Abstract
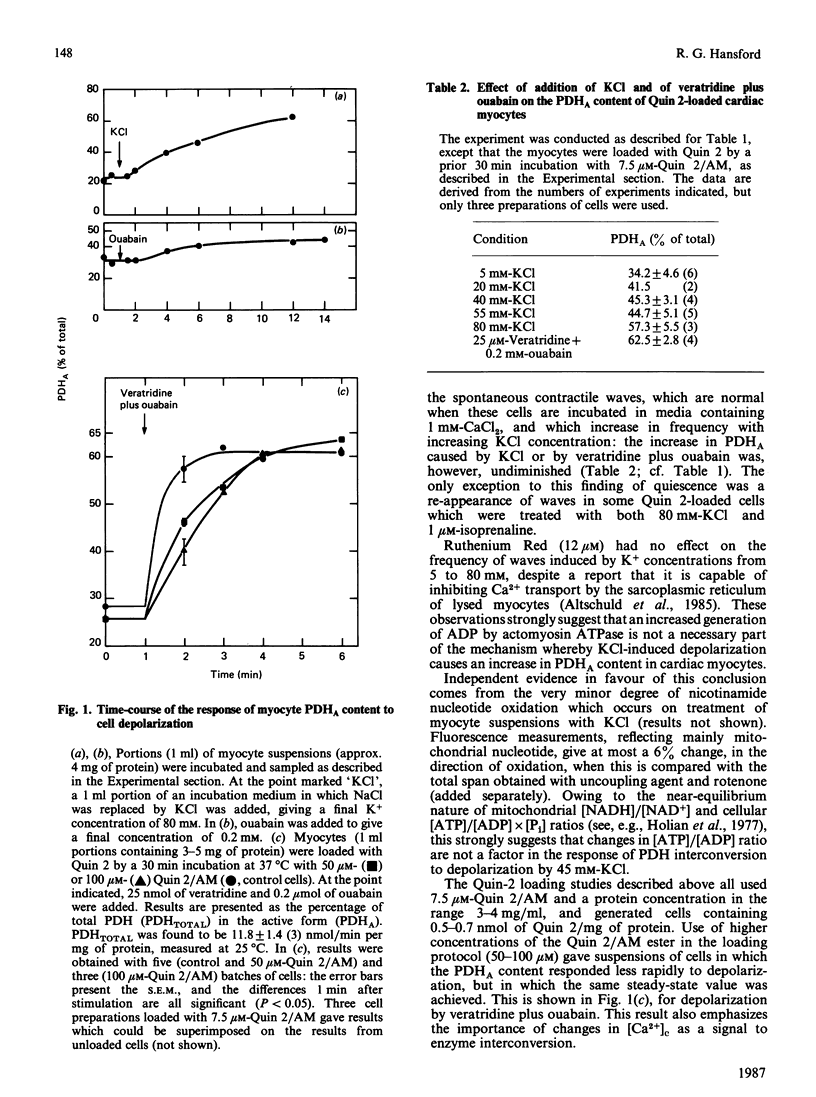
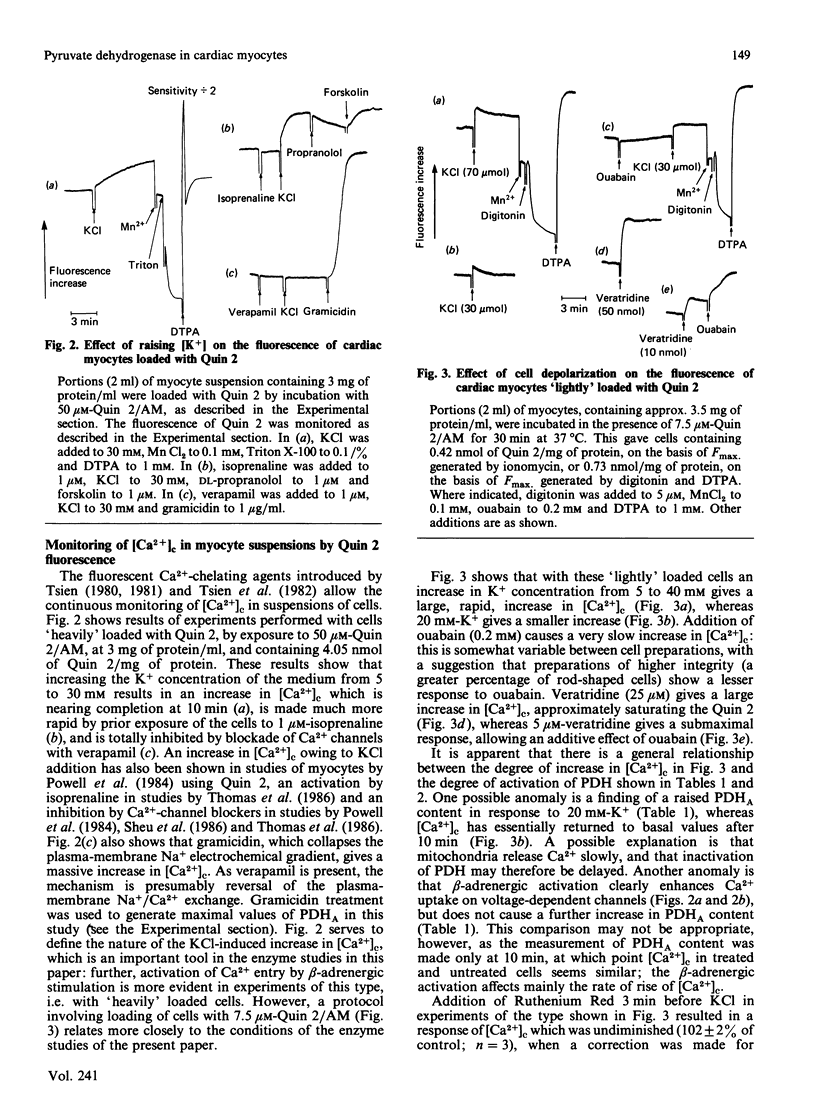
The proportion of pyruvate dehydrogenase existing in the active form (PDHA) in suspensions of unstimulated cardiac myocytes oxidizing glucose is approx. 30%. Depolarization of the cells with concentrations of K+ above physiological values leads to an increase in the content of PDHA. Overloading of the cells with Na+ by treatment with veratridine and ouabain gives the same result. Each of these interventions is shown in experiments with Quin 2-loaded myocytes to lead to an increase in cytosolic free Ca2+ concentration ([Ca2+]c). Treatment of the cells with Ruthenium Red, an inhibitor of Ca2+ transport into mitochondria, largely prevents an increase in PDHA in response to addition of KCl or of veratridine plus ouabain. Ruthenium Red does not attenuate the increase in [Ca2+]c that occurs under these conditions. By contrast, treatment of the cells with ryanodine, an inhibitor of sarcoplasmic-reticulum Ca2+ transport and therefore of contraction, does not diminish the response of PDHA content to agents which raise [Ca2+]c; nor does loading of the cells with the Ca2+-chelating agent Quin 2, which also prevents contraction, at appropriate concentrations. It is concluded that an increase in [Ca2+]c causes an increase in PDHA content of cardiac myocytes independently of an increase in mechanical work. In the normal physiological situation the activation of dehydrogenases by Ca2+ is thought to help to maintain the balance of energy supply and demand during periods of increased work-load, which are associated with an increased myoplasmic [Ca2+]c.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuld R. A., Wenger W. C., Lamka K. G., Kindig O. R., Capen C. C., Mizuhira V., Vander Heide R. S., Brierley G. P. Structural and functional properties of adult rat heart myocytes lysed with digitonin. J Biol Chem. 1985 Nov 15;260(26):14325–14334. [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Bünger R., Permanetter B., Sommer O., Yaffe S. Adaptive changes of pyruvate oxidation in perfused heart during adrenergic stimulation. Am J Physiol. 1982 Jan;242(1):H30–H36. doi: 10.1152/ajpheart.1982.242.1.H30. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escueta A. V., Appel S. H. Biochemical studies of synapses in vitro. II. Potassium transport. Biochemistry. 