Abstract
Purpose
Recently, a 3D‐concentric ring trajectory (CRT)‐based free induction decay (FID)‐MRSI sequence was introduced for fast high‐resolution metabolic imaging at 7 T. This technique provides metabolic ratio maps of almost the entire brain within clinically feasible scan times, but its robustness has not yet been thoroughly investigated. Therefore, we have assessed quantitative concentration estimates and their variability in healthy volunteers using this approach.
Methods
We acquired whole‐brain 3D‐CRT‐FID‐MRSI at 7 T in 15 min with 3.4 mm nominal isometric resolution in 24 volunteers (12 male, 12 female, mean age 27 ± 6 years). Concentration estimate maps were calculated for 15 metabolites using internal water referencing and evaluated in 55 different regions of interest (ROIs) in the brain. Data quality, mean metabolite concentrations, and their inter‐subject coefficients of variation (CVs) were compared for all ROIs.
Results
Of 24 datasets, one was excluded due to motion artifacts. The concentrations of total choline, total creatine, glutamate, myo‐inositol, and N‐acetylaspartate in 44 regions were estimated within quality thresholds. Inter‐subject CVs (mean over 44 ROIs/minimum/maximum) were 9%/5%/19% for total choline, 10%/6%/20% for total creatine, 11%/7%/24% for glutamate, 10%/6%/19% for myo‐inositol, and 9%/6%/19% for N‐acetylaspartate.
Discussion
We defined the performance of 3D‐CRT‐based FID‐MRSI for metabolite concentration estimate mapping, showing which metabolites could be robustly quantified in which ROIs with which inter‐subject CVs expected. However, the basal brain regions and lesser‐signal metabolites in particular remain as a challenge due susceptibility effects from the proximity to nasal and auditory cavities. Further improvement in quantification and the mitigation of B 0/B 1‐field inhomogeneities will be necessary to achieve reliable whole‐brain coverage.
Keywords: 7 T, healthy brain, high resolution, inter‐subject reproducibility, MRS, MRSI
We have assessed the typical amplitude and variability of quantitative concentration estimates of 3D‐CRT‐FID‐MRSI at 7 T in healthy volunteers. We successfully estimated, using an internal water reference, metabolite concentrations in 44 brain ROIs that agree with previous literature and determined inter‐subject coefficients of variation. Our results are a step towards future metabolic high‐resolution atlases of the human brain.
Abbreviations
- Asp
aspartate
- CRLB
Cramér‐Rao lower bound
- CRT
concentric ring trajectory
- CSF
cerebrospinal fluid
- EPSI
echo‐planar spectroscopic imaging
- FID
free induction decay
- FWHM
full width at half maximum
- GABA
γ‐aminobutyric acid
- Gln
glutamine
- Glu
glutamate
- Gly
glycine
- GM
gray matter
- GSH
glutathione
- mIns
myo‐inositol
- MRSI
magnetic resonance spectroscopic imaging
- NAA
N‐acetylaspartate
- NAAG
N‐acetylaspartylglutamate
- ROI
region of interest
- SAR
specific absorption rate
- Ser
serine
- sIns
scyllo‐inositol
- SNR
signal‐to‐noise ratio
- SVS
single‐voxel spectroscopy
- Tau
taurine
- tCho
phosphocholine + glycerophosphocholine
- tCr
total creatine (creatine + phosphocreatine)
- T E
echo time
- T R
repetition time
- WET
water suppression enhanced through T 1 effects
- WM
white matter
1. INTRODUCTION
Proton magnetic resonance spectroscopic imaging (MRSI) in the brain based on the direct acquisition of the free induction decay (FID) signal has been introduced to overcome many technical challenges with ultra‐high‐field systems. 1 , 2 Such challenges include lower B 0/B 1 + homogeneity, more restricted specific absorption rate (SAR), and shorter T 2 times. 3 This simple acquisition scheme further reduces SAR, eliminates signal loss due to T 2 relaxation and J coupling, improves spatial selection, and enables short repetition times (T R). 1 , 2 Over the last several years, technical improvements have concentrated on faster data acquisition to reach shorter measurement times, increased brain coverage, and higher spatial resolutions, which are attractive for clinical metabolic brain mapping. 4 Starting with parallel imaging techniques, 5 , 6 , 7 , 8 echo‐planar spectroscopic imaging (EPSI), 9 and T R reduction, 10 , 11 , 12 these recent innovations have concentrated on spatial‐spectral encoding using spiral, 13 , 14 rosette, 15 and concentric ring trajectories (CRTs). 16 , 17 , 18 , 19 , 20 Research culminated in a CRT‐based 3D‐MRSI sequence at 7 T that can cover the whole brain with ~3 mm isotropic resolution, acquired in 10‐15 min. 21 , 22
7 T MRSI, with an increased signal‐to‐noise ratio (SNR) and spectral dispersion, compared with lower‐field MR scanners, enables imaging of a wide range of metabolites, eg improving the separation of N‐acetylaspartate (NAA) from N‐acetylaspartylglutamate (NAAG), glutamate (Glu) from glutamine (Gln), or glycine (Gly) from myo‐inositol (mIns). 22 , 23 , 24 Based on these benefits, 7 T MRSI has been successfully applied to research applications ranging from γ‐aminobutyric acid (GABA) mapping 25 , 26 to the resolution of metabolism in tumors, 9 , 22 , 23 , 24 , 27 multiple sclerosis, 28 and epilepsy. 29
While many technical milestones have been reached, open questions remain with respect to the stability of these whole‐brain and/or high‐resolution MRSI methods. This includes inter‐subject variations of metabolite concentrations in different brain regions, as well as intra‐subject variations over time. So far, we had only investigated these variations for FID‐MRSI in a small study at 3 T. 30 Historically, the results of MRSI quantification have varied greatly depending on study design, data evaluation, and investigated brain regions. 31 , 32 , 33 , 34 , 35 , 36 , 37 To facilitate the use of MRSI for clinical and research applications, we have to first establish the normal concentrations of metabolites within different brain regions of healthy volunteers instead of relative signals as previously.
To evaluate the performance and inter‐subject stability of our 7 T 3D‐CRT‐FID‐MRSI approach, we conducted a study with a larger subject cohort, detailed quantification estimation and regional evaluation.
1.1. Purpose
The purpose was to acquire whole‐brain, 3D‐CRT‐based FID‐MRSI at 7 T in a wider cohort (24 volunteers) than in our previous volunteer studies and to derive for the first time concentration estimates for our method, and further to assess the local MRSI data quality in an array of different small and large brain regions in order to evaluate the quantification robustness and inter‐subject variability for the concentration estimates of individual metabolites.
The results will define the performance limits of our 3D‐CRT‐based FID‐MRSI method at 7 T in regard to which metabolites can be confidently and reliably mapped in which regions of the brain.
2. EXPERIMENTAL
2.1. Subject recruitment
This study was conducted with the approval of the local institutional review board. Subjects were included in this study when no contraindications for 7 T MRI (eg claustrophobia, ferromagnetic implants, non‐ferromagnetic metal head implants >12 mm, or pregnancy) were reported. Written and informed consent was obtained from all 24 young healthy volunteers (12 male, 12 female, mean age 27 ± 6 years, Table 1). We chose a young cohort due to expected good compliance for motionlessness and easy reproducibility.
TABLE 1.
Overview of all volunteer subjects measured in this study. In some volunteers, individual ROIs had to be excluded due to poor segmentation of the GM from the WM
| Volunteer | Sex | Age [years] | Regions excluded due to poor GM/WM segmentation |
|---|---|---|---|
| 1 | Male | 24 | |
| 2 | Male | 34 | |
| 3 | Male | 23 | |
| 4 | Male | 23 | |
| 5 | Female | 33 | Parietal GM, WM, GM + WM |
| 6 | Female | 23 | |
| 7 | Female | 24 | |
| 8 | Female | 29 | Parietal GM, WM, GM + WM |
| 9 | Male | 33 | Frontal, motor, parietal GM, WM, GM + WM |
| 10 | Female | 19 | |
| 11 | Female | 21 | |
| 12 | Female | 20 | |
| 13 | Male | 31 | |
| 14 | Male | 23 | |
| 15 | Male | 38 | |
| 16 | Male | 27 | |
| 17 | Male | 35 | |
| 18 | Male | 39 | |
| 19 | Female | 22 | |
| 20 | Female | 23 | Parietal GM, WM, GM + WM |
| 21 | Female | 24 | |
| 22 | Male | 34 | |
| 23 | Female | 23 | |
| 24 | Female | 20 | Frontal, motor, parietal GM, WM, GM + WM |
2.2. 7 T MRSI measurement protocol
We performed the measurement protocol using a 7 T whole‐body MR imager (Magnetom, Siemens Healthineers, Erlangen, Germany), located at the High Field MR Centre of the Medical University of Vienna, featuring a gradient system with a 70 mT/m maximum gradient strength per direction and a 200 mT/m/s slew rate as well as a 32‐channel head receive coil array (Nova Medical, Wilmington, MA). The protocol included B 1 + maps for flip‐angle optimization, B 0 maps, and magnetization prepared two rapid gradient echoes (MP2RAGE) as the T 1‐weighted morphological MRI reference (T R 5000 ms, T E 4.13 ms, T I1 700 ms, T I2 2700 ms, 0.75 × 0.75 × 0.75 mm3 resolution, 8 min 2 s with GRAPPA factor 3).
Our 3D‐CRT‐FID‐MRSI sequence (a detailed description of sequence design and implementation can be found in Reference 21 ) employed in‐plane 2D‐CRT, and through‐plane phase encoding of an ellipsoidal k‐space resulted in a 64 × 64 × 39 matrix over a field of view of 220 × 220 × 133 mm3. This corresponds to a nominal spatial resolution of 3.4 × 3.4 × 3.4 mm3 and an effective resolution of 4.7 × 4.7 × 4.7 mm3 or 0.1 cm3. A slab of 110 mm thickness starting at the superior part the brain was selected with slices oriented in parallel to the horns of the corpus callosum. Other MRSI scan parameters included T R of 450 ms, scan time of 15 min, acquisition delay of 1.3 ms, 39°excitation flip angle (calculated as nominal average Ernst angle of NAA, tCr (creatine + phosphocreatine), tCho (phosphocholine + glycerophosphocholine), Glu, mIns 5 ), readout duration of 345 ms, spectral bandwidth of 2778 Hz, variable temporal interleaves (eg 1‐3 depending on the respective ring radii to maintain the spectral bandwidth for larger readout circles), and 7 T‐optimized WET water suppression. 10 , 38 No lipid suppression was employed during acquisition to allow a short T R. A second MRSI scan, without water suppression, included a T R of 200 ms, a readout duration/spectral bandwidth of 158 ms/606 Hz, and an Ernst angle of 27°, but with otherwise identical spatial coverage, and was acquired in 3 min 18 s as an internal water reference. This second scan was necessary as we required an unsuppressed water signal as reference.
2.3. Data processing and quantification
For offline MRSI processing, we utilized our in‐house‐developed software pipeline 39 that is based on MATLAB (MathWorks, Natick, MA), Bash (Free Software Foundation, Boston, MA), FSL (Analysis Group, FMRIB, Oxford, UK), and MINC (MINC Tools, McConnell Brain Imaging Center, Montreal, QC, Canada). This pipeline included iMUSICAL (interleaved multichannel spectroscopic data combined by matching image calibration data) coil combination based on interleaved water calibration scans, 17 , 20 k‐space reconstruction with in‐plane convolution gridding 20 , 40 (weighting non‐Cartesian points in relation to the Cartesian target point), off‐resonance correction to compensate the time delay of acquisition samples 41 and spatial Hamming filtering, as well as post‐measurement lipid signal removal by L2 regularization 42 , 43 prior to spectral quantification. Reconstruction did not include eddy current correction, as CRTs are inherently resistant to these. 18 It further did not include B 0 or B 1 correction (while differences in local flip angles can lead to differences in the effective T 1 weighting, we previously found only little impact for FID‐MRSI 44 ). A graphical overview of the reconstruction pipeline is available in Supporting Figure 1.
