Abstract
Lassa and Ebola viruses cause acute, often fatal, hemorrhagic fever diseases, for which no effective vaccines are currently available. Although lethal human disease outbreaks have been confined so far to sub-Saharan Africa, they also pose significant epidemiological concern worldwide as demonstrated by several instances of accidental importation of the viruses into North America and Europe. In the present study, we developed experimental individual vaccines for Lassa virus and bivalent vaccines for Lassa and Ebola viruses that are based on an RNA replicon vector derived from an attenuated strain of Venezuelan equine encephalitis virus. The Lassa and Ebola virus genes were expressed from recombinant replicon RNAs that also encoded the replicase function and were capable of efficient intracellular self-amplification. For vaccinations, the recombinant replicons were incorporated into virus-like replicon particles. Guinea pigs vaccinated with particles expressing Lassa virus nucleoprotein or glycoprotein genes were protected from lethal challenge with Lassa virus. Vaccination with particles expressing Ebola virus glycoprotein gene also protected the animals from lethal challenge with Ebola virus. In order to evaluate a single vaccine protecting against both Lassa and Ebola viruses, we developed dual-expression particles that expressed glycoprotein genes of both Ebola and Lassa viruses. Vaccination of guinea pigs with either dual-expression particles or with a mixture of particles expressing Ebola and Lassa virus glycoprotein genes protected the animals against challenges with Ebola and Lassa viruses. The results showed that immune responses can be induced against multiple vaccine antigens coexpressed from an alphavirus replicon and suggested the possibility of engineering multivalent vaccines based upon alphavirus vectors for arenaviruses, filoviruses, and possibly other emerging pathogens.
Lassa and Ebola viruses were discovered in Africa in 1969 and 1976, respectively, and were immediately noted for their extreme pathogenic potential (19, 21, 26). Lassa virus is a member of the Arenaviridae family, with enveloped, spherical virions 90 to 120 nm in diameter and a segmented RNA genome. Lassa fever accounts for 10 to 15% of adult medical admissions in West Africa, resulting in up to 300,000 infections and several thousand deaths per year (23). The natural host for the virus is the multimammate rat Mastomys natalensis, and human infection occurs by exposure to virus-contaminated food, water, or soil (24). Many biological features of the Lassa virus, including the pathogenic mechanisms, remain to be elucidated. Ebola virus, a member of the family Filoviridae, is in many respects, even less well defined. The filamentous, enveloped Ebola virus virions are 80 nm in diameter, with an average length of 920 nm. The natural host for the virus and a mode of primary human infection remain unknown. Human mortality rates during Ebola virus outbreaks approach 90% (4). Although Lassa and Ebola viruses are unrelated taxonomically, development of vaccines for both viruses has often been considered in parallel (4, 26). Both viruses are endemic in partially overlapping areas of sub-Saharan Africa, with Ebola virus registered in Zaire, Gabon, Côte d'Ivoire, and Sudan and Lassa virus found primarily in Sierra Leone, Liberia, and Nigeria. Both viruses are assigned to the highest categories of laboratory containment because of the severity of diseases and the fact that no vaccines are currently available. Vaccines against Lassa and Ebola viruses would benefit populations in areas of endemicity as well as at-risk medical personnel. Further, vaccines may be of critical importance to prevent spread of these viruses within or outside Africa. Cases of Lassa fever have already been registered in the United States and Europe (1, 2, 26). Strain Reston of Ebola virus was introduced in the United States in 1989 (18).
The development of safe and efficacious vaccines for Lassa and Ebola viruses has proved difficult. Inadequate efficacy and safety concerns surround the development of live attenuated or inactivated virus vaccines (4, 26). Substantial protection against infection with Lassa virus was achieved using recombinant vaccinia viruses expressing Lassa virus nucleoprotein (LNP) or glycoprotein (LGP) genes (3, 5, 10, 11, 25). Protection against infection with Ebola virus was observed using Ebola virus nucleoprotein (ENP) or glycoprotein (EGP) that was expressed from recombinant vaccinia viruses, DNA vectors, or a combination of DNA vector and recombinant adenovirus (12, 31, 33, 35). These studies successfully identified viral antigens that are potentially useful in vaccine development. However, the use of live, nonattenuated virus vectors also raised safety concerns. In several cases, incomplete protection was observed or only mild challenge conditions were evaluated.
Previously, we described an RNA replicon vaccine vector derived from attenuated Venezuelan equine encephalitis virus (VEE), an alphavirus (28). The VEE vector system consists of an RNA replicon expression vector and a bipartite RNA packaging helper, all three RNAs produced in vitro from transcription plasmids. The replicon RNA encodes a vaccine-relevant gene and the VEE replicase-transcriptase that controls self-replication and transcription of the heterologous gene. Such replicons are packaged into VEE-like replicon particles (VRP) using the VEE capsid and envelope proteins, which are expressed from helper RNAs. During vaccination, VRP serve as a vehicle for delivery, amplification, and expression in vivo of the vaccine-relevant gene. In contrast to live virus vectors, gene expression is confined to the cells initially infected with VRP, with no spread of infection. Previous studies showed that the VRP envelope, which is derived from live attenuated strain V3014 of VEE (9), targets gene expression to lymph nodes, including professional antigen-presenting dendritic cells, and is capable of eliciting high-level humoral, mucosal, and cell-mediated immune responses to the expressed antigen (8, 13, 22). Immune response to two different antigens was detected after sequential inoculations with VRP (28). However, covaccination with VRP has not yet been tested, and there have been no reports of a combined vaccine for Lassa and Ebola viruses, in part due to the lack of a rodent model suitable for both viruses. Recently, strain 13 guinea pigs were developed as a model for both Lassa and Ebola viruses (6, 17).