1969 Feb;8(2):725–733. doi: 10.1021/bi00830a038. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hansford R. G., Castro F. Role of Ca2+ in pyruvate dehydrogenase interconversion in brain mitochondria and synaptosomes. Biochem J. 1985 Apr 1;227(1):129–136. doi: 10.1042/bj2270129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feed-back inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Arch Biochem Biophys. 1978 Nov;191(1):65–81. doi: 10.1016/0003-9861(78)90068-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Effect of micromolar concentrations of free Ca2+ ions on pyruvate dehydrogenase interconversion in intact rat heart mitochondria. Biochem J. 1981 Mar 15;194(3):721–732. doi: 10.1042/bj1940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Hiltunen J. K., Hassinen I. E. Energy-linked regulation of glucose and pyruvate oxidation in isolated perfused rat heart. Role of pyruvate dehydrogenase. Biochim Biophys Acta. 1976 Aug 13;440(2):377–390. doi: 10.1016/0005-2728(76)90072-4. [DOI] [PubMed] [Google Scholar]
- Holian A., Owen C. S., Wilson D. F. Control of respiration in isolated mitochondria: quantitative evaluation of the dependence of respiratory rates on [ATP], [ADP], and [Pi]. Arch Biochem Biophys. 1977 May;181(1):164–171. doi: 10.1016/0003-9861(77)90494-5. [DOI] [PubMed] [Google Scholar]
- Illingworth J. A., Ford W. C., Kobayashi K., Williamson J. R. Regulation of myocardial energy metabolism. Recent Adv Stud Cardiac Struct Metab. 1975;8:271–290. [PubMed] [Google Scholar]
- Illingworth J. A., Mullings R. Pyruvate dehydrogenase activation after an increase in cardiac output. Biochem Soc Trans. 1976;4(2):291–292. doi: 10.1042/bst0040291. [DOI] [PubMed] [Google Scholar]
- Katz A. M. Contractile proteins of the heart. Physiol Rev. 1970 Jan;50(1):63–158. doi: 10.1152/physrev.1970.50.1.63. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Neely J. R. Effects of increased cardiac work on pyruvate dehydrogenase activity in hearts from diabetic animals. J Mol Cell Cardiol. 1983 Jun;15(6):347–357. doi: 10.1016/0022-2828(83)90319-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Neely J. R. Mechanism of pyruvate dehydrogenase activation by increased cardiac work. J Mol Cell Cardiol. 1983 Jun;15(6):369–382. doi: 10.1016/0022-2828(83)90321-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long W. M., Bagby G. J., Spitzer J. J. Contribution of viable and nonviable heart myocytes to substrate oxidation. Am J Physiol. 