Each spectrum was separately quantified via LCModel (v6.3‐1, LCMODEL, Oakville, Ontario, Canada) 45 over evaluation ranges of 0.2‐1.2 ppm and 1.8‐3.88 ppm (excluding the lipid spectral range of 1.2‐1.8 ppm due to possible remaining lipid signal after L2 regularization; the upper limit of 3.88 was necessary due to water suppression effects). Our basis set, simulated in NMRscope‐B 46 accounting for the first‐order phase caused by the acquisition delay of 1.3 ms, included the following neurochemicals: tCr, tCho, NAA, NAAG, Glu, Gln, mIns, scyllo‐inositol (sIns), GABA, glutathione (GSH), Gly, taurine (Tau), cysteine, serine (Ser), and aspartate (Asp). An average macromolecular background based on prior studies with metabolite‐nulled measurements was included to improve quantification. 47 , 48 Water was quantified separately from the unsuppressed reference scan, using LCModel as well, with a water basis simulated as above. SNR (using the pseudo‐replica method with receiver noise prescans acquired at the start of the MRSI sequence 6 ) and full width at half maximum (FWHM) were calculated voxel‐wise from the LCModel fits of NAA and tCr at 3.02 ppm.
For the calculation of concentration estimates using the internal water concentration as a reference, T 1‐weighted images were segmented into gray matter (GM)/white matter (WM)/cerebrospinal fluid (CSF) using FSL's FAST tool and the segmentations were resampled to the MRSI resolution. Only the GM/WM segmentations were used for further analysis. Molar concentrations of 36.1 moL/L for GM, 43.3 moL/L for WM, and 53.8 moL/L for CSF were assumed based on literature. 49 The T 1 relaxation times, which are necessary for the correction of saturation effects at short T R, were taken from the literature or estimated as an average of known T 1 values for metabolites without published human 7 T values (Table 2). 50 , 51 Due to the use of an ultra‐short acquisition delay of 1.3 ms, we did not deem T 2 corrections necessary. Metabolite concentrations were estimated as previously described 52 , 53 : in short, for every MRSI voxel, tissue segmentation was used to calculate T 1 correction factors for all metabolites and water, according to the GM and WM content, and applied to the signal derived from the LCModel fitting results. Concentration estimates for each spectrum were then calculated as the ratio of metabolite‐to‐water signal multiplied by the local water concentration and the correction factor calculated before influenced by the respective GM/WM/CSF fraction as well as T 1 relaxation.
TABLE 2.
T 1 times for metabolites and water for GM and WM used for concentration estimates in this study. Lacking reported literature values for human in vivo MRS at 7 T, we used an average of known 7 T T 1 times (ie NAA, tCr, tCho, mIns, Glu, Gln, GSH, Tau, NAAG) for Gly and Ser
| Compound | T 1 GM [ms] | T 1 WM [ms] | Reference |
|---|---|---|---|
| Asp | 1000 | 1000 | Estimated from 51 |
| tCho | 1510 | 1320 | 50 |
| tCr 3 ppm | 1780 | 1740 | 50 |
| GABA | 1100 | 1200 | 94 |
| Glu | 1610 | 1750 | 50 |
| Gln | 1540 | 1740 | 50 |
| Gly | 1400 | 1400 | Average estimate from known compounds |
| GSH | 1140 | 1060 | 50 |
| mIns | 1280 | 1190 | 50 |
| NAA | 1535 | 1545 | , 50 average of resonances |
| NAAG | 1210 | 940 | 50 |
| Ser | 1400 | 1400 | Average estimate from known compounds |
| Tau | 2150 | 2090 | 50 |
| Water | 2000 | 1550 | 50 |
We created 3D maps of concentration estimates for all metabolites and filtered these based on a spectral quality mask, motivated by recent consensus recommendations, 54 which excluded voxels with at least one of these parameter restrictions: tCr SNR < 5, tCr FWHM > 0.15 ppm, metabolite Cramér‐Rao lower bound (CRLB) > 40%, and metabolite fit value > 13 median absolute deviations. For display purposes, these maps were interpolated tri‐linearly to fourfold resolution in MINC's register tool.
FreeSurfer (6.0, Laboratory for Computational Neuroimaging at the Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA) 55 , 56 was used for automated segmentation of structural images based on cortical and subcortical brain atlases. 56 , 57 In‐house MATLAB codes were used for mask extraction within each region of interest (ROI). We defined 55 ROIs, as shown in Table 3, including small and large structures/cortices, GM and WM regions separated and merged, and ROIs per hemisphere, in order to investigate which brain regions and ROI sizes could be reliably imaged. Metabolic maps were interpolated to the 0.8 mm resolution of structural images using nearest‐neighbor interpolation. Interpolated maps were overlaid with derived masks and mean metabolite concentrations were calculated within each ROI. 58
TABLE 3.
Evaluation of region fitting quality, including rejected regions. ROIs were separated based on the percentage of voxels that fulfilled the criteria of all of NAA, tCr, tCho, and mIns with CRLBs < 40%. Further listed is the estimated GM/WM content of our segmentations, mean ROI size (for comparison, effective voxel size = 0.1 cm3) as well as the percentage of voxels for a metabolite in an ROI that had a CRLB of <20% for NAA, tCr, tCho, and Ins, and <40% for all others
| ROI > 80% | GM [%] | WM [%] | Mean ROI size [cm3] | tCho [%] | tCr [%] | GABA [%] | Glu [%] | Gln [%] | Gly [%] | GSH [%] | mIns [%] | NAA [%] | NAAG [%] | Ser [%] | Tau [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subcortical WM (left) | 35 | 63 | 101.4 | 85 | 81 | 47 | 83 | 50 | 33 | 46 | 78 | 84 | 59 | 61 | 52 |
| Subcortical WM (right) | 35 | 62 | 101.0 | 87 | 84 | 50 | 85 | 52 | 34 | 50 | 82 | 85 | 62 | 62 | 57 |
| Subcortical WM (bilateral) | 35 | 63 | 202.4 | 86 | 82 | 48 | 84 | 51 | 33 | 48 | 80 | 85 | 61 | 61 | 54 |
| Motor subcortex WM | 31 | 68 | 18.4 | 98 | 97 | 74 | 97 | 50 | 54 | 54 | 97 | 98 | 90 | 78 | 78 |
| Motor cortex GM | 63 | 27 | 18.7 | 86 | 83 | 62 | 86 | 55 | 36 | 32 | 81 | 87 | 70 | 67 | 70 |
| Motor cortex/subcortex GM+WM | 47 | 47 | 37.1 | 92 | 90 | 68 | 92 | 53 | 45 | 43 | 89 | 92 | 80 | 72 | 74 |
| Parietal subcortex WM | 35 | 64 | 47.6 | 98 | 97 | 64 | 96 | 60 | 54 | 58 | 96 | 98 | 84 | 78 | 77 |
| Parietal cortex GM | 63 | 27 | 66.0 | 91 | 89 | 62 | 91 | 66 | 45 | 42 | 87 | 92 | 72 | 71 | 76 |
| Parietal cortex/subcortex GM + WM | 50 | 44 | 103.6 | 94 | 93 | 63 | 93 | 63 | 49 | 49 | 91 | 95 | 77 | 74 | 77 |
| Cingulate subcortex WM | 31 | 69 | 13.8 | 91 | 87 | 53 | 88 | 37 | 26 | 64 | 84 | 91 | 64 | 61 | 40 |
| Cingulate cortex GM | 77 | 23 | 11.0 | 91 | 89 | 66 | 92 | 60 | 29 | 52 | 85 | 91 | 68 | 68 | 62 |
| Cingulate cortex/subcortex GM + WM | 51 | 49 | 24.9 | 91 | 88 | 59 | 90 | 47 | 27 | 58 | 84 | 91 | 66 | 64 | 50 |
| Visual subcortex WM | 33 | 60 | 13.6 | 90 | 82 | 29 | 84 | 39 | 33 | 40 | 74 | 89 | 49 | 71 | 56 |
| Primary somatosensory subcortex WM | 39 | 57 | 7.2 | 95 | 93 | 64 | 95 | 61 | 41 | 40 | 93 | 95 | 80 | 74 | 72 |
| Primary somatosensory cortex/subcortex GM + WM | 49 | 41 | 16.8 | 87 | 84 | 57 | 87 | 57 | 36 | 32 | 83 | 88 | 69 | 66 | 67 |
| Thalamus | 42 | 58 | 7.4 | 90 | 84 | 49 | 84 | 37 | 14 | 51 | 75 | 87 | 58 | 53 | 27 |
| Putamen | 55 | 45 | 5.1 | 89 | 86 | 39 | 90 | 67 | 11 | 53 | 72 | 87 | 46 | 54 | 32 |
| Non‐lobe WM | 10 | 90 | 31.9 | 94 | 91 | 45 | 86 | 34 | 31 | 73 | 86 | 93 | 68 | 61 | 36 |
| ROI > 66% | |||||||||||||||
| Cortical GM (left) | 65 | 26 | 124.3 | 69 | 63 | 40 | 69 | 48 | 23 | 30 | 59 | 69 | 44 | 50 | 47 |
| Cortical GM (right) | 61 | 25 | 123.9 | 78 | 74 | 46 | 77 | 55 | 28 | 36 | 71 | 77 | 52 | 55 | 57 |
| Cortical GM (bilateral) | 63 | 25 | 248.2 | 73 | 69 | 43 | 73 | 52 | 25 | 33 | 65 | 73 | 48 | 52 | 52 |
| Cortical GM + subcortical WM (left) | 51 | 43 | 225.7 | 76 | 71 | 43 | 75 | 49 | 27 | 37 | 68 | 76 | 51 | 55 | 49 |
| Cortical GM + subcortical WM (right) | 49 | 43 | 224.9 | 82 | 78 | 48 | 81 | 54 | 30 | 42 | 76 | 81 | 57 | 58 | 57 |
| Cortical GM + subcortical WM (bilateral) | 50 | 43 | 443.8 | 79 | 75 | 46 | 78 | 51 | 29 | 40 | 72 | 78 | 54 | 56 | 53 |
| Subcortical GM (left) | 51 | 48 | 14.8 | 83 | 78 | 35 | 78 | 43 | 11 | 52 | 65 | 78 | 44 | 42 | 25 |
| Subcortical GM (right) | 50 | 50 | 14.4 | 77 | 68 | 32 | 68 | 40 | 9 | 45 | 57 | 69 | 32 | 39 | 19 |
| Subcortical GM (bilateral) | 51 | 49 | 29.3 | 80 | 73 | 33 | 72 | 41 | 10 | 49 | 61 | 74 | 38 | 40 | 22 |
| Auditory subcortex WM | 43 | 53 | 7.0 | 85 | 79 | 39 | 81 | 62 | 19 | 41 | 75 | 79 | 42 | 55 | 42 |
| Auditory cortex GM | 62 | 24 | 12.2 | 73 | 67 | 35 | 70 | 55 | 15 | 31 | 60 | 68 | 33 | 45 | 41 |
| Auditory cortex/subcortex GM + WM | 54 | 36 | 19.2 | 77 | 71 | 36 | 74 | 58 | 16 | 35 | 66 | 72 | 36 | 49 | 41 |
| Occipital subcortex WM | 34 | 61 | 22.4 | 86 | 79 | 30 | 80 | 40 | 30 | 39 | 73 | 84 | 45 | 64 | 51 |
| Occipital cortex GM | 59 | 28 | 29.5 | 75 | 66 | 30 | 73 | 41 | 22 | 28 | 60 | 74 | 40 | 56 | 49 |
| Occipital cortex/subcortex GM + WM | 48 | 43 | 51.9 | 80 | 71 | 30 | 76 | 41 | 26 | 33 | 65 | 78 | 42 | 59 | 50 |
| Temporal subcortex WM | 38 | 59 | 33.8 | 72 | 67 | 33 | 70 | 50 | 19 | 39 | 64 | 69 | 43 | 47 | 35 |
| Temporal cortex/subcortex GM + WM | 52 | 41 | 77.0 | 66 | 60 | 32 | 64 | 49 | 17 | 33 | 56 | 62 | 38 | 44 | 35 |
| Frontal subcortex WM | 34 | 64 | 80.8 | 82 | 80 | 50 | 83 | 51 | 28 | 46 | 77 | 82 | 57 | 55 | 50 |
| Frontal cortex GM | 62 | 23 | 96.4 | 66 | 62 | 41 | 68 | 48 | 17 | 31 | 59 | 67 | 41 | 43 | 44 |
| Frontal cortex/subcortex GM + WM | 49 | 43 | 177.2 | 73 | 70 | 45 | 74 | 50 | 22 | 38 | 67 | 74 | 48 | 48 | 47 |
| Visual cortex GM | 55 | 28 | 17.1 | 76 | 65 | 26 | 76 | 39 | 22 | 27 | 58 | 79 | 42 | 61 | 49 |
| Visual cortex/subcortex GM + WM | 45 | 43 | 30.7 | 82 | 73 | 27 | 80 | 39 | 27 | 33 | 65 | 84 | 45 | 65 | 52 |
| Primary somatosensory cortex GM | 57 | 28 | 9.6 | 80 | 77 | 52 | 81 | 55 | 32 | 26 | 75 | 82 | 61 | 60 | 63 |
| Pallidum | 13 | 87 | 1.9 | 81 | 79 | 37 | 75 | 43 | 4 | 53 | 55 | 76 | 32 | 35 | 13 |
| Hippocampus | 72 | 26 | 4.1 | 81 | 69 | 23 | 65 | 37 | 12 | 51 | 56 | 66 | 27 | 28 | 23 |
| Corpus callosum | 29 | 71 | 1.8 | 83 | 73 | 41 | 77 | 25 | 17 | 58 | 68 | 82 | 49 | 48 | 19 |
| Mean | 47 | 47 | 67.0 | 83 | 78 | 45 | 80 | 49 | 27 | 43 | 73 | 81 | 54 | 57 | 49 |
Below quality threshold: brain stem, cerebellum (left, right, bilateral), cerebral WM (left, right, bilateral), amygdala, nucleus accumbens, temporal cortex (GM), ventral diencephalon.