In this study, we developed VRP-based vaccines for Lassa virus and assessed their immunogenicity and protective capability against lethal Lassa virus challenge in guinea pigs. In addition, we configured the VEE replicons for the combined expression of vaccine-relevant genes and evaluated the protective capability of combination and bivalent vaccines against both Lassa and Ebola viruses.
MATERIALS AND METHODS
Cells and viruses.
Baby hamster kidney (BHK-21), Vero, and Vero-E6 cell lines were obtained from the American Type Culture Collection (Manassas, Va.) and maintained in minimal essential medium with Earle's salts (EMEM), 10% fetal bovine serum (FBS), penicillin (200 U/ml), streptomycin (200 U/ml), and gentamicin sulfate (10 μg/ml) at 37°C in 5% CO2. Lassa virus was from the original Josiah strain Lassa virus isolate, passage 5 in Vero cells. Ebola virus was previously adapted to lethal virulence in strain 13 guinea pigs by serial passage of the 1976 Zaire (Mayinga) isolate (6).
VEE replicons and helpers.
For construction of the LGP-replicon, cDNA clone LS1337 containing the Josiah strain Lassa virus LGP gene (D. Auperin, Centers for Disease Control and Prevention, Atlanta, Ga.) was digested with ApaI, treated with T4 DNA polymerase, and digested with BamHI. The 1.5-kb LGP gene fragment was cloned into HindIII (treated with T4 polymerase)-BamHI sites within a ClaI-flanked polylinker in the shuttle vector and then subcloned as a ClaI fragment into the ClaI site of the VEE replicon clone (28). The dual-expression vector p2 × 26S was constructed by cloning into the ClaI site of the annealed oligonucleotides 5′-CGATACTTAAGGGCGCGCCTATAACTCTCTACGGCTAACCTGAATGGACTATCGAAGATATCGGCGC-3′ and 5′-CGGCGCCGATATCTTCGATAGTCCATTCAGGTTAGCCGTAGAGAGTTATAGGCGCGCCCTTAAGTAT-3′. pEGP/LGP was constructed by cloning the EGP and LGP genes from the EGP- and LGP-replicon cDNA clones as ClaI fragments into the ClaI and NarI sites of p2 × 26S, respectively. Runoff in vitro transcriptions; LNP-, hemagglutinin (HA) gene-, EGP-, and ENP-replicons; and the VEE c and gp helpers were described previously (27, 28).
Protein expression and production of VRP.
BHK cells were transfected by electroporation and incubated for 30 h (27, 28). Intracellular proteins were metabolically labeled for 1 h with 25 μCi of [35S]Met in Met-depleted medium. Cells were lysed in a buffer containing 50 mM Tris-HCl (pH 6.8), 5% 2-mercaptoethanol, 10% glycerol, and 1% sodium dodecyl sulfate, and proteins were separated on 7% or 4 to 12% polyacrylamide gels. Western blotting was carried out using Lassa virus-specific serum from convalescent rhesus monkey or a cocktail of LGP-specific mouse monoclonal antibodies (L52-121-22-BA02, L52-2121-22-BA02, and L52-135-17A [U.S. Army Medical Research Institute for Infectious Diseases]) or EGP-specific mouse serum (U.S. Army Medical Research Institute for Infectious Diseases), followed by the appropriate peroxidase-labeled secondary antibodies.
Culture supernatants containing VRP were clarified by centrifugation at 4000 × g for 10 min, and VRP were concentrated and partially purified by pelleting at 28,000 rpm for 5 h in an SW28 rotor through 20% (wt/wt) sucrose in phosphate-buffered saline (pH 7.4). VRP titers were determined by immunofluorescence assay (IFA). BHK cells were grown to subconfluency in eight-well chamber slides, and VRP were diluted at 10-fold increments in the EMEM containing 10% FBS and absorbed (0.1 ml/well) onto BHK cell monolayers for 1 h at 37°C. Then, 0.3 ml of the medium was added per well and incubation was continued for 16 h. Cells were fixed with cold acetone and probed with a cocktail of LGP-specific mouse monoclonal antibodies (L52-121-22-BA02, L52-2121-22-BA02, and L52-135-17A), each at a 1:100 dilution, or with rhesus monkey LNP-specific serum or guinea pig EGP-specific serum at a 1:25 dilution. Fluorescein-labeled secondary antibodies to mouse, human, or guinea pig immunoglobulin G (IgG) (heavy and light chain [H+L]) were used at a 1:25 dilution. For double-staining IFA, a mixture of LGP- and EGP-specific antibodies was used, followed by a mixture of rhodamine-labeled antibody to mouse IgG (H+L) and fluorescein-labeled antibody to guinea pig IgG (H+L).
Immunizations.
VRP were diluted in phosphate-buffered saline, pH 7.4. Strain 13 female guinea pigs (body weight, 300 to 400 g) were inoculated subcutaneously (s.c.) at day 0 with a total of 0.5 ml containing 107 infectious units (IU) of VRP. At 28-day intervals, two booster inoculations were administered. Passive immunization was carried out by intraperitoneal (i.p.) administration of 5 ml of the immune serum 4 h before viral challenge. Immune serum was prepared by inoculating strain 13 guinea pigs (four per group) three times at 28-day intervals with 107 IU of LGP- or LNP-VRP. At day 72, animals were anesthetized and exsanguinated, and serum was assayed and pooled.
