi virus were slightly elevated in postchallenge sera from A/Aichi virus-immunized mice (Fig. 10A). This may be related to the antibody responses to the A/Aichi challenge virus. All A/Sydney virus-immunized mice had HI titers to the A/Aichi virus in postchallenge sera (Fig. 10B), an indication of immune responses to the challenge virus and possibly viral replication. HI titers to the A/Sydney virus in the postchallenge sera were as expected, i.e., absent in A/Aichi virus-immunized mice and unchanged in A/Sydney virus-immunized mice. These data indicate that HI antibodies may not be responsible for protection in A/Sydney virus-immunized mice. Cross-reacting antibodies between these two viruses could be detected in both the pre- and postchallenge sera by ELISA (data not shown). Whether the protection was afforded by CTL responses to conserved antigens or cross-reacting non-HI antibodies requires further investigation.
FIG. 10.
Pre- and postchallenge HI titers to A/Aichi and A/Sydney viruses. Mice were immunized with the A/Aichi (A) or A/Sydney (B) influenza virus using EPI. Prechallenge (day 42) and postchallenge (day 61) sera from the study as described in the legend to Fig. 9 were analyzed by an HI assay. Data are serum HI titers from individual mice; each solid line indicates the geometric mean titer for eight animals.
DISCUSSION
Since protective antigens from many viruses have been identified, an immunization method that can effectively generate a broad range of immune responses, including CTL responses, will lead to rapid development of more efficacious vaccines. We have demonstrated in this study that EPI delivers antigens directly to LCs and induces CTL and antibody responses to several antigens, including a peptide, a recombinant protein, and inactivated viruses. We have further demonstrated that the CTL activities are associated with the activation of CD8+ T cells.
Priming a CTL response normally requires endogenously produced proteins or peptides that are presented to the immune system under the restriction of MHC class I molecules. DNA vaccines induce CTL responses primarily through the endogenous pathway (19), although the alternative pathway of antigen presentation may play a role (12, 49). Some adjuvants may help subunit vaccines to induce CTL responses through cytosolic antigen delivery (34). CTL induction via EPI may be related to the intracellular delivery of antigens to LCs in the epidermis. Cytosolic localization of antigen-coated gold particles in the viable epidermis was shown by TEM. Localization of antigen-coated gold particles in LCs at the vaccination site and, at a later time point, in the draining lymph nodes was demonstrated by immunofluorescence assays. Antigens on the gold particles delivered to the cytosol of the LC may lead to MHC class I-restricted antigen presentation to the T cells and induction of CTL responses.
The epidermis of the skin is a potent immunologic organ and an effective site of induction of immune responses. Particle-mediated delivery of DNA vaccines to the epidermis induces serum antibody responses and cellular immune responses to a variety of vaccines (17). In the present study, EPI induced augmented antibody and CTL responses to several viral antigens. LCs are believed to play an important role in the immune responses to EPI. LCs, which account for 2 to 4% of all epithelial cells in the viable epidermis, are known potent APCs (2, 5, 11, 14, 53). McKinney and Streilein have demonstrated that 10 adoptively transferred allogeneic LCs are sufficient to generate allogeneic CTL responses in vivo (33). Timares et al. have shown that 500 to 1,000 transferred LCs can generate a full range of immune responses (53). With their dendrites extending into the interstitial spaces between the keratinocytes, LCs form a dense network in the epidermis. The density of LCs on most human body surfaces, as well as mouse skin, is approximately 1,000 cells/mm2 (8, 11, 30). In the present study, EPI delivered antigens to a skin target area of approximately 113 mm2, which comprises approximately 105 LCs. Thus, EPI appears to require a successful “hit” in 1% or fewer of the LCs in the target site in order to elicit a full range of immune responses. Our data show that approximately 50% of LCs were directly targeted by EPI.