2.4. Data evaluation
2.4.1. General overview of measurement quality
Metabolite maps of all subjects were controlled by a reader (G.H.) for the presence of lipid and movement artifacts. Quantification of all metabolites listed in the basis set was evaluated and metabolites that were not fit in at least 10% of brain voxels were discarded from further evaluation. Cysteine was discarded as well due to general doubts about its quantification. We compared concentration estimate maps with uncorrected metabolite maps for differences in data quality and contrast. Representative spectra and metabolite maps were selected for display.
2.4.2. Quantification quality within ROIs
Regions
Region‐specific data quality was assessed by calculating the percentage of voxels within an ROI that had CRLBs less than 40% for all of NAA, tCr, tCho, and mIns. ROIs with more than 80% of voxels above that threshold were defined as good and those with 66‐79% as acceptable, and those with less than 60% were rejected. Rejected ROIs were excluded from further evaluation. To also quantify the performance of individual metabolite fitting, the percentage of voxels with CRLBs less than 20% for NAA, tCr, tCho, Ins, and CRLBs less than 40% for all others were determined for every ROI.
2.4.3. Metabolites
Only metabolites that were fit in more than 66% of voxels (mean of all regions) were considered as qualified for the main analysis, but the remaining ones are included in the Supporting Information (Supporting Tables 3 and 4).
2.4.4. Quantification estimates
For all metabolites in all qualified ROIs, regional means per subject and inter‐subject mean of means for all subjects not excluded in Section 2.4.1 were calculated. The range of observed ROI concentration estimates was compared with literature values. To facilitate the comparison with other studies that used ratios to tCr, we additionally calculated metabolite ratios to tCr.
2.4.5. Inter‐subject coefficients of variation
As a measure for the expected variability in metabolite estimates based on physiologic differences among subjects and the stability of our MRSI method, inter‐subject coefficients of variation (CVs) of mean ROI‐specific concentration estimates and ratios to tCr were calculated and compared for all qualified ROIs and metabolites based on the mean concentration estimates/ratios per subject for every ROI. This mean and its standard deviation were then used for CV calculation per ROI over all subjects.
3. RESULTS
3.1. General overview of measurement quality
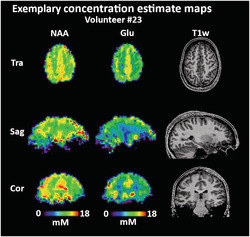
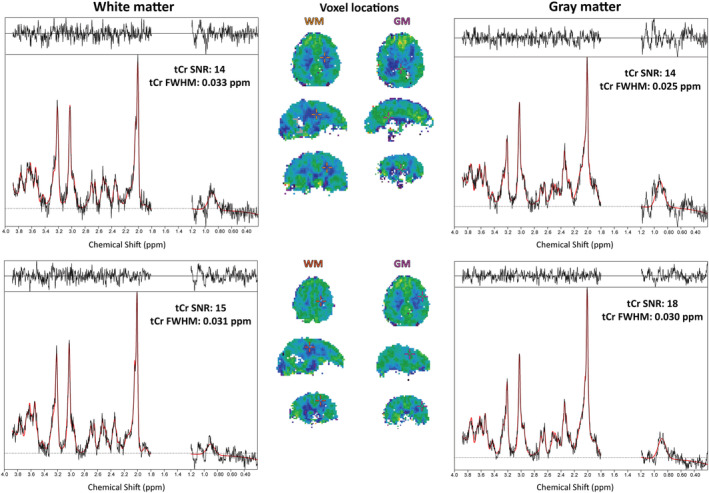
One dataset (Volunteer 1) was impaired by strong movement artifacts and had to be excluded from further analysis. In five subjects, some ROIs had to be excluded (Table 1), as the GM/WM classification based on T 1‐weighted imaging had failed in these ROIs. In these ROIs, mean concentration estimates were calculated for the remaining subjects. Total Cr SNR and FWHM over all volunteer brain voxels within the quality mask were 11 ± 5 and 0.06 ± 0.02 ppm. Asp and sIns were the only metabolites that were completely excluded from further analysis. Generally, fitting was of good quality, as illustrated by sample spectra in Figure 1 and Supporting Figure 2. Comparison of metabolite maps before T 1 correction and water referencing with concentration estimate maps showed that the concentration estimate maps showed a slight reduction of inhomogeneities and fewer outliers at the brain periphery (Figure 2). The latter was related to the inclusion of GM/WM segmentation, which removed CSF‐dominant voxels from the maps. As our extensive 3D metabolite maps cannot satisfactorily be displayed with a limited number of figures, we supplied multiple complete datasets for review (see Section 4.5).
FIGURE 1.
Sample spectra for pure GM and WM voxels in Volunteer 11. The spectra were first‐order phased for viewing convenience. GM/WM differences are especially visible for Glu. Spectral phasing is due to the 1.3 ms acquisition delay. In these examples, only small residual lipid signals remain visible
FIGURE 2.
Comparison of metabolite maps prior to water referencing and T 1 corrections with concentration estimate maps in one volunteer for the main metabolites. T 1 corrections and water referencing appear to reduce regional variations (eg the transversal tCho maps) while concentration estimates are in general accord with previous literature. Due to the inclusion of segmentation data in the map calculation, more fringe voxels are filtered out, causing the concentration estimate maps to appear smaller
3.2. Quantification quality within ROIs
3.2.1. Regions
Of the 55 segmented regions, 18 fulfill the criteria for “good,” 26 for “acceptable,” and 11 were rejected, as detailed in Table 3, including the mean ROI sizes. Concentration estimate standard deviations are generally higher for smaller regions. The rejected regions were mostly situated in the lower parts of the brain, were small, or were close to the nasal cavities/eyes. Of all metabolites, tCho, tCr, Glu, mIns, and NAA fulfilled the qualification criterion of being fit in more than 66% of voxels (Table 3). An overview over tCr SNR, FWHM, and metabolite CRLBs in relation to the resulting concentration estimate maps is given in Supporting Figure 3.
3.2.2. Metabolites
Of all cortices, the parietal, motor, and cingulate cortices performed the best.
3.3. Quantification estimates
Our concentration estimates for the five qualified metabolites over 44 ROIs are presented in Table 4 and graphically summarized in Figure 4. The highest apparent concentrations were found for tCr, Glu, and NAA. Over all ROIs, the minimum and maximum obtained means [mM] were 1.37‐2.42 for tCho, 5.93‐9.36 for tCr, 6.18‐10.14 for Glu, 4.31‐6.60 for mIns, and 7.12‐10.86 for NAA. We found a high variability between different ROIs. Our estimates were generally within the range of published research for all metabolites. A comparison of our results with previously published concentration estimates 35 , 37 , 59 is shown in Table 5. Metabolite ratios to tCr per ROI are summarized in Supporting Table 1.
TABLE 4.
Mean concentration estimates per ROI [mM] and their standard deviations for all qualified metabolites in all qualified ROIs
| ROI | tCho | tCr | Glu | mIns | NAA |
|---|---|---|---|---|---|
| Subcortical WM (left) | 1.89 ± 0.76 | 7.24 ± 2.72 | 7.68 ± 3.35 | 5.15 ± 1.92 | 9.78 ± 3.77 |
| Subcortical WM (right) | 1.95 ± 0.71 | 7.25 ± 2.50 | 7.67 ± 3.23 | 5.35 ± 1.77 | 9.67 ± 3.44 |
| Subcortical WM (bilateral) | 1.92 ± 0.73 | 7.24 ± 2.62 | 7.67 ± 3.29 | 5.24 ± 1.85 | 9.73 ± 3.61 |
| Motor subcortex WM | 2.00 ± 0.47 | 7.96 ± 1.61 | 7.90 ± 2.25 | 5.45 ± 1.15 | 10.72 2.11 |
| Motor cortex GM | 1.71 ± 0.50 | 7.55 ± 2.06 | 8.42 ± 2.58 | 5.39 ± 1.72 | 10.15 ± 2.79 |
| Motor cortex/subcortex GM+WM | 1.87 ± 0.50 | 7.78 ± 1.83 | 8.13 ± 2.41 | 5.42 ± 1.43 | 10.48 ± 2.44 |
| Parietal subcortex WM | 1.84 ± 0.51 | 7.51 ± 2.00 | 7.64 ± 2.61 | 5.54 ± 1.38 | 10.21 ± 2.66 |
| Parietal cortex GM | 1.64 ± 0.50 | 7.57 ± 2.23 | 8.55 ± 2.81 | 5.61 ± 1.63 | 10.09 ± 3.02 |
| Parietal cortex/subcortex GM+WM | 1.74 ± 0.52 | 7.54 ± 2.11 | 8.07 ± 2.74 | 5.58 ± 1.50 | 10.15 ± 2.83 |
| Cingulate subcortex WM | 2.32 ± 0.81 | 7.55 ± 2.75 | 8.06 ± 3.70 | 5.99 ± 2.01 | 10.55 ± 3.57 |
| Cingulate cortex GM | 2.22 ± 0.74 | 8.45 ± 2.62 | 10.14 ± 3.57 | 6.60 ± 2.05 | 10.86 ± 3.41 |
| Cingulate cortex/subcortex GM+WM | 2.28 ± 0.78 | 7.94 ± 2.73 | 8.98 ± 3.79 | 6.26 ± 2.05 | 10.68 ± 3.51 |
| Visual subcortex WM | 1.54 ± 0.57 | 6.74 ± 2.44 | 6.64 ± 3.18 | 4.60 ± 1.62 | 9.31 ± 3.69 |
| Primary somatosensory subcortex WM | 1.73 ± 0.50 | 7.57 ± 1.93 | 7.88 ± 2.27 | 5.21 ± 1.27 | 10.06 ± 2.57 |
| Primary somatosensory cortex/subcortex GM+WM | 1.65 ± 0.52 | 7.40 ± 2.14 | 7.90 ± 2.48 | 5.16 ± 1.46 | 9.73 ± 2.84 |
| Thalamus | 2.40 ± 0.83 | 8.81 ± 3.07 | 9.18 ± 4.15 | 6.24 ± 2.21 | 10.60 ± 4.00 |
