Abstract
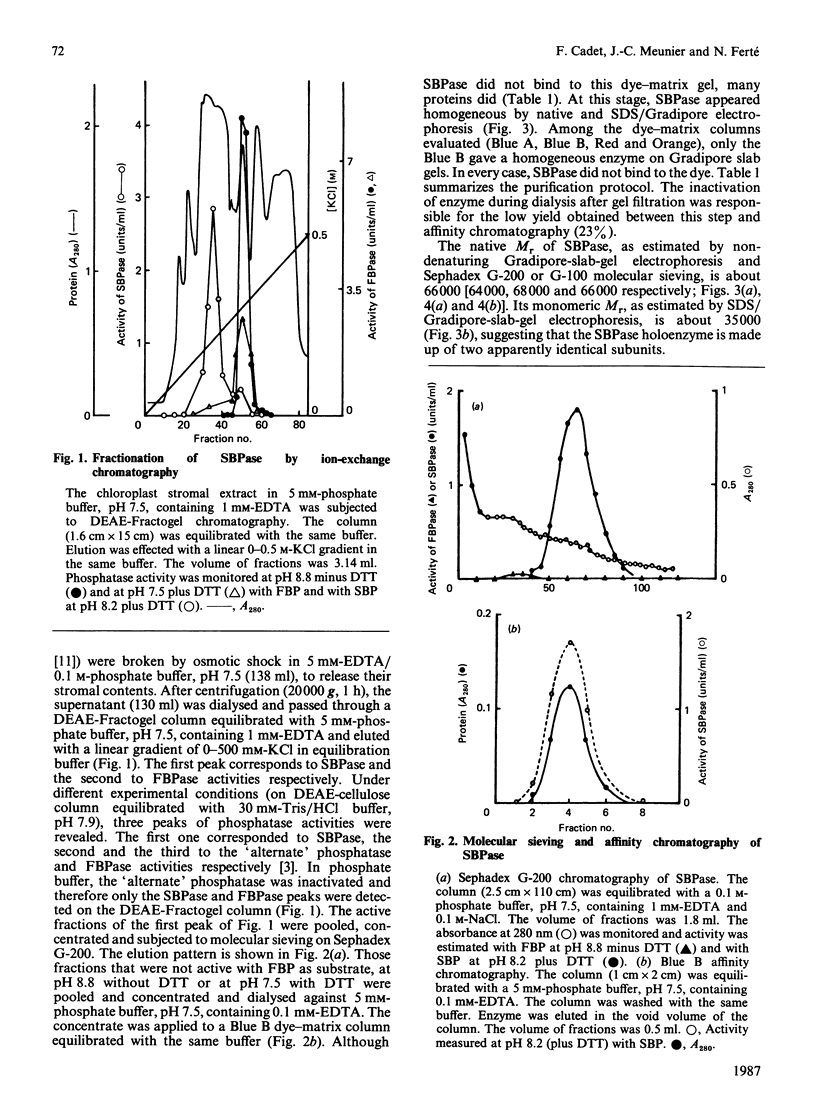
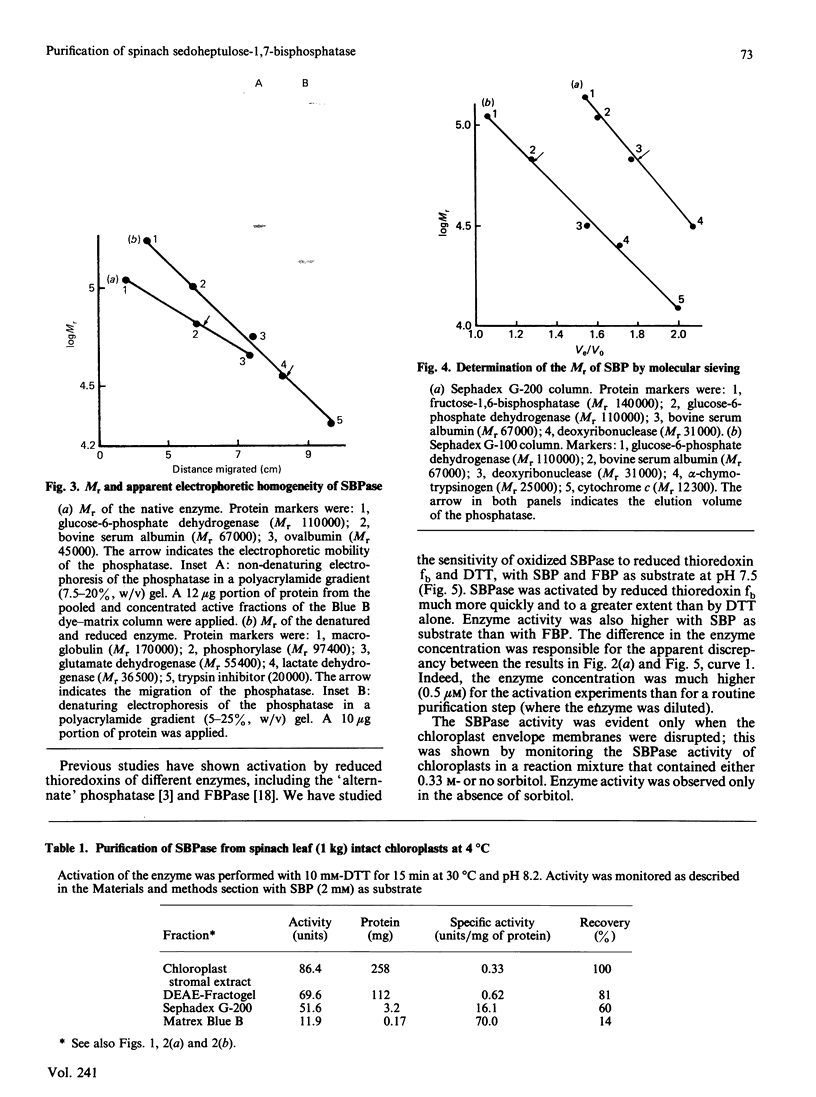
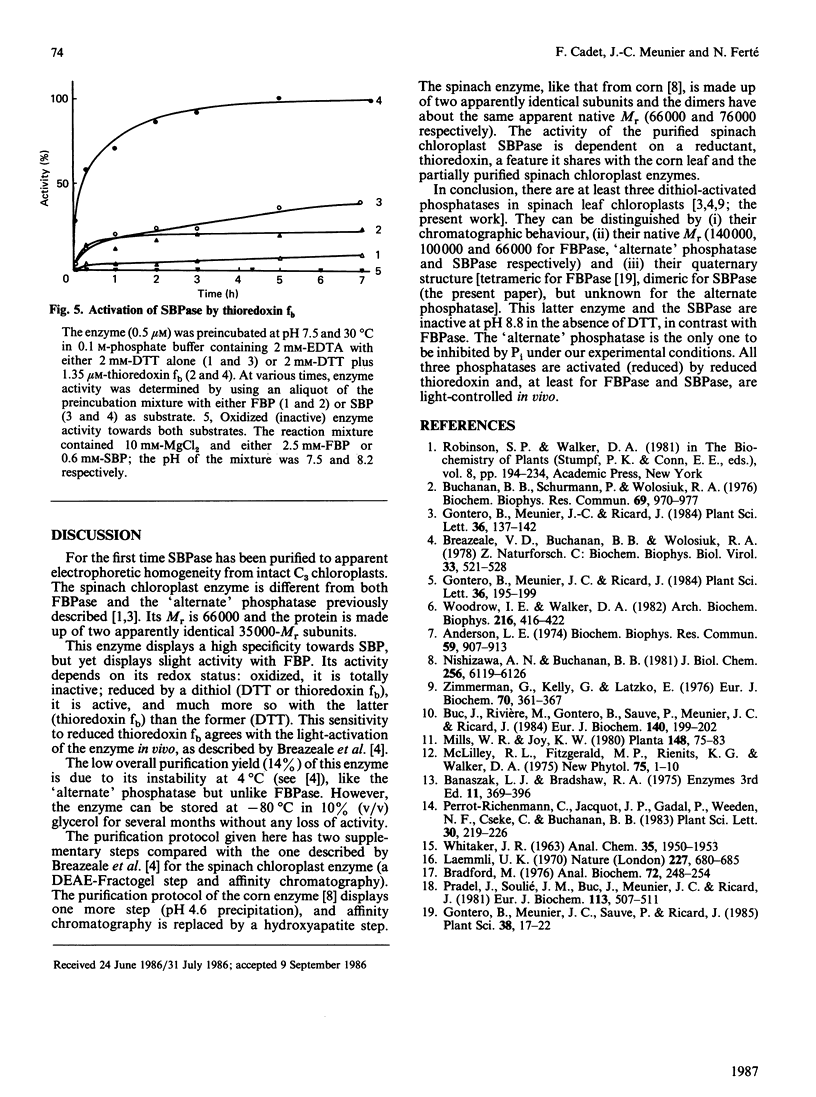
Higher-plant sedoheptulose-1,7-bisphosphatase was isolated and purified over 200-fold from spinach (Spinacia oleracea) chloroplast stromal extracts to apparent electrophoretic homogeneity by DEAE-Fractogel, molecular sieving on Sephadex G-200 and Blue B dye-matrix affinity chromatography. It is a protein of Mr 66,000, made up of two apparently identical subunits (Mr 35,000). The enzyme is activated by reduced thioredoxin fb in the presence of dithiothreitol. Its specificity towards sedoheptulose 1,7-bisphosphate versus fructose 1,6-bisphosphate is high, but not absolute.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E. Activation of pea leaf chloroplast sedoheptulose 1,7-diphosphate phosphatase by light and dithiothreitol. Biochem Biophys Res Commun. 