Serological tests and plaque assays.
IgG enzyme-linked immunosorbent assay (ELISA) was performed with gradient-purified and irradiated Zaire 1995 strain Ebola virus (14, 27) or Josiah strain Lassa virus as the substrate antigen. Sera were initially diluted 1:50 and then serially diluted 1:3, and a reaction stronger than the average reaction with negative control serum plus two standard deviations was considered positive. For Western blotting, guinea pig sera were pooled and assayed at 1:500 dilution. Neutralizing antibodies for Lassa virus were determined by 80% plaque reduction neutralization assay (PRNT80). Sera were initially diluted 1:10 and then serially diluted 1:2 in Hanks' balanced salt solution containing 10 mM HEPES and 10% guinea pig complement. Diluted serum (0.5 ml) was incubated with 103 PFU of Lassa virus for 1 h at 37°C in a total volume of 1 ml. Virus was absorbed on Vero cells in six-well plates (0.2 ml/well) for 1 h at 37°C, overlaid with 2 ml of 0.5% agarose in basal medium Eagle containing 10 mM HEPES and 5% FBS, and incubated for 4 days. A second overlay containing 5% neutral red was applied, plaques were counted 24 h later, and the serum dilution required to achieve 80% plaque reduction was determined. Neutralizing antibody for VEE and Ebola viruses was determined similarly, except that for incubation with VEE, serum was heat inactivated for 30 min at 56°C and serially diluted 1:2 in Hanks' balanced salt solution containing 25 mM HEPES and 2% heat-inactivated FBS, and cells were incubated for 1 day before the second overlay. For incubation with Ebola virus, serum was diluted in EMEM containing 5% FBS, and Vero-E6 cells were used, which were incubated for 10 days before staining with saline containing 5% FBS and 5% neutral red.
Virus challenge.
Challenge was carried out 28 days after the final dose of VRP or 4 h after passive immunization as previously described for Lassa (3, 5) and Ebola (27) viruses in a guinea pig model. Guinea pigs were challenged s.c. with 160 50% lethal doses (LD50), equivalent to 1,000 PFU of Josiah strain Lassa virus, or with 1,000 LD50 (104 PFU) of guinea pig-adapted Mayinga strain Ebola virus. The virus was administered in a total volume of 0.5 ml in EMEM containing 2% FBS. Animals were observed daily for 31 days as described elsewhere (5, 27), and survival and changes in the appearance and behavior of the animals were recorded. Blood samples were taken on the days indicated after challenge and viremia levels were determined by plaque assay.
Research was conducted in compliance with the Animal Welfare Act and other regulations relating to experiments involving animals.
RESULTS
Preparation of VRP vaccines for Lassa virus.
As experimental vaccines for Lassa virus, we prepared and evaluated VRP expressing LGP or LNP genes. The LGP or LNP gene was cloned downstream from the VEE replicase and 26S promoter in the transcription plasmid containing the VEE replicon cDNA (Fig. 1A). The LGP-replicon, LNP-replicon, c helper, and gp helper RNAs were prepared by in vitro transcription of the recombinant plasmids using T7 RNA polymerase. The LGP-VRP were prepared by cotransfecting BHK-21 cells with LGP-replicon along with the VEE c helper and gp helper RNAs. Similarly, the LNP-VRP were prepared by cotransfecting BHK cells with LNP-replicon, c helper, and gp helper RNAs. At 30 h posttransfection, the titers of the LGP-VRP and LNP-VRP in the medium from cotransfected cells were 107 and 108 IU/ml, respectively. To confirm that live VEE did not regenerate by recombination between the replicon and helper RNAs, VRP preparations were tested and found negative for VEE, by IFA with VEE-specific antibodies and by plaque assay, both in transfection supernatants and after a blind passage in BHK cells (data not shown).
FIG. 1.
VRP and expression of LGP and LNP. (A) Production of VRP for expression of LGP or LNP genes using the replicon, c helper, and gp helper RNAs transcribed from cDNA clones. RNAs are shown with solid lines; indicated are the T7 RNA polymerase promoter (PT7), the VEE 26S promoter (open arrow), the location of the EGP or LNP gene (Lassa), and the encapsidation signal (ψ). (B) Expression of LGP and LNP by Coomassie staining and autoradiography (upper panel) and Western blotting (bottom panel). Proteins were labeled with [35S]Met and separated on a polyacrylamide gel. Each lane was loaded with an equivalent of 104 cells. For Western blotting, convalescent rhesus monkey serum (anti-LNP), or monoclonal antibodies to LGP (anti-LGP)- or EGP (anti-EGP)-specific mouse serum were used. Numbers to the right of the panels are molecular masses (kilodaltons). NC, negative control untransfected cells.
To evaluate expression of LGP and LNP genes, we labeled proteins with [35S]methionine at 24 h posttransfection and analyzed them by Coomassie staining, autoradiography, and Western blotting (Fig. 1B). Expression of LGP and LNP was detected by direct staining of whole-cell extracts with Coomassie stain and by autoradiography. This was confirmed by Western blotting. In the cells expressing LGP, the major 79-kDa protein was detected, which corresponded to the intracellular GPc precursor (10). Minor bands of approximately 38 to 44 kDa were also detected in the Western blot, which are consistent with the expected proteolytic processing of the precursor into G1 and G2 proteins (25). The LNP was observed as a major protein band of 63 kDa by Coomassie staining, autoradiography, and Western blotting.
Also shown on Fig. 1B is expression of EGP in the cells transfected with EGP-replicon and expression of both EGP and LGP from the dual-expression EGP/LGP-replicon, which will be discussed below.