| Putamen | 2.27 ± 0.86 | 9.36 ± 3.32 | 9.20 ± 3.59 | 5.09 ± 2.04 | 9.58 ± 3.77 |
| Non‐lobe WM | 2.42 ± 0.76 | 7.59 ± 2.53 | 6.18 ± 2.75 | 5.34 ± 1.71 | 9.88 ± 3.14 |
| Cortical GM (left) | 1.64 ± 0.76 | 6.92 ± 3.06 | 8.13 ± 3.99 | 5.06 ± 2.28 | 9.16 ± 4.47 |
| Cortical GM (right) | 1.75 ± 0.64 | 7.25 ± 2.58 | 8.35 ± 3.35 | 5.49 ± 1.91 | 9.52 ± 3.49 |
| Cortical GM (bilateral) | 1.69 ± 0.71 | 7.07 ± 2.85 | 8.23 ± 3.70 | 5.26 ± 2.12 | 9.33 ± 4.05 |
| Cortical GM+ subcortical WM (left) | 1.78 ± 0.77 | 7.09 ± 2.89 | 7.89 ± 3.67 | 5.11 ± 2.10 | 9.49 ± 4.14 |
| Cortical GM+ subcortical WM (right) | 1.86 ± 0.69 | 7.25 ± 2.54 | 7.98 ± 3.31 | 5.41 ± 1.84 | 9.60 ± 3.47 |
| Cortical GM+ subcortical WM (bilateral) | 1.81 ± 0.73 | 7.16 ± 2.73 | 7.93 ± 3.50 | 5.25 ± 1.98 | 9.54 ± 3.83 |
| Subcortical GM (left) | 2.32 ± 0.95 | 8.61 ± 3.64 | 8.20 ± 4.06 | 5.58 ± 2.48 | 9.50 ± 4.21 |
| Subcortical GM (right) | 2.02 ± 0.97 | 7.40 ± 3.68 | 7.24 ± 4.16 | 4.99 ± 2.48 | 7.83 ± 4.32 |
| Subcortical GM (bilateral) | 2.17 ± 0.97 | 7.99 ± 3.72 | 7.72 ± 4.15 | 5.28 ± 2.51 | 8.66 ± 4.35 |
| Auditory subcortex WM | 1.91 ± 0.72 | 7.13 ± 2.48 | 8.14 ± 3.23 | 5.13 ± 1.79 | 8.81 ± 3.45 |
| Auditory cortex GM | 1.67 ± 0.69 | 6.69 ± 2.60 | 8.10 ± 3.43 | 4.88 ± 1.95 | 8.13 ± 3.56 |
| Auditory cortex/subcortex GM+WM | 1.78 ± 0.71 | 6.89 ± 2.56 | 8.11 ± 3.34 | 4.99 ± 1.88 | 8.44 ± 3.53 |
| Occipital subcortex WM | 1.61 ± 0.68 | 6.61 ± 2.66 | 6.68 ± 3.35 | 4.59 ± 1.79 | 8.83 ± 3.91 |
| Occipital cortex GM | 1.46 ± 0.65 | 6.55 ± 2.92 | 7.26 ± 3.72 | 4.57 ± 2.00 | 8.58 ± 4.31 |
| Occipital cortex/subcortex GM+WM | 1.54 ± 0.67 | 6.58 ± 2.79 | 6.95 ± 3.55 | 4.58 ± 1.89 | 8.71 ± 4.12 |
| Temporal subcortex WM | 1.95 ± 0.89 | 6.89 ± 3.17 | 7.30 ± 3.68 | 4.83 ± 2.15 | 8.56 ± 4.06 |
| Temporal cortex/subcortex GM+WM | 1.84 ± 0.87 | 6.74 ± 3.19 | 7.47 ± 3.91 | 4.82 ± 2.21 | 8.33 ± 4.63 |
| Frontal subcortex WM | 1.99 ± 0.79 | 7.39 ± 2.69 | 7.99 ± 3.41 | 5.20 ± 1.92 | 9.91 ± 3.81 |
| Frontal cortex GM | 1.74 ± 0.79 | 6.98 ± 3.01 | 8.29 ± 3.98 | 5.22 ± 2.29 | 9.44 ± 3.98 |
| Frontal cortex/subcortex GM+WM | 1.88 ± 0.80 | 7.21 ± 2.84 | 8.12 ± 3.67 | 5.21 ± 2.09 | 9.70 ± 3.89 |
| Visual cortex GM | 1.37 ± 0.55 | 6.74 ± 2.72 | 7.28 ± 3.46 | 4.53 ± 1.81 | 9.17 ± 3.99 |
| Visual cortex/subcortex GM+WM | 1.46 ± 0.57 | 6.73 ± 2.57 | 6.90 ± 3.32 | 4.56 ± 1.71 | 9.23 ± 3.83 |
| Primary somatosensory cortex GM | 1.57 ± 0.53 | 7.22 ± 2.33 | 7.91 ± 2.69 | 5.11 ± 1.63 | 9.37 ± 3.06 |
| Pallidum | 2.11 ± 0.94 | 8.88 ± 3.75 | 8.18 ± 4.30 | 4.31 ± 2.03 | 8.43 ± 4.06 |
| Hippocampus | 2.18 ± 1.01 | 7.37 ± 3.35 | 6.83 ± 3.67 | 5.78 ± 2.74 | 7.12 ± 3.73 |
| Corpus callosum | 2.07 ± 0.88 | 5.93 ± 2.62 | 6.54 ± 3.46 | 5.25 ± 2.31 | 9.50 ± 4.28 |
| Mean | 1.88 | 7.37 | 7.85 | 5.23 | 9.43 |
| Min | 1.37 | 5.93 | 6.18 | 4.31 | 7.12 |
| Max | 2.42 | 9.36 | 10.14 | 6.60 | 10.86 |
FIGURE 4.
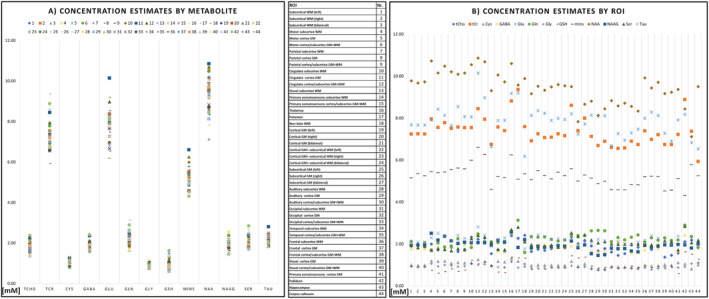
Scatterplots of the metabolite concentration estimates presented in Table 4 and Supporting Table 3. A, Estimated concentrations per metabolite; B, estimated concentrations per ROI
TABLE 5.
Overview of concentration estimation results of this study compared with those in the literature. A breakdown of reference concentration estimate and literature source ROIs is given in Section 4. An analysis per ROI is presented in Table 7
| Metabolite | This study: lowest [mM] | Literature: lowest [mM] | This study: highest [mM] | Literature: highest[mM] | Agreement | References |
|---|---|---|---|---|---|---|
| tCho | 1.4 | 0.5 | 2.4 | 4 | High | Kreis 1993, Hetherington 1994, Pouwels 1998, Gasparovic 2006, Minati 2010, van de Bank 2015, Lecocq 2015, Volk 2018 |
| tCr | 5.9 | 1.8 | 9.4 | 14 | High | Kreis 1993, Hetherington 1994, Pouwels 1998, Gasparovic 2006, Minati 2010, van de Bank 2015, Lecocq 2015, Volk 2018, Dhamala 2019 |
| GABA | 1.6 | 1.3 | 2.5 | 3.5 | High | van Zijl 1997, van de Bank 2015, Dhamala 2019, Gonen 2020 |
| Glu | 6.2 | 5 | 10.1 | 12 | High | Pouwels 1998, Choi 2006, van de Bank 2015, Volk 2018, Dhamala 2019, Gonen 2020 |
| Gln | 1.6 | 1 | 3.1 | 5 | High | Pouwels 1998, Choi 2006, van de Bank 2015, Dhamala 2019 |
| Gly | 0.8 | 1 | 1.2 | 1 | High | van Zijl 1997 |
| GSH | 0.6 | 0.7 | 1.6 | 2.2 | Moderate | Terpstra 2005, Emir 2011, van de Bank 2015, Rai 2018, Dhamala 2019, Gonen 2020 |
| mIns | 4.3 | 3 | 6.6 | 9 | High | Kreis 1993, Pouwels 1998, Minati 2010, van de Bank 2015, Lecocq 2015, Volk 2018, Dhamala 2019 |
| NAA | 7.1 | 5 | 10.9 | 17 | High | Kreis 1993, Hetherington 1994, Pouwels 1998, Gasparovic 2006, Minati 2010, van de Bank 2015, Lecocq 2015, Volk 2018, Dhamala 2019 |
| NAAG | 1.5 | 0.5 | 2.6 | 3 | High | Pouwels 1997, Pouwels 1998, Edden 2007, Dhamala 2019 |
| Ser | 1.7 | — | 2.9 | — | — | — |
| Tau | 1.8 | 1.5 | 2.8 | 2.3 | Moderate | van Zijl 1997, van de Bank 2015 |
3.4. Inter‐subject coefficients of variation
The inter‐subject CVs in Table 6 show a good comparability of the regional analyses between subjects for most cases. These CVs were the lowest for tCr and NAA and, generally, in the range of 10‐20%. In the majority of “good” ROIs, tCho, tCr, Glu, mIns, and NAA CVs were 10% or less. Figure 3 illustrates these findings over multiple subjects. The CVs for ratios to tCr (Supporting Table 2) were very similar, with differences between the means over all ROIs not exceeding 4%.
TABLE 6.
Inter‐subject CVs of the concentration estimates per ROI displayed in Table 4. As expected, higher SNR/concentration metabolites corresponded to the lowest CVs
| ROI | tCho [%] | tCr [%] | Glu [%] | mIns [%] | NAA [%] |
|---|---|---|---|---|---|
| Subcortical WM (left) | 7 | 7 | 7 | 8 | 7 |
| Subcortical WM (right) | 6 | 6 | 8 | 6 | 6 |
| Subcortical WM (bilateral) | 6 | 6 | 7 | 7 | 6 |
| Motor subcortex WM | 8 | 7 | 7 | 7 | 7 |
| Motor cortex GM | 6 | 8 | 8 | 8 | 9 |
| Motor cortex/subcortex GM + WM | 7 | 7 | 7 | 8 | 8 |
| Parietal subcortex WM | 11 | 10 | 10 | 10 | 10 |
| Parietal cortex GM | 10 | 10 | 11 | 10 | 11 |
| Parietal cortex/subcortex GM + WM | 10 | 10 | 11 | 10 | 11 |
| Cingulate subcortex WM | 9 | 10 | 12 | 10 | 9 |
| Cingulate cortex GM | 9 | 10 | 12 | 10 | 10 |
| Cingulate cortex/subcortex GM + WM | 9 | 10 | 12 | 10 | 9 |
| Visual subcortex WM | 13 | 15 | 14 | 14 | 8 |
| Primary somatosensory subcortex WM | 8 | 9 | 8 | 10 | 8 |
| Primary somatosensory cortex/subcortex GM + WM | 8 | 9 | 8 | 10 | 9 |
| Thalamus | 19 | 20 | 21 | 19 | 16 |
| Putamen | 9 | 9 | 15 | 13 | 11 |
| Non‐lobe WM | 10 | 7 | 15 | 10 | 8 |
| Cortical GM (left) | 7 | 8 | 7 | 8 | 8 |
| Cortical GM (right) | 6 | 6 | 7 | 6 | 6 |
| Cortical GM (bilateral) | 6 | 6 | 7 | 7 | 7 |
| Cortical GM + subcortical WM (left) | 7 | 7 | 7 | 8 | 8 |
| Cortical GM + subcortical WM (right) | 6 | 6 | 7 | 6 | 6 |
| Cortical GM + subcortical WM (bilateral) | 6 | 6 | 7 | 7 | 6 |
| Subcortical GM (left) | 10 | 9 | 14 | 13 | 10 |
| Subcortical GM (right) | 15 | 15 | 16 | 14 | 15 |
| Subcortical GM (bilateral) | 12 | 11 | 15 | 13 | 11 |
| Auditory subcortex WM | 8 | 9 | 9 | 10 | 9 |
| Auditory cortex GM | 7 | 8 | 8 | 8 | 9 |
| Auditory cortex/subcortex GM + WM | 7 | 8 | 9 | 9 | 9 |
| Occipital subcortex WM | 12 | 12 | 12 | 12 | 7 |
| Occipital cortex GM | 11 | 12 | 10 | 12 | 7 |
| Occipital cortex/subcortex GM + WM | 11 | 12 | 11 | 12 | 6 |
| Temporal subcortex WM | 6 | 8 | 7 | 9 | 8 |
| Temporal cortex/subcortex GM + WM | 5 | 8 | 7 | 8 | 8 |
| Frontal subcortex WM | 8 | 7 | 10 | 9 | 8 |
| Frontal cortex GM | 8 | 7 | 9 | 8 | 9 |
| Frontal cortex/subcortex GM + WM | 8 | 7 | 9 | 9 | 8 |
| Visual cortex GM | 13 | 17 | 12 | 15 | 8 |
| Visual cortex/subcortex GM + WM | 13 | 16 | 12 | 14 | 7 |
| Primary somatosensory cortex GM | 8 | 10 | 9 | 10 | 9 |
| Pallidum | 14 | 12 | 24 | 15 | 19 |
| Hippocampus | 16 | 17 | 18 | 18 | 16 |
| Corpus callosum | 16 | 16 | 19 | 15 | 17 |
| Mean | 9 | 10 | 11 | 10 | 9 |
| Min. | 5 | 6 | 7 | 6 | 6 |
| Max. | 19 | 20 | 24 | 19 | 19 |
FIGURE 3.