Another means of generating CTL responses by EPI may involve cross-priming by antigens delivered to the keratinocytes in the viable epidermis. DNA vaccine studies have indicated that the transfer of fibroblasts or myoblasts expressing a vaccine antigen into naïve mice induced antigen-specific CTL and antibody responses (15, 54). It is believed that cross-priming may occur when professional APCs process antigens secreted by keratinocytes or by phagocytosis of the damaged cells (2, 29, 53). Keratinocytes account for the majority of cells in the viable epidermis and should receive most of the gold particles after EPI. The antigens entering the keratinocytes may elicit immune responses by being passed to professional APCs.
Priming of CD8+ CTL responses by APCs typically requires cognate T helper cells to provide an activation signal via CD40-CD40 ligand interaction (6, 7, 27, 40, 47). When T helper cells are not present, APCs must be activated by alternative means, e.g., viral infection or adjuvants (7, 56). It is hypothesized that proinflammatory cytokines produced during viral infection or by adjuvant stimulation may directly activate APCs, circumventing the need for T helper cells. It is remarkable to see a CTL response to a minimal CTL epitope after EPI, since the NP peptide contains no T helper cell epitope. It will be interesting for future studies to investigate if T helper cells are involved in the CTL priming following EPI with the NP peptide. We have preliminary data indicating that EPI elicits proinflammatory cytokines at the vaccination site (unpublished data), which may activate APCs to prime a CTL response independently of T helper cells.
We did not expect to see an antibody response to the NP peptide following EPI, since the NP peptide is not a known B-cell epitope and the 9-amino-acid peptide is probably a little too small for class II presentation. Further, there was no evidence that the NP peptide could activate T helper cells, which is typically required for an antibody response. A previous study using i.m. injection of a minigene encoding the same NP peptide failed to demonstrate an antibody response (23). It is surprising for EPI to induce such a high IgG titer to the NP peptide. In addition to targeted delivery to LCs, EPI may enhance the immunogenicity of the peptide by presenting the peptide to the immune system as an amorphous aggregate and ligomers on the gold surface. This resembles the previously described high-density multiple antigenic peptide system (52), a method designed to improve the immunogenicity of a peptide vaccine and its ability to elicit antibodies recognizing the native antigens.
We have demonstrated in this study that EPI delivers protein antigens intracellularly to the viable epidermal cells, including LCs, and induces both the cellular and the humoral immune response. Therefore, EPI offers the potential benefits of live-attenuated vaccines without the associated safety problems. Although the scope of this study was limited to hepatitis B and influenza viruses in a mouse model, our results could have significant implications for the prophylaxis and therapy of these and many other important viral diseases in humans. It is also feasible to apply this technology to the development of vaccines for diseases caused by intracellular bacterial and protozoan pathogens, and possibly to cancer therapy.
ACKNOWLEDGMENTS
We thank Amy Bucklen, Cindy Zuleger, and Edna Gonzalez for technical assistance, Ralph Braun and Scott Umlauf for evaluation of the manuscript and helpful discussions, Y. Kawaoka (University of Wisconsin) for providing the A/Aichi virus used in the challenge study, and Randall J. Massey (University of Wisconsin) for conducting the TEM work.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:167–178. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert M L, Pearce S F, Francisco L M, Sauter B, Roy P, Silverstein R L, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αVβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 5.Becker Y. HIV-peplotion vaccine. A novel approach to vaccination against AIDS by transepithelial transport of viral peptides to Langerhans cells for induction of cytolytic T cells by HLA class I and CD1 molecules for long term protection. Adv Exp Med Biol. 1996;397:97–104. [PubMed] [Google Scholar]