Vaccination and protection against infection with Lassa virus.
We used LGP-VRP and LNP-VRP to vaccinate female strain 13 guinea pigs. Four groups of five guinea pigs were evaluated as follows. The first group was inoculated with 107 IU of LGP-VRP, the second group was inoculated with 107 IU of LNP-VRP, the third group was inoculated with a mixture of 107 IU LGP-VRP and 107 IU LNP-VRP, and the fourth (control) group was inoculated with 107 IU of ENP-VRP. We administered a total of three injections s.c., at 4-week intervals. All animals remained healthy and showed no adverse effects after vaccination with VRP. Prechallenge serum antibodies to Lassa virus were detected by ELISA and Western blotting in all animals except the controls, whereas neutralizing antibodies were not detectable by PRNT80 (data not shown). At 4 weeks after the last inoculation, animals were challenged s.c. with 160 LD50 of Lassa virus. All the control animals became infected and died with severe disease symptoms and high viremia (Fig. 2). In contrast, no symptoms of disease were detected in any of the animals inoculated with either LGP-VRP, LNP-VRP, or a mixture of LGP- and LNP-VRP. Most of the animals had no detectable viremia, except that three animals immunized with LGP-VRP, one animal immunized with LNP-VRP, and one animal immunized with LGP- and LNP-VRP had viremia at levels of 50 to 100 PFU/ml at day 7 postchallenge.
FIG. 2.
Protection of guinea pigs with LGP-VRP and LNP-VRP against lethal challenge with Lassa virus. Viremia (A) and survival (B) are shown. Guinea pigs were immunized s.c. with LGP-VRP (LGP), LNP-VRP (LNP), a mixture of both LGP-VRP and LNP-VRP (LGP+LNP), or control ENP-VRP expressing ENP. The animals were challenged s.c. with 160 LD50 of Lassa virus. Error bars, standard deviations.
Passive immunization against infection with Lassa virus.
LGP and LNP immune sera for passive immunization were prepared by inoculating guinea pigs with LGP-VRP and LNP-VRP, respectively. Pooled LGP- or LNP-specific serum was injected i.p. into two groups of five guinea pigs, 5 ml per animal, reconstituting 25 to 30% of the total serum volume (30). A third group remained untreated and was used as a control. The animals were challenged 4 h after serum transfer with 160 LD50 of Lassa virus. After challenge, all serum recipients and untreated control animals became infected, developed viremia, and died with severe disease (Fig. 3). This result showed that in spite of the fact that active immunization with VRP resulted in high-level protection against lethal challenge with Lassa virus, passive transfer of significant volumes of sera from vaccinated animals did not elicit any detectable protective effect in serum recipients against Lassa virus challenge.
FIG. 3.
Survival of guinea pigs after passive i.p. immunization with LGP-specific serum (LGP serum) or LNP-specific serum (LNP serum). Controls received no serum. The animals were challenged s.c. with 160 LD50 of Lassa virus 4 h after serum administration.
Combination and dual-expression vaccines for Lassa and Ebola viruses.
To develop a single vaccine against infection with Ebola and Lassa viruses, we evaluated two vaccine candidates: a combination vaccine composed of a mixture of LGP-VRP and EGP-VRP and a dual-expression EGP/LGP-VRP, which expressed both EGP and LGP genes from the same replicon RNA. A previous study showed that EGP-VRP expressing the EGP gene protects guinea pigs and mice against Ebola virus infection (27). To configure the VEE replicon as a dual-expression vector and to introduce both EGP and LGP genes into the replicon RNA, we constructed a cloning vector, p2 × 26S, encoding the VEE replicon with two copies of the 26S promoter and restriction sites downstream from each promoter (Fig. 4A). The EGP and LGP genes were cloned into the p2 × 26S vector, and the EGP/LGP-replicon RNA encoding both EGP and LGP genes was obtained by in vitro transcription. The dual-expression EGP/LGP-VRP were prepared by cotransfecting BHK cells with the EGP/LGP-replicon and the c and gp helper RNAs. The titer of EGP/LGP-VRP in the medium from cotransfected BHK cells was 5 × 107 IU/ml.
FIG. 4.
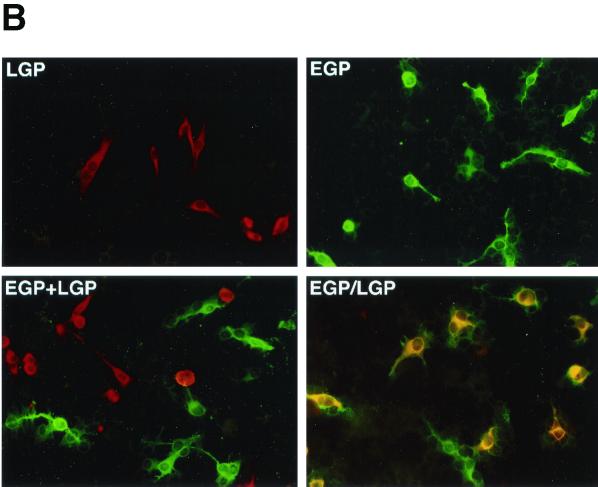
Dual-expression VEE replicon RNA and coexpression of EGP and LGP. (A) The dual-expression cloning vector p2 × 26S and construction of the dual-expression EGP/LGP-replicon RNA. Indicated are the T7 RNA polymerase promoter (PT7), the VEE 26S promoters (open arrows), and the encapsidation signal (ψ). (B) Expression of EGP and LGP in BHK cells, by double-staining immunofluorescence. Cells were infected at a multiplicity of infection of 0.1 with LGP-VRP (LGP), EGP-VRP (EGP), a combination of both (LGP+EGP), or dual-expression EGP/LGP-VRP (EGP/LGP). Cells were fixed with acetone and probed with a cocktail of mouse LGP-specific monoclonal antibodies and guinea pig EGP-specific serum. Antigen-expressing cells were stained with a mixture of rhodamine-conjugated antibody to mouse IgG and fluorescein-conjugated antibody to guinea pig IgG.