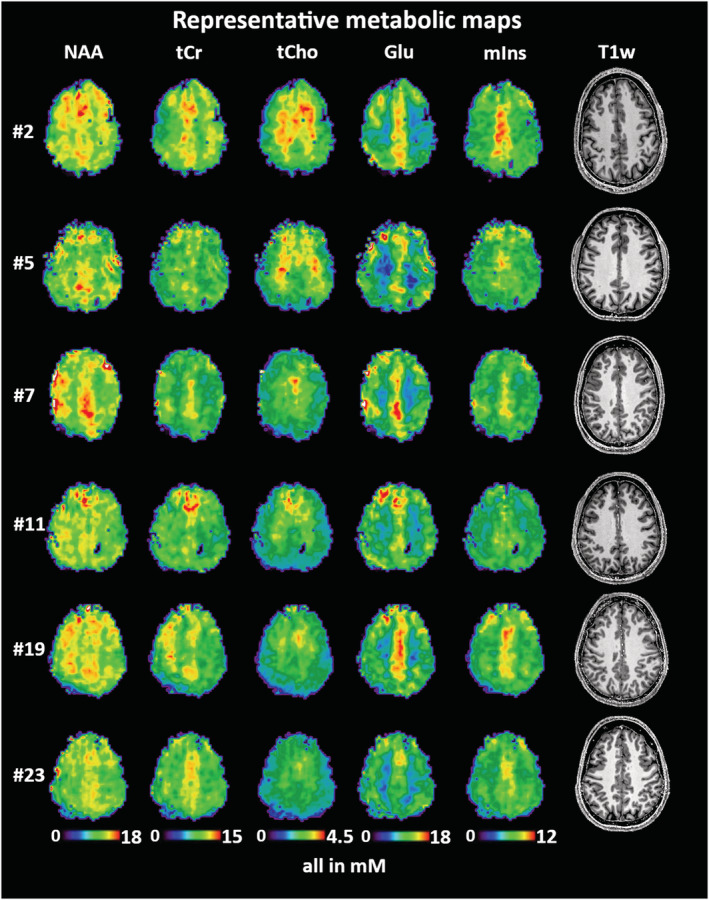
Visualization of data consistency of concentration estimates in six volunteer subjects for NAA, tCr, tCho, Glu, and mIns. Presented is a transversal MRSI slice location directly above the ventricles in all subjects. Full map datasets for a detailed inspection of all metabolite concentration estimate maps of Volunteers 7, 11, 13, 19, and 23 are available at Zenodo (https://doi.org/10.5281/zenodo.5006923)
4. DISCUSSION
We have assessed the average and region‐specific concentration estimates, as well as their inter‐subject variability, for five neuro‐metabolites that can be reliably mapped in the human brain using our 3D‐CRT‐based FID‐MRSI sequence at 7 T. To improve upon previous work, 21 we have expanded the subject cohort, also evaluated less abundant metabolites, and assessed concentration estimates instead of ratios and data quality over a large number of automatically segmented brain ROIs to provide a more detailed understanding of the performance of 7 T 3D‐CRT‐based FID‐MRSI. The additional inclusion of T 1 corrections and internal water referencing allowed a more quantitative assessment (ie of concentration estimates). In summary, we found our method to yield acceptable results in 23 of 24 volunteer subjects for five metabolites in 44 predefined ROIs. Our concentration estimates are in the range of previous reports, while inter‐subject CVs indicated a good level of stability in many of these metabolites and ROIs. Still, the quantification quality of GABA, Gln, Gly, GSH, NAAG, Ser, and Tau in healthy subjects cannot be considered sufficient for this MRSI application, necessitating different approaches or methodological improvements if these are required. These results are important to define the limits of stability, sensitivity, and regional reliability for our MRSI method for future research applications. As the sum of our generated imaging data is hard to convey within few figures, we invite the readers to look at the supplementary full datasets provided by us on Zenodo.
To contextualize our resultant metabolite distributions, we conducted an extensive comparison with previous research. Concentration estimates for many metabolites in many brain regions have been reported over the last decades, sometimes with contradicting results due to different processing methods, subject cohorts, partial volume effects, acquisition schemes (eg MRSI or single‐voxel spectroscopy, SVS), and scanner types. Different quantification algorithms are known to affect reported results. 60 Dhamala et al 37 found that correlations varied strongly for different metabolites between different MRS methods in the same subjects. A comparison with our 7 T‐FID‐MRSI method with 3.4 mm nominal isometric resolution, a T R of 450 ms, and an acquisition delay of 1.3 ms remains challenging. Nonetheless, our results are, overall, consistent with previously reported concentration estimates 31 , 32 , 33 , 34 , 35 , 36 , 37 , 53 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 as summarized in Table 5, and mostly found in the middle or lower range of references. For metabolites separable in our study, such as Glu/Gln and NAA/NAAG, they individually agree with previous literature (Tables 5 and7), and further comparing their sums agrees well with studies that cannot separate them.
TABLE 7.
Comparison of this study's concentration estimates and their standard deviations in specific ROIs to literature. The details of this comparison are elaborated on in Section 4
| Source | tCho | tCr | Glu | mIns | NAA |
|---|---|---|---|---|---|
| Occipital GM | |||||
| This study | 1.46 ± 0.65 | 6.55 ± 2.92 | 7.26 ± 3.72 | 4.57 ± 2.00 | 8.58 ± 4.31 |
| Kreis 1993 | 1.41 ± 0.05 | 7.95 ± 0.11 | — | 6.24 ± 0.21 | 9.06 ± 0.12 |
| Pouwels 1998 | 0.88 ± 0.10 | 6.90 ± 0.70 | 8.60 ± 1.10 | 4.10 ± 0.60 | 9.20 ± 0.90 |
| Lecocq 2015 | 1.20‐1.40 | 7.30 8.70 | — | 3.50‐4.60 | 9.50‐12.10 |
| Occipital WM | |||||
| This study | 1.61 ± 0.68 | 6.61 ± 2.66 | 6.68 ± 3.35 | 4.59 ± 1.79 | 8.83 ± 3.91 |
| Pouwels 1998 | 1.64 ± 0.21 | 5.50 0.80 | 6.00 ± 1.20 | 4.10 ± 0.80 | 7.80 ± 0.90 |
| Lecocq 2015 | 1.20‐1.40 | 7.30 8.70 | — | 3.50‐4.60 | 9.50‐12.10 |
| Frontal GM | |||||
| This study | 1.74 ± 0.79 | 6.98 ± 3.01 | 8.29 ± 3.98 | 5.22 ± 2.29 | 9.44 ± 3.98 |
| Pouwels 1998 | 1.38 ± 0.17 | 6.40 ± 0.70 | 8.50 ± 1.00 | 4.30 ± 0.90 | 7.70 ± 1.00 |
| Lecocq 2015 | 1.50 ± 2.50 | 6.70 ± 8.60 | — | 4.00 ± 7.80 | 9.50 ± 12.50 |
| Frontal WM | |||||
| This study | 1.99 ± 0.79 | 7.39 ± 2.69 | 7.99 ± 3.41 | 5.20 ± 1.92 | 9.91 ± 3.81 |
| Pouwels 1998 | 1.78 ± 0.41 | 5.70 ± 0.50 | 7.00 ± 2.60 | 3.80 ± 0.90 | 8.10 ± 0.90 |
| Minati 2010 | 3.60 ± 0.80 | 11.50 ± 2.40 | — | 7.60 ± 2.00 | 14.20 ± 2.00 |
| Lecocq 2015 | 1.50 ± 2.50 | 6.70 ± 8.60 | — | 4.00 ± 7.80 | 9.50 ± 12.50 |
| Parietal lobe | |||||
| This study | 1.74 ± 0.52 | 7.54 ± 2.11 | 8.07 ± 2.74 | 5.58 ± 1.50 | 10.15 ± 2.83 |
| Lecocq 2015 | 0.80‐1.10 | 4.10‐6.50 | — | 1.90‐3.20 | 8.30‐10.70 |
| Volk 2018 | 1.65 ± 0.05 | 7.54 ± 0.14 | 12.55 ± 0.22 | 3.70 ± 0.08 | 11.59 ± 0.13 |
| Thalamus | |||||
| This study | 2.40 ± 0.83 | 8.81 ± 3.07 | 9.18 ± 4.15 | 6.24 ± 2.21 | 10.60 ± 4.00 |
| Minati 2010 | 3.40 ± 0.80 | 12.00 ± 1.10 | — | 6.60 ± 1.80 | 16.30 ± 2.00 |
| Lecocq 2015 | 1.20‐1.30 | 5.90‐6.60 | — | 3.00‐3.30 | 5.90‐6.60 |
| Temporal cortex | |||||
| This study | 1.84 ± 0.87 | 6.74 ± 3.19 | 7.47 ± 3.91 | 4.82 ± 2.21 | 8.33 ± 4.63 |
| Minati 2010 | 3.60 ± 1.10 | 12.00 ± 4.00 | — | 7.90 ± 3.00 | 14.10 ± 2.50 |
| Lecocq 2015 | 1.40‐2.30 | 7.50‐8.80 | — | 4.20‐5.30 | 8.80‐10.90 |
| Cingulate subcortex | |||||
| This study | 2.28 ± 0.78 | 7.94 ± 2.73 | 8.98 ± 3.79 | 6.26 ± 2.05 | 10.68 ± 3.51 |
| Hetherington 1994 | 2.30 ± 0.40 | 7.70 ± 0.90 | — | — | 13.50 ± 0.90 |
| van de Bank 2015 | 1.30 ± 0.10 | 8.10 ± 0.50 | 9.40 ± 0.80 | 6.40 ± 0.60 | 12.10 ± 1.00 |
| Lecocq 2015 | 1.00‐2.50 | 5.90‐9.30 | — | 3.20‐6.40 | 7.90‐11.50 |
| Gonen 2020 | — | — | 10.20 ± 1.80 | — | — |
Some publications used more ROIs without GM/WM separation per region and the results are therefore presented as a range of their findings.
Going from overall reported values to specific ROIs, as compared in Table 7, we found similar concentrations (within the respective standard deviations of each other) in occipital GM and WM, 31 , 32 , 35 frontal WM, 32 , 35 parietal lobe, 36 thalamus, 34 temporal cortex, 35 and cingulate cortex 33 , 63 for most studies. We found disagreements beyond a standard deviation for the cingulate cortex with van de Bank et al, 68 for the frontal WM, thalamus, and temporal cortex with Minati et al, 34 and for the parietal cortex as well as the thalamus with Lecocq et al. 35
For metabolite ratios to tCr, we also compared our results with the 7 T MRSI results of Bhogal et al, 70 as seen in Table 8. Over the six compared ROIs, our ratios are consistently higher for all except GSH/tCr, which is mixed. The effect is most pronounced for Ins + Gly. The generally higher ratios could be sourced in lower quantification estimates of tCr or differences in the MRSI acquisition.
TABLE 8.
