Abstract
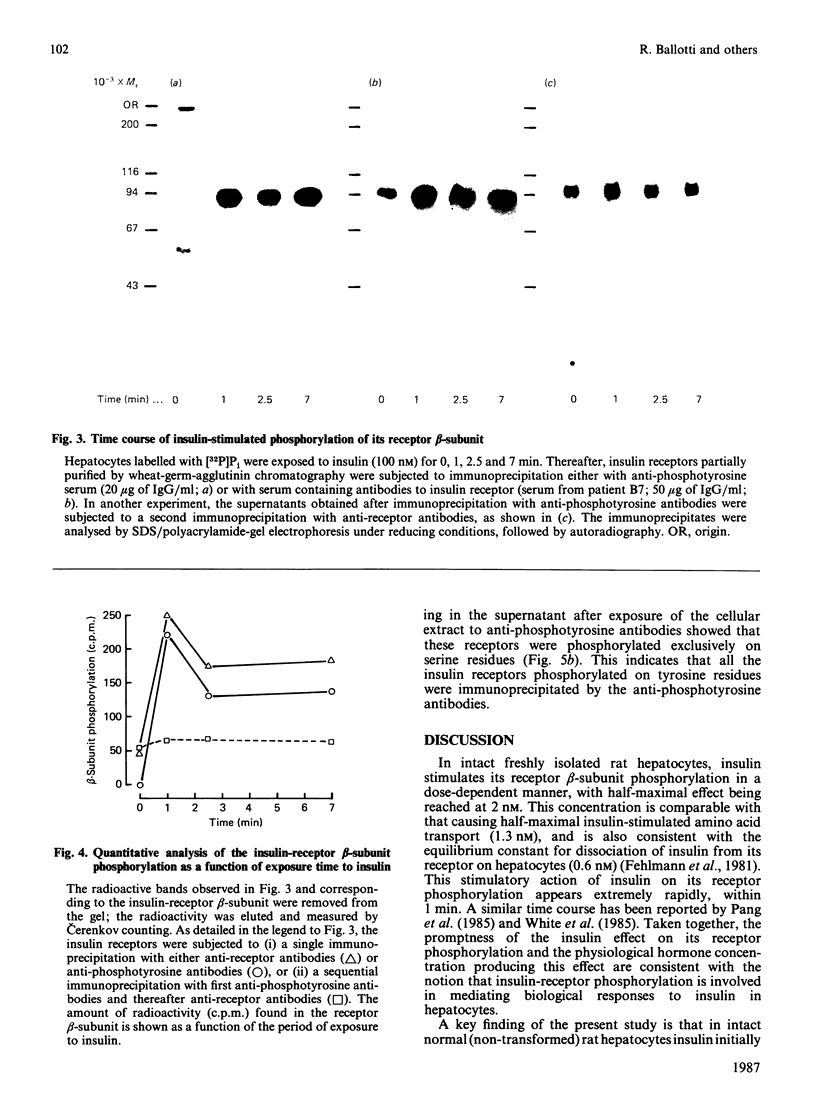
We studied the phosphorylation of the beta subunit of the insulin receptor in intact freshly isolated rat hepatocytes, labelled with [32P]Pi. Insulin receptors partially purified by wheat-germ agglutinin chromatography were immunoprecipitated with either antibodies to insulin receptor or antibodies to phosphotyrosine. Receptors derived from cells incubated in the absence of insulin contained only phosphoserine. Addition of insulin to hepatocytes led to a dose-dependent increase in receptor beta-subunit phosphorylation, with half-maximal stimulation being observed at 2 nM-insulin. Incubation of cells with 100 nM-insulin showed that, within 1 min of exposure to the hormone, maximal receptor phosphorylation occurred, which was followed by a slight decrease and then a plateau. This insulin-induced stimulation of its receptor phosphorylation was largely accounted for by phosphorylation on tyrosine residues. Sequential immunoprecipitation of receptor with anti-phosphotyrosine antibodies and with anti-receptor antibodies, and phosphoamino acid analysis of the immunoprecipitated receptors, revealed that receptors that failed to undergo tyrosine phosphorylation were phosphorylated on serine residues. The demonstration of a functional hormone-sensitive insulin-receptor kinase in normal cells strongly supports a role for this receptor enzymic activity in mediating biological effects of insulin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Leone G. R., Martin D. B. Effects of epinephrine and insulin on phosphopeptide metabolism in adipocytes. J Biol Chem. 1976 Mar 10;251(5):1511–1515. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W., Belsham G. J. A partial view of the mechanism of insulin action. Diabetologia. 1981 Oct;21(4):347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Morin O., Kitabgi P., Freychet P. Insulin and glucagon receptors of isolated rat hepatocytes: comparison between hormone binding and amino acid transport stimulation. Endocrinology. 1981 Jul;109(1):253–261. doi: 10.1210/endo-109-1-253. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Van Obberghen E. Protein kinase activity of the insulin receptor. Biochem J. 1986 Apr 1;235(1):1–11. doi: 10.1042/bj2350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzano H., Kowalski A., Fehlmann M., Van Obberghen E. Two different protein kinase activities are associated with the insulin receptor. Biochem J. 1983 Dec 15;216(3):575–582. doi: 10.1042/bj2160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring H., Kirsch D., Obermaier B., Ermel B., Machicao F. Tumor-promoting phorbol esters increase the Km of the ATP-binding site of the insulin receptor kinase from rat adipocytes. J Biol Chem. 1986 Mar 15;261(8):3869–3875. [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Flier J. S., Grunfeld C., Harmon J. T., Harrison L. C., Karlsson F. A., Kasuga M., King G. L., Lang U. C. Insulin receptors, receptor antibodies, and the mechanism of insulin action. Recent Prog Horm Res. 1981;37:477–538. doi: 10.1016/b978-0-12-571137-1.50015-3. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Fujita-Yamaguchi Y., Blithe D. L., Kahn C. R. Tyrosine-specific protein kinase activity is associated with the purified insulin receptor. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2137–2141. doi: 10.1073/pnas.80.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Cam A., Freychet P. Neutral amino acid transport. Characterization of the A and L systems in isolated rat hepatocytes. J Biol Chem. 1977 Jan 10;252(1):148–156. [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A., White M. F., Kahn C. R. Predominance of tyrosine phosphorylation of insulin receptors during the initial response of intact cells to insulin. J Biol Chem. 1985 Jun 10;260(11):7131–7136. [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Rosen O. M. Insulin receptor is an insulin-dependent tyrosine protein kinase: copurification of insulin-binding activity and protein kinase activity to homogeneity from human placenta. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3327–3331. doi: 10.1073/pnas.81.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Obberghen E., Ksauga M., Le Cam A., Hedo J. A., Itin A., Harrison L. C. Biosynthetic labeling of insulin receptor: studies of subunits in cultured human IM-9 lymphocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1052–1056. doi: 10.1073/pnas.78.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Obberghen E., Rossi B., Kowalski A., Gazzano H., Ponzio G. Receptor-mediated phosphorylation of the hepatic insulin receptor: evidence that the Mr 95,000 receptor subunit is its own kinase. Proc Natl Acad Sci U S A. 1983 Feb;80(4):945–949. doi: 10.1073/pnas.80.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Takayama S., Kahn C. R. Differences in the sites of phosphorylation of the insulin receptor in vivo and in vitro. J Biol Chem. 1985 Aug 5;260(16):9470–9478. [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]