- 6.Bennett S R, Carbone F R, Karamalis F, Flavell R A, Miller J F, Heath W R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 7.Bennett S R, Carbone F R, Karamalis F, Miller J F, Heath W R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergstresser P R, Fletcher C R. Surface densities of Langerhans cells in relation to rodent epidermal sites with special immunologic properties. J Investig Dermatol. 1980;74:77–80. doi: 10.1111/1523-1747.ep12519909. [DOI] [PubMed] [Google Scholar]
- 9.Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Endres R L, Erickson C A, Weis K F, McGregor M W, Kawaoka Y, Payne L G. Epidermal immunization by a needle-free powder delivery technology: immunogenicity of influenza vaccine and protection in mice. Nat Med. 2000;6:1187–1190. doi: 10.1038/80538. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Yuan J, Wang Y, Silvers W K. Distribution of ATPase-positive Langerhans cells in normal adult skin. Br J Dermatol. 1985;113:707–711. doi: 10.1111/j.1365-2133.1985.tb02406.x. [DOI] [PubMed] [Google Scholar]
- 12.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 13.Demkowicz W E, Jr, Littaua R A, Wang J, Ennis F A. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627–2631. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flohe S B, Bauer C, Moll H. Antigen-pulsed epidermal Langerhans cells protect susceptible mice from infection with the intracellular parasite Leishmania major. Eur J Immunol. 1998;28:3800–3811. doi: 10.1002/(SICI)1521-4141(199811)28:11<3800::AID-IMMU3800>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Fu T M, Ulmer J B, Caulfield M J, Deck R R, Friedman A, Wang S, Liu X, Donnelly J J, Liu M A. Priming of cytotoxic T lymphocytes by DNA vaccines: requirements for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol Med. 1997;3:362–371. [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller J T, Fuller D H, McCabe D, Haynes J R, Widera G. Immune responses to hepatitis B virus surface and core antigens in mice, monkeys, and pigs after Accell particle-mediated DNA immunization. Ann N Y Acad Sci. 1995;772:282–284. doi: 10.1111/j.1749-6632.1995.tb44760.x. [DOI] [PubMed] [Google Scholar]
- 17.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girard M. Antiviral vaccines. Med Trop. 1999;59:522–526. [PubMed] [Google Scholar]
- 19.Gurunathan S, Klinman D M, Seder R A. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 20.Gurunathan S, Wu C Y, Freidag B L, Seder R A. DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol. 2000;12:442–447. doi: 10.1016/s0952-7915(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 21.Harty J T, Bevan M J. Responses of CD8+ T cells to intracellular bacteria. Curr Opin Immunol. 1999;11:89–93. doi: 10.1016/s0952-7915(99)80016-8. [DOI] [PubMed] [Google Scholar]
- 22.Harty J T, Tvinnereim A R, White D W. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki A, Dela Cruz C S, Young A R, Barber B H. Epitope-specific cytotoxic T lymphocyte induction by minigene DNA immunization. Vaccine. 1999;17:2081–2088. doi: 10.1016/s0264-410x(98)00411-3. [DOI] [PubMed] [Google Scholar]
- 24.Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 25.Kagi D, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 26.Kagi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 27.Keene J A, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kensil C R, Wu J Y, Anderson C A, Wheeler D A, Amsden J. QS-21 and QS-7: purified saponin adjuvants. Dev Biol Stand. 1998;92:41–47. [PubMed] [Google Scholar]
- 29.Klinman D M, Sechler J M, Conover J, Gu M, Rosenberg A S. Contribution of cells at the sites of DNA vaccination to the generation of antigen-specific immunity and memory. J Immunol. 1998;160:2388–2392. [PubMed] [Google Scholar]
- 30.Koyama Y, Nagao S, Ohashi K, Takahashi H, Marunouchi T. Sex differences in the densities of epidermal Langerhans cells of the mouse. J Investig Dermatol. 1987;88:541–544. doi: 10.1111/1523-1747.ep12470104. [DOI] [PubMed] [Google Scholar]
- 31.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macklin M D, McCabe D, McGregor M W, Neumann V, Meyer T, Callan R, Hinshaw V S, Swain W F. Immunization of pigs with a particle-mediated DNA vaccine to influenza A virus protects against challenge with homologous virus. J Virol. 1998;72:1491–1496. doi: 10.1128/jvi.72.2.1491-1496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKinney E C, Streilein J M. On the extraordinary capacity of allogeneic epidermal Langerhans cells to prime cytotoxic T cells in vivo. J Immunol. 1989;143:1560–1564. [PubMed] [Google Scholar]