Comparison of this study's metabolite ratios and standard deviations of tCho, Glu, Glu + Gln, NAA + NAAG, Ins + Gly, and GSH to tCr in six ROIs compared with similar 7 T MRSI results of Reference. 70 The results of our study are consistently higher for all except GSH/tCr, with two higher/lower/same ratio regions each
| Source | tCho/tCr | Glu/tCr | (Glu + Gln)/tCr | (NAA + NAAG)/tCr | (Ins + Gly)/tCr | GSH/tCr |
|---|---|---|---|---|---|---|
| (Cortical) GM | ||||||
| This study | 0.24 ± 0.01 | 1.17 ± 0.09 | 1.50 ± 0.10 | 1.59 ± 0.09 | 0.86 ± 0.04 | 0.11 ± 0.01 |
| Bhogal 2020 | 0.17 ± 0.05 | 0.97 ± 0.20 | 1.20 ± 0.27 | 1.13 ± 0.28 | 0.41 ± 0.16 | 0.15 ± 0.06 |
| (Subcortical) WM | ||||||
| This study | 0.27 ± 0.02 | 1.07 ± 0.09 | 1.34 ± 0.10 | 1.62 ± 0.09 | 0.85 ± 0.04 | 0.12 ± 0.01 |
| Bhogal 2020 | 0.22 ± 0.04 | 0.72 ± 0.14 | 0.88 ± 0.17 | 1.27 ± 0.20 | 0.41 ± 0.11 | 0.15 ± 0.04 |
| Corpus callosum | ||||||
| This study | 0.35 ± 0.03 | 1.13 ± 0.28 | 1.38 ± 0.10 | 1.97 ± 0.27 | 1.06 ± 0.13 | 0.21 ± 0.04 |
| Bhogal 2020 | 0.23 ± 0.04 | 0.73 ± 0.13 | 0.90 ± 0.16 | 1.26 ± 0.22 | 0.45 ± 0.09 | 0.17 ± 0.04 |
| Pallidum | ||||||
| This study | 0.24 ± 0.02 | 0.90 ± 0.17 | 1.20 ± 0.20 | 1.15 ± 0.15 | 0.56 ± 0.07 | 0.17 ± 0.03 |
| Bhogal 2020 | 0.18 ± 0.01 | 0.58 ± 0.10 | 0.71 ± 0.12 | 0.85 ± 0.19 | 0.31 ± 0.06 | 0.15 ± 0.04 |
| Thalamus | ||||||
| This study | 0.28 ± 0.03 | 1.07 ± 0.24 | 1.34 ± 0.26 | 1.53 ± 0.16 | 0.83 ± 0.14 | 0.15 ± 0.04 |
| Bhogal 2020 | 0.22 ± 0.03 | 0.75 ± 0.10 | 0.93 ± 0.12 | 1.07 ± 0.12 | 0.36 ± 0.09 | 0.15 ± 0.02 |
| Putamen | ||||||
| This study | 0.24 ± 0.01 | 0.98 ± 0.12 | 1.30 ± 0.14 | 1.23 ± 0.08 | 0.63 ± 0.05 | 0.14 ± 0.03 |
| Bhogal 2020 | 0.19 ± 0.02 | 0.78 ± 0.10 | 0.97 ± 0.13 | 0.84 ± 0.14 | 0.32 ± 0.06 | 0.15 ± 0.03 |
For some metabolites, previous concentration estimates are only reported sparsely. Our 0.76‐1.16 mM of Gly align well to the 1.02 mM in Reference 64 and to a Gly/tCr of 0.14 in Reference, 71 which compares well with our range of 0.08‐0.17 for Gly/tCr. We established concentrations of 1.68‐2.85 mM for Ser, with a ratio to tCr of 0.8 in Reference, 72 which that was published in the MRS literature. Our Ser/tCr of 0.21‐0.36 is notably lower. For Tau, we found 1.77‐2.81 mM, which is higher than the 1.48 mM reported in Reference, 64 but well aligned with the 2.3 mM for Tau + glucose in Reference. 68
Considering the multitude of applied methods and overall limited sample sizes, our results are in general agreement with the current state of knowledge in the field but are for the first time based on concentration estimation for high‐resolution 7 T FID‐MRSI. We see a further need to investigate metabolites such as GSH, Ser, and Tau, which are difficult to quantify and for which MRS‐based concentration estimates are scarce.
Our inter‐subject CVs were the smallest for metabolites with the highest SNR (ie, NAA, tCr, tCho, Glu, and mIns are in the <10% range) but approached 30% for other metabolites in some ROIs (see Table 6). Comparison with the literature is difficult, as most studies report intra‐subject or inter‐system CVs, such as van de Bank et al, 68 who reported CVs for SVS on four different 7 T systems in the posterior cingulate cortex of 3‐4% for NAA, tCho, tCr, mIns, and Glu, 22.2% for GABA, 14.4% for GSH, and 8.8% for Gln. Another example, but at 3 T, is the study by Zhang et al, 73 who calculated intra‐subject CVs for whole‐brain EPSI in 10 volunteers over three scans in 47 ROIs and found NAA CVs of 3.3%‐17.8%, tCho CVs of 3.7%‐31.0%, tCr CVs of 3.1%‐18.0% (with mean ROI CVs < 10%), and mIns CVs of 5.9%‐54.0%. Inter‐subject CVs for metabolic ratios at 3 T were reported by Veenith et al, 74 with mean CVs of 21.24% for tCho/tCr and 13.30% for tNAA/tCr. Except for higher mIns CVs, these results are similar to our findings, but our 7 T MRSI featured a higher resolution and more quantifiable metabolites and was also affected by additional physiologic inter‐subject variation. Considering that the CV calculation did not account for diurnal effects, 36 or age 75 , 76 , 77 and sex differences, 78 , 79 these results seem convincing but still include methodological artifacts such as subject motion. Intra‐subject CVs from a test‐retest study will be necessary to complete the picture. In another MRSI study at 3 T, we found that the application of motion correction improved the CVs of metabolite ratios to tCr by 30%. 30 Another source of local variability would be the combination of T 1 weighting with our short T R and a lack of knowledge of local tissue metabolite T 1 values. 52 , 80 Although we tried to correct our T 1 estimates and reference water concentrations for voxel‐wise GM/WM fractions, we assume that additional variation exists based on this. 53 More precise concentration estimates could be obtained via the direct mapping of tissue water content. 81 Due to our echo‐less acquisition approach with negligible acquisition delay, our results can be considered to be robust to T 2 effects. This is a potential advantage in the study of the aging brain or of pathologies that can cause local iron deposits, which affect metabolite and water T 2 values. 82
4.1. Limitations
Our study has some limitations. T 1 values for multiple metabolites had to be estimated without previous reports. The assumption of a single metabolite T 1 over the whole brain is also a rough approximation. This could have biased the estimated concentrations.
Quantification is further limited by the precision of the internal water referencing, which relies on the assumption of tissue water relaxation times and concentrations, effective local excitation, and water signal quantification. Beyond the general variability of MRS quantification results based on the fit model parameters, L2 regularization is also known to influence metabolite signal estimation, especially NAA, 83 even if just by removing lipid signals. The comparison of quantification estimates between different field strengths, acquisition schemes, processing pipelines, resolutions, and brain segmentations limits the comparability to the even subset of MRS studies that report concentration estimates.
Our study is limited to the analysis of inter‐subject variations and does not, therefore, report on intra‐subject variations. Measuring intra‐subject variation could also help to separate the more subject‐specific (eg physiological) from the method‐specific contributions to variability.
The exclusion of 11 ROIs, predominantly basal brain regions such as brain stem or cerebellum, from further analysis was necessary due to the lack of spectral fit confidence, limiting the mappable brain coverage. This shows that B 0‐ and B 1‐field inhomogeneities (the first caused by the proximity to the nasal and auditory cavities, the second by the limitations of single channel transmit coils at 7 T) remain a significant ultra‐high‐field challenge, which will have to be resolved via hardware improvements 84 , 85 , 86 and/or further improved high‐resolution approaches. 87
Filtering of outliers based on a set of rigid criteria is insufficient. In the future, automated quality assessment of voxels based on deep learning will be necessary to evaluate datasets of this scale adequately. The necessary interpolation between MRSI and reference imaging combined with MRSI partial volume effects, even at our high resolution, are further confounding factors for the regional analysis as relevant GM/WM fractions remain within the ROIs (Table 3), reducing the expected GM/WM contrast, eg for Glu. While we obtained results for difficult‐to quantify metabolites in healthy tissue (ie GABA, Gln, Gly, GSH, NAAG, Ser, and Tau), the percentage of voxels within the quality criteria remained overall low (Table 1).
Subject motion is another factor not yet accounted for in our study, and significant improvements are expected in stability, 88 , 89 which is of particular importance for studies in children and elderly patients. 26 In particular, real‐time correction has been shown to significantly enhance data quality in high‐resolution 3D‐CRT‐based FID‐MRSI at 3 T. 30
4.2. Conclusions/outlook
We have stablished the brain region‐specific concentration estimates and their variability in a large number of healthy young volunteers for our whole‐brain MRSI‐based metabolic maps at 7 T for the first time. While not all brain ROIs performed well enough to be considered, especially basal regions such as the cerebellum, we could successfully quantify five metabolites—tCho, tCr, NAA, Glu, and Ins—in 44 ROIs, with all others not being quantified with a sufficient quality in a substantial number of voxels. Our estimated concentrations are consistent with previous research.
This was a necessary first step to define the reliability and to guide future basic and clinical research of the brain metabolism and to show the capability of our high‐resolution 3D‐MRSI technique in the current discussion of MRSI standardization. 52 , 90 , 91 Our results can guide future study planning, targeting specific brain regions and metabolites of interest on the one hand and the eventual development of a 7 T‐MRSI based metabolic brain atlas on the other. The next avenues of research could be the investigation of intra‐subject variability, improved quantification by direct water concentration mapping, 81 also in pathologies, and better definition of specific relaxation times. With these improvements, the metabolites that currently lack reliability could be reevaluated and an in‐depth study of sex and age differences could be carried out. In summary, we have shown new insights into the expectable results and stability of our fast high‐resolution MRSI at 7 T, but this approach still requires more sophistication.
CONFLICT OF INTEREST
We have no conflicts of interest to report.
SUPPLEMENTARY MATERIALS/DATA AVAILABILITY STATEMENT
Individual subject concentration estimate map datasets in MINC and NIFTI format including SNR, FWHM, and CRLB maps are available on Zenodo: https://doi.org/10.5281/zenodo.5006923. 92 A summary of the MRSI method used according to the MRSinMRS experts' consensus 93 can be found in Supporting Table 5.
Supporting information
Figure S1. Flow chart of the post‐processing and evaluation pipeline. Details for every step can be found in the Experimental section.
Figure S2. More sample spectra from different volunteers and brain regions, of different qualities, including a spectrum of the excluded volunteer featuring a dominant lipid artefact at the edge of the excluded fitting range.
Figure S3. Examples of MRSI fitting quality markers (tCr SNR and FWHM, metabolite CRLBs) in two volunteers. A) complements data shown in Figure 2. As the CRLB maps show the difficulty of expressing the dynamic range of values, we have also made CRLB maps available for the subject in the supplementary data available at Zenodo.
Table S1. Mean ratios to tCr and their standard deviations for all other metabolites, based on the overall ROI concentration estimates.
Table S2. Inter‐subject CVs for ratios to tCr per ROI, with high similarity to concentration estimate CVs, as described in Tbl.5.
Table S3. Mean concentration estimates per ROI [mM] and their standard deviations for all quantified metabolites in all qualified ROIs.
Table S4. Inter‐subject CVs of the concentration estimates per ROI displayed in Sup.Tbl. 3. As expected, higher SNR/concentration metabolites corresponded to the lowest CVs.
Table S5. A summary of the MRSI method according to the MRSinMRS expert's consensus proposed standard93.
ACKNOWLEDGEMENTS
This work was supported by the Austrian Science Fund (FWF) grants KLI‐646, P 30701 and P 34198.
Hangel G, Spurny‐Dworak B, Lazen P, et al. Inter‐subject stability and regional concentration estimates of 3D‐FID‐MRSI in the human brain at 7 T. NMR in Biomedicine. 2021;34(12):e4596. 10.1002/nbm.4596
Funding information Austrian Science Fund (FWF), Grant/Award Numbers: P 34198, P 30701, KLI‐646
DATA AVAILABILITY STATEMENT
Individual subject ROI quantification tables, as well as metabolite and concentration estimate map datasets in MINC and NIFTI format, are available at Zenodo https://doi.org/10.5281/zenodo.500692392. A summary of the MRSI method used according to the MRSinMRS expert’s consensus can be found in Sup.Tbl. 5.