Coexpression of Ebola and Lassa virus antigens.
Initially, expression from the dual-expression EGP/LGP-replicon was characterized by Western blotting. The banding patterns and levels of expression of EGP and LGP from the dual-expression replicon were comparable to those observed from the individual EGP- or LGP-replicons, although the processed forms of the proteins were detected in larger amounts (Fig. 1B). For example, Western blot with anti-LGP antibodies showed larger quantities of G1/G2 proteins in the cells transfected with EGP/LGP-replicon than in the cells transfected with LGP-replicon. Similarly, Western blotting with anti-EGP antibody showed accumulation of a 45-kDa protein in the cell transfected with EGP/LGP-replicon, which may represent a proteolytic fragment of EGP and was also detected on overexposed Western blots of cells transfected with EGP-replicon (not shown). In the latter cells, minor bands of 48 to 54 kDa were also detected, which may represent secreted Ebola virus sGP protein and/or proteolytic fragments of sGP or EGP (Fig. 1B). We were unable to detect the 26-kDa Ebola virus GP2 protein in the cell extracts (Fig. 1B) or in the purified irradiated Ebola virus virions (not shown) using our antibodies; however, the presence of multiple bands in the vicinity of 140 to 160 kDa is consistent with the expected processing of EGP into GP1 and GP2 (34).
Expression of the EGP and LGP was confirmed by IFA, by infecting BHK cells with either EGP-VRP or LGP-VRP alone, with a combination of EGP- and LGP-VRP, or with the dual-expression EGP/LGP-VRP. At 16 h postinfection, we probed the infected cells with a mixture of antibodies to Lassa and Ebola virus proteins in a double-staining IFA (Fig. 4B). As expected, cells infected with EGP-VRP or LGP-VRP expressed only EGP or LGP, respectively. In the majority of cells infected with the combination of EGP- and LGP-VRP, the EGP and LGP antigens were expressed within separate cells. In contrast, in the majority of cells infected with the dual-expression EGP/LGP-VRP, the EGP and LGP antigens were coexpressed within the same cells. Less than 0.1% of cells infected with EGP/LGP-VRP expressed only one antigen, either EGP or LGP, which reflects the low rate of spontaneous deletion or inactivation of either gene within the dual-expression replicon.
Coimmunization and antibody responses against Ebola and Lassa viruses.
Five groups of 10 female guinea pigs were vaccinated s.c. with a total of three injections of either (i) 107 IU of EGP-VRP, (ii) 107 IU of LGP-VRP, (iii) a combination of 107 IU EGP-VRP and 107 IU LGP-VRP, (iv) 107 IU of the dual-expression EGP/LGP-VRP, or (v) 107 IU of negative control HA-VRP expressing the influenza A virus HA gene. The animals were inoculated, and serum samples were collected at 4-week intervals.
Antibody to LGP was detected by Western blotting after two inoculations with LGP-VRP, the combination of LGP- and EGP-VRP, or the dual-expression EGP/LGP-VRP (Fig. 5A). The antibodies recognized the full-length LGP. The reactivity increased after a third inoculation with either LGP-VRP or a combination of EGP- and LGP-VRP but not with dual-expression EGP/LGP-VRP. This result suggested that the dual-expression vaccine may induce an antibody response differing from that of a combination vaccine. Antibody to Ebola virus antigen was readily detected after the first immunization with EGP-VRP, the combination of EGP- and LGP-VRP, or the EGP/LGP-VRP. The reactivity increased dramatically after a booster inoculation. Antibodies from the animals immunized with EGP-VRP also recognized a 48-kDa protein, which is coexpressed in EGP-expressing cells (Fig. 1B). Prechallenge antibodies to Lassa and Ebola viruses were also detected by ELISA; however, no neutralizing antibodies to Lassa virus and low titers of neutralizing antibodies to Ebola virus were observed (Table 1).
FIG. 5.
Development of serum antibodies in guinea pigs immunized s.c. with EGP-VRP (EGP), LGP-VRP (LGP), a combination of both, and dual-expression EGP/LGP-VRP. Sera were collected at days 28, 56, and 94. (A) Serum antibodies to EGP and LGP antigens, as shown by Western blotting. Proteins from BHK cells infected with LGP- or EGP-VRP were separated on a polyacrylamide gel (104 cells/lane) and probed with pooled guinea pig serum. (B) VEE-neutralizing antibody titers, as shown by PRNT80. Error bars, standard deviations.
TABLE 1.
Prechallenge titers of serum antibody to Lassa and Ebola virus antigens
| Immunogena | Titer of antibody tob:
|
|||
|---|---|---|---|---|
| Lassa virus
|
Ebola virus
|
|||
| ELISA | PRNT80 | ELISA | PRNT80 | |
| LGP | 3.0 ± 0.2 | 10 | NT | <10 |
| EGP | NT | <10 | 3.1 ± 0.2 | 20 |
| EGP + LGP | 3.3 ± 0.1 | <10 | 3.1 ± 0.2 | 20 |
| EGP/LGP | 2.7 ± 0.5 | <10 | 3.4 ± 0.2 | 20 |
| HA | <1.7 | <10 | <1.7 | <10 |
LGP, 107 IU of LGP-VRP; EGP, 107 IU of EGP-VRP; EGP + LGP, a mixture of 107 IU of EGP-VRP and 107 IU of LGP-VRP; EGP/LGP, 107 IU of a dual-expression EGP/LGP-VRP.