1974 Aug 5;59(3):907–913. doi: 10.1016/s0006-291x(74)80065-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buc J., Rivière M., Gontero B., Sauve P., Meunier J. C., Ricard J. Affinity chromatography, on fructose-bisphosphatase-Sepharose, of two chloroplastic thioredoxins F. Purification and comparative molecular properties. Eur J Biochem. 1984 Apr 2;140(1):199–202. doi: 10.1111/j.1432-1033.1984.tb08086.x. [DOI] [PubMed] [Google Scholar]
- Buchanan R. B., Schfürmann P., Wolosiuk R. A. Appearance of sedoheptulose 1,7-diphosphatase activity on conversion of chloroplast fructose 1,6-diphosphatase from dimer form to monomer form. Biochem Biophys Res Commun. 1976 Apr 19;69(4):970–978. doi: 10.1016/0006-291x(76)90468-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishizawa A. N., Buchanan B. B. Enzyme regulation in C4 photosynthesis. Purification and properties of thioredoxin-linked fructose bisphosphatase and sedoheptulose bisphosphatase from corn leaves. J Biol Chem. 1981 Jun 25;256(12):6119–6126. [PubMed] [Google Scholar]
- Pradel J., Soulié J. M., Buc J., Meunier J. C., Ricard J. On the activation of fructose-1,6-bisphosphatase of spinach chloroplasts and the regulation of the Calvin cycle. Eur J Biochem. 1981 Jan;113(3):507–511. doi: 10.1111/j.1432-1033.1981.tb05092.x. [DOI] [PubMed] [Google Scholar]
- Woodrow I. E., Walker D. A. Activation of wheat chloroplast sedoheptulose bisphosphatase: a continuous spectrophotometric assay. Arch Biochem Biophys. 1982 Jul;216(2):416–422. doi: 10.1016/0003-9861(82)90230-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase. Eur J Biochem. 1976 Nov 15;70(2):361–367. doi: 10.1111/j.1432-1033.1976.tb11025.x. [DOI] [PubMed] [Google Scholar]