1980 May;238(5):H740–H744. doi: 10.1152/ajpheart.1980.238.5.H740. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat heart. Evidence from studies with isolated mitochondria that adrenaline activates the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes by increasing the intramitochondrial concentration of Ca2+. Biochem J. 1984 Feb 15;218(1):235–247. doi: 10.1042/bj2180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The activation of pyruvate dehydrogenase in the perfused rat heart by adrenaline and other inotropic agents. Biochem J. 1981 Feb 15;194(2):639–643. doi: 10.1042/bj1940639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., England P. J. Ruthenium Red inhibits the activation of pyruvate dehydrogenase caused by positive inotropic agents in the perfused rat heart. Biochem J. 1983 Aug 15;214(2):581–585. doi: 10.1042/bj2140581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montini J., Bagby G. J., Spitzer J. J. Importance of exogenous substrates for the energy production of adult rat heart myocytes. J Mol Cell Cardiol. 1981 Oct;13(10):903–911. doi: 10.1016/0022-2828(81)90289-3. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Ota M., Narahashi T., Keeler R. F. Effects of veratrum alkaloids on membrane potential and conductance of squid and crayfish giant axons. J Pharmacol Exp Ther. 1973 Jan;184(1):143–154. [PubMed] [Google Scholar]
- Pearce F. J., Walajtys-Rode E., Williamson J. R. Effects of work and acidosis on pyruvate dehydrogenase activity in perfused rat hearts. J Mol Cell Cardiol. 1980 May;12(5):499–510. doi: 10.1016/0022-2828(80)90006-1. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Roche T. E., Reed L. J. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem Biophys Res Commun. 1972 Oct 17;49(2):563–571. doi: 10.1016/0006-291x(72)90448-2. [DOI] [PubMed] [Google Scholar]
- Powell T., Tatham P. E., Twist V. W. Cytoplasmic free calcium measured by quin2 fluorescence in isolated ventricular myocytes at rest and during potassium-depolarization. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1012–1020. doi: 10.1016/0006-291x(84)91192-6. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Denton R. M., Pask H. T., Severson D. L. Calcium ions and the regulation of pyruvate dehydrogenase. Biochem Soc Symp. 1974;(39):75–88. [PubMed] [Google Scholar]