Titers were determined by ELISA (means ± standard deviations [log10]) and by PRNT80 (dilution−1). NT, not tested.
We also found that sera from immunized guinea pigs were capable of neutralizing VEE (Fig. 5B). Despite this neutralizing activity, anamnestic responses to EGP and LGP antigens were detected after booster immunizations (Fig. 5A).
Before challenge, we divided each of five groups of the immunized animals into two subgroups; one was challenged with Lassa virus, and the second was challenged with Ebola virus.
Protection against infection with Lassa virus.
The animals were challenged s.c. with 160 LD50 of Lassa virus. All guinea pigs inoculated with EGP-VRP or HA-VRP developed symptoms of severe disease and high viremia and died within 13 to 26 days (Fig. 6A and B). Animals inoculated with LGP-VRP, the combination of LGP- and EGP-VRP, or the dual-expression EGP/LGP-VRP survived lethal challenge with no symptoms of disease. The exception was one animal in the group inoculated with a combination of LGP- and EGP-VRP, which died at day 14 postchallenge (Fig. 6B). Interestingly, this animal had no detectable viremia at days 7 and 14 postchallenge. In the same group, the remaining four animals had viremias of 102 to 103 PFU/ml at day 7, and two of these remained viremic (102 PFU/ml) at day 14, but all animals cleared the virus by day 21 postchallenge. In the group immunized with LGP-VRP, one animal had viremia of 102 PFU/ml at day 7, but there was no detectable viremia in any of the animals at days 14 and 21 postchallenge. Similarly, in the group immunized with the dual-expression EGP/LGP-VRP, two out of five animals had viremia of 102 PFU/ml at day 7, but all animals were aviremic at days 14 and 21 postchallenge (Fig. 6A).
FIG. 6.
Protection of guinea pigs against lethal challenges with Lassa and Ebola viruses. Animals were immunized s.c. with either EGP-VRP (EGP), LGP-VRP (LGP), a combination of both (EGP+LGP), dual-expression EGP/LGP-VRP (EGP/LGP), or control HA-VRP expressing influenza virus HA. (A) Viremia after challenge with 160 LD50 of Lassa virus. (B) Survival after challenge with 160 LD50 of Lassa virus. (C) Viremia after challenge with 1,000 LD50 of Ebola virus. (D) Survival after challenge with 1,000 LD50 of Ebola virus. *, no viremia detected in nonsurviving animals. (A and C) Error bars, standard deviations.
Protection against infection with Ebola virus.
The second subgroup of the immunized animals was challenged s.c. with 1,000 LD50 of guinea pig-adapted Ebola virus. The animals inoculated with either LGP-VRP or HA-VRP developed severe disease and high viremia and died within 8 to 11 days (Fig. 6C and D). In contrast, animals immunized with either EGP-VRP or the combination of LGP- and EGP-VRP remained healthy for the entire 31-day observation period, with no viremia detected at any time. Animals immunized with EGP/LGP-VRP were also symptom-free and aviremic for the entire postchallenge period. However, there were two late deaths in this group (days 21 and 29 postchallenge) with no symptoms of disease or viremia. Immunohistochemical analysis of the samples from serum, liver, spleen, kidney, adrenal glands, lungs, and brain tissues from these two animals did not reveal any Ebola virus antigen. Lesions characteristic of Ebola virus-infected guinea pigs were also not evident in these tissues, suggesting that the cause of death may be due to undetected pathological changes or may be unrelated to Ebola virus.
DISCUSSION
The Lassa and Ebola viruses belong to separate families of viruses, with very distinct replication strategies, virion architectures, and biological characteristics. Both viruses can cause severe hemorrhagic diseases associated with significant human mortality rates (4). Although experimental vaccinations have shown promise (3, 5, 10–12, 25, 31, 33, 35), vaccines for Lassa and Ebola viruses are currently not available. This is in part due to the fact that both viruses require maximum biosafety containment, which radically reduces the numbers of laboratories researching these pathogens, and in part due to the safety and efficacy concerns of existing vaccine candidates. The purpose of this study was to develop and evaluate novel vaccine candidates for Lassa virus as well as bivalent experimental vaccines for both Ebola and Lassa viruses. Our approach was based on a VRP replicon vector derived from an attenuated strain of VEE, an alphavirus from the Togaviridae family (28).
Lassa virus vaccines.