REFERENCES
- 1. Henning A, Fuchs A, Murdoch JB, Boesiger P. Slice‐selective FID acquisition, localized by outer volume suppression (FIDLOVS) for 1H‐MRSI of the human brain at 7 T with minimal signal loss. NMR Biomed. 2009;22(7):683‐696. 10.1002/nbm.1366 [DOI] [PubMed] [Google Scholar]
- 2. Bogner W, Gruber S, Trattnig S, Chmelik M. High‐resolution mapping of human brain metabolites by free induction decay 1H MRSI at 7T. NMR Biomed. 2012;25(6):873‐882. 10.1002/nbm.1805 [DOI] [PubMed] [Google Scholar]
- 3. Moser E, Stahlberg F, Ladd ME, Trattnig S. 7‐T MR—from research to clinical applications? NMR Biomed. 2012;25(5):695‐716. 10.1002/nbm.1794 [DOI] [PubMed] [Google Scholar]
- 4. Bogner W, Otazo R, Henning A. Accelerated MR spectroscopic imaging—a review of current and emerging techniques. NMR Biomed. 2021;34(5):e4314. 10.1002/nbm.4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hangel G, Strasser B, Považan M, et al. Lipid suppression via double inversion recovery with symmetric frequency sweep for robust 2D‐GRAPPA‐accelerated MRSI of the brain at 7 T. NMR Biomed. 2015;28(11):1413‐1425. 10.1002/nbm.3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strasser B, Považan M, Hangel G, et al. (2 + 1)D‐CAIPIRINHA accelerated MR spectroscopic imaging of the brain at 7T. Magn Reson Med. 2017;78(2):429‐440. 10.1002/mrm.26386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nassirpour S, Chang P, Henning A. MultiNet PyGRAPPA: multiple neural networks for reconstructing variable density GRAPPA (a 1H FID MRSI study). NeuroImage. 2018;183:336‐345. 10.1016/j.neuroimage.2018.08.032 [DOI] [PubMed] [Google Scholar]
- 8. Posse S, Otazo R, Tsai S‐Y, Yoshimoto AE, Lin F‐H. Single‐shot magnetic resonance spectroscopic imaging with partial parallel imaging. Magn Reson Med. 2009;61(3):541‐547. 10.1002/mrm.21855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. An Z, Tiwari V, Ganji SK, et al. Echo‐planar spectroscopic imaging with dual‐readout alternated gradients (DRAG‐EPSI) at 7 T: application for 2‐hydroxyglutarate imaging in glioma patients. Magn Reson Med. 2018;79(4):1851‐1861. 10.1002/MRM.26884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hangel G, Strasser B, Považan M, et al. Ultra‐high resolution brain metabolite mapping at 7 T by short‐TR Hadamard‐encoded FID‐MRSI. NeuroImage. 2016;168:199‐210. 10.1016/j.neuroimage.2016.10.043 [DOI] [PubMed] [Google Scholar]
- 11. Nassirpour S, Chang P, Henning A. High and ultra‐high resolution metabolite mapping of the human brain using 1H FID MRSI at 9.4T. NeuroImage. 2016;168:211‐221. 10.1016/j.neuroimage.2016.12.065 [DOI] [PubMed] [Google Scholar]
- 12. Iqbal Z, Nguyen D, Hangel G, Motyka S, Bogner W, Jiang S. Super‐resolution 1H magnetic resonance spectroscopic imaging utilizing deep learning. Front Oncol. 2019;9:1010. 10.3389/fonc.2019.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andronesi OC, Gagoski BA, Sorensen AG. Neurologic 3D MR spectroscopic imaging with low‐power adiabatic pulses and fast spiral acquisition. Radiology. 2012;262(2):647‐661. 10.1148/radiol.11110277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Esmaeili M, Strasser B, Bogner W, Moser P, Wang Z, Andronesi OC. Whole‐slab 3D MR spectroscopic imaging of the human brain with spiral‐out‐in sampling at 7T. J Magn Reson Imaging. 2021;53(4):1237‐1250. 10.1002/jmri.27437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schirda CV, Zhao T, Andronesi OC, et al. In vivo brain rosette spectroscopic imaging (RSI) with LASER excitation, constant gradient strength readout, and automated LCModel quantification for all voxels. Magn Reson Med. 2016;76(2):380‐390. 10.1002/mrm.25896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiew M, Jiang W, Burns B, et al. Density‐weighted concentric rings k‐space trajectory for 1H magnetic resonance spectroscopic imaging at 7 T. NMR Biomed. 2018;31(1):e3838. 10.1002/nbm.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moser P, Bogner W, Hingerl L, et al. Non‐Cartesian GRAPPA and coil combination using interleaved calibration data—application to concentric‐ring MRSI of the human brain at 7T. Magn Reson Med. 2019;82(5):1587‐1603. 10.1002/mrm.27822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furuyama JK, Wilson NE, Thomas MA. Spectroscopic imaging using concentrically circular echo‐planar trajectories in vivo. Magn Reson Med. 2012;67(6):1515‐1522. 10.1002/mrm.23184 [DOI] [PubMed] [Google Scholar]
- 19. Emir UE, Burns B, Chiew M, Jezzard P, Thomas MA. Non‐water‐suppressed short‐echo‐time magnetic resonance spectroscopic imaging using a concentric ring k‐space trajectory. NMR Biomed. 2017;30(7):e3714. 10.1002/nbm.3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hingerl L, Bogner W, Moser P, et al. Density‐weighted concentric circle trajectories for high resolution brain magnetic resonance spectroscopic imaging at 7T. Magn Reson Med. 2018;79(6):2874‐2885. 10.1002/mrm.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hingerl L, Strasser B, Moser P, et al. Clinical high‐resolution 3D‐MR spectroscopic imaging of the human brain at 7 T. Invest Radiol. 2020;55(4):239‐248. 10.1097/RLI.0000000000000626 [DOI] [PubMed] [Google Scholar]
- 22. Hangel G, Cadrien C, Lazen P, et al. High‐resolution metabolic imaging of high‐grade gliomas using 7T‐CRT‐FID‐MRSI. NeuroImage Clin. 2020;28:102433. 10.1016/j.nicl.2020.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gruber S, Heckova E, Strasser B, et al. Mapping an extended neurochemical profile at 3 and 7 T using accelerated high‐resolution proton magnetic resonance spectroscopic imaging. Invest Radiol. 2017;52(10):631‐639. 10.1097/RLI.0000000000000379 [DOI] [PubMed] [Google Scholar]
- 24. Hangel G, Jain S, Springer E, et al. High‐resolution metabolic mapping of gliomas via patch‐based super‐resolution magnetic resonance spectroscopic imaging at 7T. NeuroImage. 2019;191:587‐595. 10.1016/j.neuroimage.2019.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moser P, Hingerl L, Strasser B, et al. Whole‐slice mapping of GABA and GABA+ at 7T via adiabatic MEGA‐editing, real‐time instability correction, and concentric circle readout. NeuroImage. 2019;184:475‐489. 10.1016/j.neuroimage.2018.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heckova E, Povazan M, Strasser B, et al. Real‐time correction of motion and imager instability artifacts during 3D γ‐aminobutyric acid‐edited MR spectroscopic imaging. Radiology. 2018;286(2):666‐675. 10.1148/radiol.2017170744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ganji SK, An Z, Tiwari V, et al. In vivo detection of 2‐hydroxyglutarate in brain tumors by optimized point‐resolved spectroscopy (PRESS) at 7T. Magn Reson Med. 2017;77(3):936‐944. 10.1002/mrm.26190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heckova E, Strasser B, Hangel GJ, et al. 7 T magnetic resonance spectroscopic imaging in multiple sclerosis. Invest Radiol. 2019;54(4):247‐254. 10.1097/RLI.0000000000000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pan JW, Duckrow RB, Gerrard J, et al. 7T MR spectroscopic imaging in the localization of surgical epilepsy. Epilepsia. 2013;54(9):1668‐1678. 10.1111/epi.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moser P, Eckstein K, Hingerl L, et al. Intra‐session and inter‐subject variability of 3D‐FID‐MRSI using single‐echo volumetric EPI navigators at 3T. Magn Reson Med. 2020;83(6):1920‐1929. 10.1002/mrm.28076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human brain. II. Metabolite concentrations. J Magn Reson B. 1993;102(1):9‐19. 10.1006/jmrb.1993.1056 [DOI] [Google Scholar]
- 32. Pouwels PJW, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998;39(1):53‐60. 10.1002/mrm.1910390110 [DOI] [PubMed] [Google Scholar]
- 33. Hetherington HP, Mason GF, Pan JW, et al. Evaluation of cerebral gray and white matter metabolite differences by spectroscopic imaging at 4.1T. Magn Reson Med. 1994;32(5):565‐571. 10.1002/mrm.1910320504 [DOI] [PubMed] [Google Scholar]
- 34. Minati L, Aquino D, Bruzzone M, Erbetta A. Quantitation of normal metabolite concentrations in six brain regions by in‐vivo 1 H‐MR spectroscopy. J Med Phys. 2010;35(3):154‐163. 10.4103/0971-6203.62128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lecocq A, Le Fur Y, Maudsley AA, et al. Whole‐brain quantitative mapping of metabolites using short echo three‐dimensional proton MRSI. J Magn Reson Imaging. 2015;42(2):280‐289. 10.1002/jmri.24809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Volk C, Jaramillo V, Merki R, O'Gorman Tuura R, Huber R. Diurnal changes in glutamate + glutamine levels of healthy young adults assessed by proton magnetic resonance spectroscopy. Hum Brain Mapp. 2018;39(10):3984‐3992. 10.1002/hbm.24225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dhamala E, Abdelkefi I, Nguyen M, Hennessy TJ, Nadeau H, Near J. Validation of in vivo MRS measures of metabolite concentrations in the human brain. NMR Biomed. 2019;32(3):e4058. 10.1002/nbm.4058 [DOI] [PubMed] [Google Scholar]
- 38. Ogg RJ, Kingsley PB, Taylor JS. WET, a T 1‐ and B 1‐insensitive water‐suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 39. Považan M, Strasser B, Hangel G, Gruber S, Trattnig S, Bogner W. Multimodal post‐processing software for MRSI data evaluation. Proc Int Soc Magn Reson Med.s 2015;23:1973. [Google Scholar]
- 40. Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a convolution function for Fourier inversion using gridding (computerised tomography application). IEEE Trans Med Imaging. 1991;10(3):473‐478. 10.1109/42.97598 [DOI] [PubMed] [Google Scholar]
- 41. Mayer D, Levin YS, Hurd RE, Glover GH, Spielman DM. Fast metabolic imaging of systems with sparse spectra: application for hyperpolarized 13C imaging. Magn Reson Med. 2006;56(4):932‐937. 10.1002/mrm.21025 [DOI] [PubMed] [Google Scholar]
- 42. Bilgic B, Gagoski B, Kok T, Adalsteinsson E. Lipid suppression in CSI with spatial priors and highly undersampled peripheral k‐space. Magn Reson Med. 2013;69(6):1501‐1511. 10.1002/mrm.24399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hangel G, Strasser B, Považan M, et al. A comparison of lipid suppression by double inversion recovery, L1‐ and L2‐regularisation for high resolution MRSI in the brain at 7 T. Paper presented at: ISMRM 24th Annual Meeting& Exhibition; May 7‐13, 2016; Singapore.
- 44. Heckova E, Strasser B, Považan M, Hangel G, Trattnig S, Bogner W. Absolute quantification of brain metabolites by 1H‐MRSI using gradient echo imaging of ~2s as a concentration reference: initial findings. Paper presented at: ISMRM 25th Annual Meeting& Exhibition; April 22‐24, 2017; Honolulu, HI.