- Reed L. J. Regulation of mammalian pyruvate dehydrogenase complex by a phosphorylation-dephosphorylation cycle. Curr Top Cell Regul. 1981;18:95–106. doi: 10.1016/b978-0-12-152818-8.50012-8. [DOI] [PubMed] [Google Scholar]
- Reinauer H., Muller-Ruchholtz E. R. Regulation of the pyruvate dehydrogenase activity in the isolated perfused heart of guinea-pigs. Biochim Biophys Acta. 1976 Aug 24;444(1):33–42. doi: 10.1016/0304-4165(76)90221-x. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Sharma V. K., Uglesity A. Na+-Ca2+ exchange contributes to increase of cytosolic Ca2+ concentration during depolarization in heart muscle. Am J Physiol. 1986 Apr;250(4 Pt 1):C651–C656. doi: 10.1152/ajpcell.1986.250.4.C651. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Kenyon J. L. Ryanodine modification of cardiac muscle responses to potassium-free solutions. Evidence for inhibition of sarcoplasmic reticulum calcium release. J Gen Physiol. 1983 Sep;82(3):385–404. doi: 10.1085/jgp.82.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J. L., Willerson J. T. Ryanodine alteration of the contractile state of rat ventricular myocardium. Comparison with dog, cat, and rabbit ventricular tissues. Circ Res. 1980 Mar;46(3):332–343. doi: 10.1161/01.res.46.3.332. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Willerson J. T., Templeton G. H., Jones L. R., Besch H. R., Jr Ryanodine: its alterations of cat papillary muscle contractile state and responsiveness to inotropic interventions and a suggested mechanism of action. J Pharmacol Exp Ther. 1979 Apr;209(1):37–47. [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasington F. D., Gazzotti P., Tiozzo R., Carafoli E. The effect of ruthenium red on Ca 2+ transport and respiration in rat liver mitochondria. Biochim Biophys Acta. 1972 Jan 21;256(1):43–54. doi: 10.1016/0005-2728(72)90161-2. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., Cooper R. H., Randle P. J. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974 Sep;141(3):761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O. H. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Hutcheson E. T., Roche T. E., Pettit F. H., Brown J. R., Reed L. J., Watson D. C., Dixon G. H. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry. 1978 Jun 13;17(12):2364–2370. doi: 10.1021/bi00605a017. [DOI] [PubMed] [Google Scholar]