We observed that all guinea pigs vaccinated with either LGP-VRP or LNP-VRP expressing LGP and LNP genes, respectively, or with a combination of LGP-VRP and LNP-VRP were protected from clinical disease after an otherwise lethal challenge with Lassa virus. Further, 40 to 90% of these animals, depending on the immunogen used, showed no evidence of viremia after challenge. However, only low titers of antibodies but no neutralizing activity were detected in the prechallenge sera from the immunized animals. Further, reconstitution of up to 30% of total guinea pig serum with the serum from vaccinated animals did not have any protective effect on serum recipients against Lassa virus challenge. Although determination of the mechanism of protection was not in the scope of this study, these results strongly suggest the role of cellular immunity. This is consistent with the previous serum transfer experiments (16, 25) and the idea that T cells control Lassa virus infection (32). However, it has been shown that highly virulent Lassa virus strains did not induce a cytotoxic T-cell response in guinea pigs (15). LaPosta et al. suggested the protective role of CD4+ killer cells, because CD4+ cells from mice immunized with vaccinia virus expressing LGP gene proliferated in response to antigens of lymphocytic choriomeningitis virus (LCMV), a related arenavirus (20). Further, immunity to LCMV was achieved in the absence of antibodies or CD8+ cytotoxic T cells to LCMV (20). Although we observed equivalent, high-level protection with LGP-VRP, LNP-VRP, or a mixture of LGP- and LNP-VRP, the third option may provide better coverage, as both LGP and LNP clearly include protective epitopes (Fig. 2). Previously, immunizations with VRP were also shown to protect against a mucosal viral infection (28), which may be advantageous for a vaccine against Lassa virus, which is known to infect via mucosae.
Previous research has shown that guinea pigs immunized with recombinant vaccinia viruses expressing either LGP or LNP also resisted challenge with Lassa virus (3, 5, 25), although mortality rates of up to 42% were observed. Interestingly, the highest mortality was observed in the animals immunized with a mixture of LGP- and LNP-expressing vaccinia viruses. Taken together, the data suggest that coimmunization with LNP and LGP deserves further studies. Immunization of macaques with vaccinia virus expressing LNP conferred little protection, whereas vaccinia virus expressing LGP protected the animals from death but, at least in some cases, not from febrile disease (10, 11, 25). This result suggests that LGP may be a better immunogen than LNP in primates when expressed from vaccinia virus. However, safety concerns, especially in immunocompromised individuals, may impede the use of live recombinant vaccinia virus as Lassa virus vaccine, especially in areas of sub-Saharan Africa with endemic human immunodeficiency virus. Progressive disseminated vaccinia virus infection has been observed in a human immunodeficiency virus-infected individual vaccinated against smallpox (29).
Bivalent vaccines for Ebola and Lassa viruses.
In addition to vaccine candidates for Lassa virus, we developed and evaluated two vaccines capable of protecting against both Lassa and Ebola viruses. These were based on VRP expressing the glycoprotein genes of Lassa and Ebola viruses, LGP and EGP, respectively. Antibody responses were detected to both EGP and LGP, with a stronger apparent response to EGP than to LGP, as shown by ELISA and Western blotting. Induction of a low antibody response to Lassa virus was also reported in guinea pigs and primates successfully immunized with recombinant vaccinia viruses expressing Lassa virus antigens (3, 11). However, despite low antibody responses to Lassa virus, both combination and dual-expression replicon vaccines substantially protected guinea pigs against Ebola and Lassa virus challenges, with 17 out of 20 immunized animals surviving lethal virus challenges. This result shows that protection can be achieved using multiple antigens coexpressed from VRP. The cause of deaths of the three remaining animals is not clear, as viremia was consistently undetectable and no virus was recovered from their tissues. More studies are needed to elucidate the mechanism of protection and to compare the efficacy of combination and dual-expression vaccines. Dual-expression vaccine ensures that both expression products are produced in the same cell. Potentially, this feature may allow coexpression of immunostimulatory proteins with vaccine antigens or expression of subunit proteins or ligand-receptor complexes as vaccines.
We challenged the animals at day 28 postimmunization, as described previously (3, 5, 27). The recent study showed that survival of nonhuman primates was not significantly affected if challenge with Lassa virus was done at 36 or 274 days after immunization with vaccinia virus expressing LGP (11). However, more studies are needed to determine the duration of VRP-induced immunity to Lassa and Ebola viruses as well as the immune response to the VRP vector proteins. Although VRP immunizations did not induce levels of antibody to VRP structural proteins in serum in BALB/c mice or guinea pigs that were detectable by ELISA or Western blotting (27, 28), in this study, we detected VEE-neutralizing activity in guinea pig serum. This is likely because of the abortive replication of the replicons rather than the presence of live VEE. Although the latter cannot be completely excluded, we have shown that the live VEE is neither present nor regenerates in the replicon preparations even after a blind passage in cultured cells. Experiments are being conducted to address this phenomenon in detail.
In addition to the high degree of protection observed, VRP vaccines for Lassa and Ebola viruses, including the dual-expression VRP, may offer other advantages. In contrast to live virus vectors, VRP are single-cycle vectors and do not replicate beyond those cells initially infected, which ensures vaccine safety. Genes are expressed in the cell cytoplasm from the RNA replicons, avoiding the possibility of gene splicing or integration into the host genome. High-level expression is achieved due to two rounds of gene amplification, the first via vector RNA replication and the second via gene transcription from the 26S promoter. Recent studies also show that VRP target cells of the lymphoid tissue in vivo, including professional antigen-presenting dendritic cells (8, 13, 22). As a result, efficient, broad-range immunity is elicited that is especially important for emerging pathogens, for which the relevant immune effector mechanisms have not been determined. Immunity to two pathogens can be achieved by sequential immunizations (28), by coimmunizations via combination of VRP vaccines, or by dual-expression VRP as shown in this study. No toxicity of VRP was detected in rodents, including intracerebrally inoculated newborn mice (27, 28). The efficacy and safety of VRP vaccines expressing Marburg virus and simian immunodeficiency virus proteins have also been demonstrated in primates (7, 14). The results warrant further testing of VRP as candidate vaccines against Lassa and Ebola viruses and suggest that development of multivalent vaccine against additional strains of Lassa and Ebola viruses and, possibly, other pathogens, may be possible on the basis of alphavirus replicon vectors.