- 34.Morein F, Bengtsson K L. Functional aspects of ISCOMS. Immunol Cell Biol. 1998;76:295–299. doi: 10.1046/j.1440-1711.1998.00756.x. [DOI] [PubMed] [Google Scholar]
- 35.Pamer E. Antigen presentation in the immune response to infectious diseases. Clin Infect Dis. 1999;28:714–716. doi: 10.1086/515207. [DOI] [PubMed] [Google Scholar]
- 36.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 37.Pertmer T M, Roberts T R, Haynes J R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieters J. MHC class II-restricted antigen processing and presentation. Adv Immunol. 2000;75:159–208. doi: 10.1016/s0065-2776(00)75004-8. [DOI] [PubMed] [Google Scholar]
- 39.Reimann J, Schirmbeck P. Alternative pathways for processing exogenous and endogenous antigens that can generate peptides for MHC class I-restricted presentation. Immunol Rev. 1999;172:131–152. doi: 10.1111/j.1600-065x.1999.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 40.Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 41.Robbins J B, Schneerson R, Szu S C. Hypothesis: how licensed vaccines confer protective immunity. Dev Exp Med Biol. 1996;397:169–182. doi: 10.1007/978-1-4899-1382-1_22. [DOI] [PubMed] [Google Scholar]
- 42.Robbins J B, Schneerson R, Szu S C. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 43.Rock K L, Rothstein L, Gamble S, Fleischacker C. Characterization of antigen-presenting cells that present exogenous antigens in association with class I MHC molecules. J Immunol. 1993;150:438–446. [PubMed] [Google Scholar]
- 44.Rotzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J, Jung G, Rammensee H G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990;348:252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- 45.Roy M S, Wu M S, Barr L J, Fuller J T, Tussey L G, Speller S, Culp J, Burkholder J, Swain W F, Dixon R M, Widera G, Vessey R, King A, Ogg G, Gallimore A, Haynes J R, Fuller D H. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine. 2000;19:764–778. doi: 10.1016/s0264-410x(00)00302-9. [DOI] [PubMed] [Google Scholar]
- 46.Schirmbeck R, Deml L, Melber K, Wolf H, Wagner R, Reimann J. Priming of class I-restricted cytotoxic T lymphocytes by vaccination with recombinant protein antigens. Vaccine. 1995;13:857–865. doi: 10.1016/0264-410x(94)00038-o. [DOI] [PubMed] [Google Scholar]
- 47.Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 48.Sheerar M G, Easterday B C, Hinshaw V S. Antigenic conservation of H1N1 swine influenza viruses. J Gen Virol. 1989;70:3297–3303. doi: 10.1099/0022-1317-70-12-3297. [DOI] [PubMed] [Google Scholar]
- 49.Sigal L J, Crotty S, Andino R, Rock K L. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 50.Simmons C P, Mastroeni P, Fowler R, Ghaem-maghami M, Lycke N, Pizza M, Rappuoli R, Dougan G. MHC class I-restricted cytotoxic lymphocyte responses induced by enterotoxin-based mucosal adjuvants. J Immunol. 1999;163:6502–6510. [PubMed] [Google Scholar]
- 51.Stenger S, Modlin R L. Cytotoxic T cell responses to intracellular pathogens. Curr Opin Immunol. 1998;10:471–477. doi: 10.1016/s0952-7915(98)80123-4. [DOI] [PubMed] [Google Scholar]
- 52.Tam J P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timares L, Takashima A, Johnston S A. Quantitative analysis of the immunopotency of genetically transfected dentritic cells. Proc Natl Acad Sci USA. 1998;95:13147–13152. doi: 10.1073/pnas.95.22.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulmer J B, Deck R R, Dewitt C M, Donnelly J J, Liu M A. Generation of MHC class I restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology. 1996;89:59–67. doi: 10.1046/j.1365-2567.1996.d01-718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ulrich J T, Cieplak W, Paczkowski N J, Taylor S M, Sanderson S D. Induction of an antigen-specific CTL response by a conformationally biased agonist of human C5a anaphylatoxin as a molecular adjuvant. J Immunol. 2000;164:5492–5498. doi: 10.4049/jimmunol.164.10.5492. [DOI] [PubMed] [Google Scholar]
- 56.Whitmire J K, Slifka M K, Grewal I S, Flavell R A, Ahmed R. CD40-ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J Virol. 1996;70:8375–8381. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]