- 45. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260‐264. 10.1002/nbm.698 [DOI] [PubMed] [Google Scholar]
- 46. Starčuk Z, Starčuková J, Štrbák O, Graveron‐Demilly D. Simulation of coupled‐spin systems in the steady‐state free‐precession acquisition mode for fast magnetic resonance (MR) spectroscopic imaging. Meas Sci Technol. 2009;20(10):104033. 10.1088/0957-0233/20/10/104033 [DOI] [Google Scholar]
- 47. Považan M, Strasser B, Hangel G, et al. Simultaneous mapping of metabolites and individual macromolecular components via ultra‐short acquisition delay 1H MRSI in the brain at 7T. Magn Reson Med. 2018;79(3):1231‐1240. 10.1002/mrm.26778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Považan M, Hangel G, Strasser B, et al. Mapping of brain macromolecules and their use for spectral processing of 1H‐MRSI data with an ultra‐short acquisition delay at 7 T. NeuroImage. 2015;121:126‐135. 10.1016/j.neuroimage.2015.07.042 [DOI] [PubMed] [Google Scholar]
- 49. Harris AD, Puts NAJ, Edden RAE. Tissue correction for GABA‐edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 2015;42(5):1431‐1440. 10.1002/jmri.24903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xin L, Schaller B, Mlynarik V, Lu H, Gruetter R. Proton T 1 relaxation times of metabolites in human occipital white and gray matter at 7 T. Magn Reson Med. 2013;69(4):931‐936. 10.1002/mrm.24352 [DOI] [PubMed] [Google Scholar]
- 51. Knight‐Scott J, Brennan P, Palasis S, Zhong X. Effect of repetition time on metabolite quantification in the human brain in 1H MR spectroscopy at 3 tesla. J Magn Reson Imaging. 2017;45(3):710‐721. 10.1002/jmri.25403 [DOI] [PubMed] [Google Scholar]
- 52. Near J, Harris AD, Juchem C, et al. Preprocessing, analysis and quantification in single‐voxel magnetic resonance spectroscopy: experts' consensus recommendations. NMR Biomed. 2021;34(5):e4257. 10.1002/nbm.4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gasparovic C, Song T, Devier D, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55(6):1219‐1226. 10.1002/mrm.20901 [DOI] [PubMed] [Google Scholar]
- 54. Wilson M, Andronesi O, Barker PB, et al. Methodological consensus on clinical proton MRS of the brain: review and recommendations. Magn Reson Med. 2019;82(2):527‐550. 10.1002/mrm.27742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dale AM, Fischl B, Sereno MI. Cortical surface‐based analysis. NeuroImage. 1999;9(2):179‐194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 56. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341‐355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- 57. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968‐980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 58. Spurny B, Heckova E, Seiger R, et al. Automated ROI‐based labeling for multi‐voxel magnetic resonance spectroscopy data using FreeSurfer. Front Mol Neurosci. 2019;12. 10.3389/fnmol.2019.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Graaf RA. In Vivo NMR Spectroscopy. Chichester, UK: Wiley; 2019. 10.1002/9781119382461 [DOI] [Google Scholar]
- 60. Zöllner HJ, Považan M, Hui SCN, Tapper S, Edden RAE, Oeltzschner G. Comparison of different linear‐combination modeling algorithms for short‐TE proton spectra. NMR Biomed. 2021;34(4):e4482. 10.1002/nbm.4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pouwels PJW, Frahm J. Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR Biomed. 1997;10(2):73‐78. [DOI] [PubMed] [Google Scholar]
- 62. Choi C, Coupland NJ, Bhardwaj PP, et al. T 2 measurement and quantification of glutamate in human brain in vivo. Magn Reson Med. 2006;56(5):971‐977. 10.1002/mrm.21055 [DOI] [PubMed] [Google Scholar]
- 63. Gonen OM, Moffat BA, Desmond PM, Lui E, Kwan P, O'Brien TJ. Seven‐tesla quantitative magnetic resonance spectroscopy of glutamate, γ‐aminobutyric acid, and glutathione in the posterior cingulate cortex/precuneus in patients with epilepsy. Epilepsia. 2020;61(12):2785‐2794. 10.1111/epi.16731 [DOI] [PubMed] [Google Scholar]
- 64. van Zijl PCM, Barker PB. Magnetic resonance spectroscopy and spectroscopic imaging for the study of brain metabolism. Ann N Y Acad Sci. 1997;820(1):75‐96. 10.1111/j.1749-6632.1997.tb46190.x [DOI] [PubMed] [Google Scholar]
- 65. Rai S, Chowdhury A, Reniers RLEP, Wood SJ, Lucas SJE, Aldred S. A pilot study to assess the effect of acute exercise on brain glutathione. Free Radic Res. 2018;52(1):57‐69. 10.1080/10715762.2017.1411594 [DOI] [PubMed] [Google Scholar]
- 66. Terpstra M, Vaughan TJ, Ugurbil K, Lim KO, Schulz SC, Gruetter R. Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: application to schizophrenia. Magn Reson Mater Phys Biol Med. 2005;18(5):276‐282. 10.1007/s10334-005-0012-0 [DOI] [PubMed] [Google Scholar]
- 67. Emir UE, Deelchand D, Henry P‐G, Terpstra M. Noninvasive quantification of T 2 and concentrations of ascorbate and glutathione in the human brain from the same double‐edited spectra. NMR Biomed. 2011;24(3):263‐269. 10.1002/nbm.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van de Bank BL, Emir UE, Boer VO, et al. Multi‐center reproducibility of neurochemical profiles in the human brain at 7 T. NMR Biomed. 2015;28(3):306‐316. 10.1002/nbm.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Edden RAE, Pomper MG, Barker PB. In vivo differentiation of N‐acetyl aspartyl glutamate from N‐acetyl aspartate at 3 Tesla. Magn Reson Med. 2007;57(6):977‐982. 10.1002/mrm.21234 [DOI] [PubMed] [Google Scholar]
- 70. Bhogal AA, Broeders TAA, Morsinkhof L, et al. Lipid‐suppressed and tissue‐fraction corrected metabolic distributions in human central brain structures using 2D 1H magnetic resonance spectroscopic imaging at 7 T. Brain Behav. 2020;10(12):e01852. 10.1002/brb3.1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gambarota G, Mekle R, Xin L, et al. In vivo measurement of glycine with short echo‐time 1H MRS in human brain at 7 T. Magn Reson Mater Phys Biol Med. 2009;22(1):1‐4. 10.1007/s10334-008-0152-0 [DOI] [PubMed] [Google Scholar]
- 72. Choi C, Dimitrov I, Douglas D, et al. In vivo detection of serine in the human brain by proton magnetic resonance spectroscopy (1H‐MRS) at 7 Tesla. Magn Reson Med. 2009;62(4):1042‐1046. 10.1002/mrm.22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang Y, Taub E, Mueller C, et al. Reproducibility of whole‐brain temperature mapping and metabolite quantification using proton magnetic resonance spectroscopy. NMR Biomed. 2020;33(7):e4313. 10.1002/nbm.4313 [DOI] [PubMed] [Google Scholar]
- 74. Veenith TV, Mada M, Carter E, et al. Comparison of inter subject variability and reproducibility of whole brain proton spectroscopy. PLoS ONE. 2014;9(12):e115304. 10.1371/journal.pone.0115304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Roalf DR, Sydnor VJ, Woods M, et al. A quantitative meta‐analysis of brain glutamate metabolites in aging. Neurobiol Aging. 2020;95:240‐249. 10.1016/j.neurobiolaging.2020.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lind A, Boraxbekk C‐J, Petersen ET, Paulson OB, Siebner HR, Marsman A. Regional myo‐inositol, creatine, and choline levels are higher at older age and scale negatively with visuospatial working memory: a cross‐sectional proton MR spectroscopy study at 7 Tesla on normal cognitive ageing. J Neurosci. 2020;40(42):8149‐8159. 10.1523/JNEUROSCI.2883-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996;58(22):2049‐2056. 10.1016/0024-3205(96)00197-X [DOI] [PubMed] [Google Scholar]
- 78. Endres D, Tebartz van Elst L, Feige B, et al. On the effect of sex on prefrontal and cerebellar neurometabolites in healthy adults: an MRS study. Front Hum Neurosci. 2016;10:367. 10.3389/fnhum.2016.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hädel S, Wirth C, Rapp M, Gallinat J, Schubert F. Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. J Magn Reson Imaging. 2013;38(6):1480‐1487. 10.1002/jmri.24123 [DOI] [PubMed] [Google Scholar]
- 80. Gasparovic C, Chen H, Mullins PG. Errors in 1H‐MRS estimates of brain metabolite concentrations caused by failing to take into account tissue‐specific signal relaxation. NMR Biomed. 2018;31(6):e3914. 10.1002/nbm.3914 [DOI] [PubMed] [Google Scholar]
- 81. Liao Y, Oros‐Peusquens A‐M, Lindemeyer J, et al. An MR technique for simultaneous quantitative imaging of water content, conductivity and susceptibility, with application to brain tumours using a 3T hybrid MR‐PET scanner. Sci Rep. 2019;9(1):88. 10.1038/s41598-018-36435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hernández‐Tamames JA, Mato Abad V, García‐Álvarez R, González‐Zabaleta J, Pereira Loureiro J, Álvarez‐Linera J. Effect of water T2 shortening in the quantification of in‐vitro proton MR spectroscopy. J Neuroimaging. 2016;26(1):58‐61. 10.1111/jon.12258 [DOI] [PubMed] [Google Scholar]
- 83. Hangel G, Strasser B, Považan M, et al. A comparison of lipid suppression by double inversion recovery, L1‐ and L2‐regularisation for high‐resolution MRSI in the brain at 7 T. Paper presented at: ISMRM 24rd Annual Meeting & Exhibition; May 7‐13, 2016; Singapore.
- 84. Juchem C, Cudalbu C, Graaf RA, et al. B0 shimming for in vivo magnetic resonance spectroscopy: experts' consensus recommendations. NMR Biomed. 2020;34(5):e4350. 10.1002/nbm.4350 [DOI] [PubMed] [Google Scholar]
- 85. Hetherington HP, Hamid H, Kulas J, et al. MRSI of the medial temporal lobe at 7 T in explosive blast mild traumatic brain injury. Magn Reson Med. 2014;71(4):1358‐1367. 10.1002/mrm.24814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. He X, Auerbach EJ, Garwood M, Kobayashi N, Wu X, Metzger GJ. Parallel transmit optimized 3D composite adiabatic spectral‐spatial pulse for spectroscopy. Magn Reson Med. 2021;86(1):17‐32. 10.1002/mrm.28682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Motyka S, Moser P, Hingerl L, et al. The influence of spatial resolution on the spectral quality and quantification accuracy of whole‐brain MRSI at 1.5T, 3T, 7T, and 9.4T. Magn Reson Med. 2019;82(2):551‐565. 10.1002/mrm.27746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bogner W, Hess AT, Gagoski B, et al. Real‐time motion‐ and B0‐correction for LASER‐localized spiral‐accelerated 3D‐MRSI of the brain at 3T. NeuroImage. 2013;88C:22‐31. 10.1016/j.neuroimage.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Andronesi OC, Bhattacharyya PK, Bogner W, et al. Motion correction methods for MRS localization and shimming: experts' recommendations. NMR Biomed. 2021;34(5). 10.1002/nbm.4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Maudsley AA, Andronesi OC, Barker PB, et al. Advanced magnetic resonance spectroscopic neuroimaging: experts' consensus recommendations. NMR Biomed. 2021;34(5):e4309. 10.1002/nbm.4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kreis R, Boer V, Choi I‐Y, et al. Terminology and concepts for the characterization of in vivo MR spectroscopy methods and MR spectra: background and experts' consensus recommendations. NMR Biomed. 2021;34(5). 10.1002/nbm.4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hangel G, Spurny B, Lazen P, et al. Supplementary MRSI data for “Inter‐subject stability and regional concentration estimates of 3D‐FID‐MRSI in the human brain at 7T”. 2021. 10.5281/zenodo.5006923 [DOI] [PMC free article] [PubMed]
- 93. Lin A, Andronesi O, Bogner W, et al. Minimum reporting standards for in vivo magnetic resonance spectroscopy (MRSinMRS): experts' consensus recommendations. NMR Biomed. 2021;34(5):e4484. 10.1002/nbm.4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. An L, Araneta MF, Victorino M, Shen J. Determination of brain metabolite T1 without interference from macromolecule relaxation. J Magn Reson Imaging. 2020;52(5):1352‐1359. 10.1002/jmri.27259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of the post‐processing and evaluation pipeline. Details for every step can be found in the Experimental section.
Figure S2. More sample spectra from different volunteers and brain regions, of different qualities, including a spectrum of the excluded volunteer featuring a dominant lipid artefact at the edge of the excluded fitting range.
Figure S3. Examples of MRSI fitting quality markers (tCr SNR and FWHM, metabolite CRLBs) in two volunteers. A) complements data shown in Figure 2. As the CRLB maps show the difficulty of expressing the dynamic range of values, we have also made CRLB maps available for the subject in the supplementary data available at Zenodo.
Table S1. Mean ratios to tCr and their standard deviations for all other metabolites, based on the overall ROI concentration estimates.
Table S2. Inter‐subject CVs for ratios to tCr per ROI, with high similarity to concentration estimate CVs, as described in Tbl.5.
Table S3. Mean concentration estimates per ROI [mM] and their standard deviations for all quantified metabolites in all qualified ROIs.
Table S4. Inter‐subject CVs of the concentration estimates per ROI displayed in Sup.Tbl. 3. As expected, higher SNR/concentration metabolites corresponded to the lowest CVs.
Table S5. A summary of the MRSI method according to the MRSinMRS expert's consensus proposed standard93.
Data Availability Statement
Individual subject ROI quantification tables, as well as metabolite and concentration estimate map datasets in MINC and NIFTI format, are available at Zenodo https://doi.org/10.5281/zenodo.500692392. A summary of the MRSI method used according to the MRSinMRS expert’s consensus can be found in Sup.Tbl. 5.