ACKNOWLEDGMENTS
We thank D. Auperin for the LGP clone, C. Lind for expert technical assistance, and K. Steele for the pathological analysis.
REFERENCES
- 1.Anonymous. Lassa fever, case imported to Germany. Wkly Epidemiol Rec. 2000;75:17–18. [PubMed] [Google Scholar]
- 2.Anonymous. Lassa fever imported to England. Commun Dis Rep Wkly. 2000;10:99. [PubMed] [Google Scholar]
- 3.Auperin D D, Esposito J J, Lange J V, Bauer S P, Knight J, Sasso D R, McCormick J B. Construction of a recombinant vaccinia virus expressing the Lassa virus glycoprotein gene and protection of guinea pigs from a lethal Lassa virus infection. Virus Res. 1988;9:233–248. doi: 10.1016/0168-1702(88)90033-0. [DOI] [PubMed] [Google Scholar]
- 4.Clegg J C S, Sanchez A. Vaccines against arenaviruses and filoviruses. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker; 1997. pp. 749–765. [Google Scholar]
- 5.Clegg J C S, Lloyd G. Vaccinia recombinant expressing Lassa-virus internal nucleocapsid protein protects guinea pigs against Lassa fever. Lancet. 1987;ii:186–188. doi: 10.1016/s0140-6736(87)90767-7. [DOI] [PubMed] [Google Scholar]
- 6.Connolly B M, Steele K E, Davis K J, Geisbert T W, Kell W M, Jaax N K, Jahrling P B. Pathogenesis of experimental Ebola virus infection in guinea pigs. J Infect Dis. 1999;179:S203–S217. doi: 10.1086/514305. [DOI] [PubMed] [Google Scholar]
- 7.Davis N L, Caley I J, Brown K W, Betts M R, Irlbeck D M, McGrath K M, Connell M J, Montefiori D C, Frelinger J A, Swanstrom R, Johnson P R, Johnston R E. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J Virol. 2000;74:371–378. doi: 10.1128/jvi.74.1.371-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis N L, Brown K W, Johnston R E. A viral vaccine vector that expresses foreign genes in lymph nodes and protects against mucosal challenge. J Virol. 1996;70:3781–3787. doi: 10.1128/jvi.70.6.3781-3787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis N L, Powell N, Greenwald G F, Willis L V, Johnson B J, Smith J F, Johnston R E. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology. 1991;183:20–31. doi: 10.1016/0042-6822(91)90114-q. [DOI] [PubMed] [Google Scholar]
- 10.Fisher-Hoch S P, McCormick J B, Auperin D, Brown B G, Castor M, Perez G, Ruo S, Conaty A, Brammer L, Bauer S. Protection of rhesus monkeys from fatal Lassa fever by vaccination with a recombinant vaccinia virus containing the Lassa virus glycoprotein gene. Proc Natl Acad Sci USA. 1989;86:317–321. doi: 10.1073/pnas.86.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher-Hoch S P, Hutwagner L, Brown B, McCormick J B. Effective vaccine for Lassa fever. J Virol. 2000;74:6777–6783. doi: 10.1128/jvi.74.15.6777-6783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilligan K J, Geisbert J B, Jahrling P B, Anderson K. Assessment of protective immunity conferred by recombinant vaccinia viruses to guinea pigs challenged with Ebola virus. In: Brown F, Burton D, Doherty P, Mekalanos J, Norrby E, editors. Vaccines. Vol. 97. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1997. pp. 87–92. [Google Scholar]
- 13.Grieder F B, Davis N L, Aronson J F, Charles P C, Sellon D C, Suzuki K, Johnston R E. Specific restrictions in the progression of Venezuelan equine encephalitis virus-induced disease resulting from single amino acid changes in the glycoproteins. Virology. 1995;206:994–1006. doi: 10.1006/viro.1995.1022. [DOI] [PubMed] [Google Scholar]
- 14.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology. 1998;251:28–37. doi: 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 15.Jahrling P B, Peters C J. Serology and virulence diversity among Old-World arenaviruses, and the relevance to vaccine development. Med Microbiol Immunol. 1986;175:165–167. doi: 10.1007/BF02122441. [DOI] [PubMed] [Google Scholar]
- 16.Jahrling P B. Protection of Lassa virus-infected guinea pigs with Lassa-immune plasma of guinea pig, primate, and human origin. J Med Virol. 1983;12:93–102. doi: 10.1002/jmv.1890120203. [DOI] [PubMed] [Google Scholar]
- 17.Jahrling P B, Smith S, Hesse R A, Rhoderick J B. Pathogenesis of Lassa virus infection in guinea pigs. Infect Immun. 1982;37:771–778. doi: 10.1128/iai.37.2.771-778.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahrling P B, Geisbert T W, Dalgard D W, Johnson E D, Ksiazek T G, Hall W C, Peters C J. Preliminary report: isolation of Ebola virus from monkeys imported to USA. Lancet. 1990;335:502–505. doi: 10.1016/0140-6736(90)90737-p. [DOI] [PubMed] [Google Scholar]
- 19.Johnson K M, Webb P A, Lange J V, Murphy F A. Isolation and partial characterization of a new virus causing acute haemorrhagic fever in Zaire. Lancet. 1977;i:569–571. doi: 10.1016/s0140-6736(77)92000-1. [DOI] [PubMed] [Google Scholar]
- 20.LaPosta V J, Auperin D D, Kamin-Lewis R, Cole G A. Cross-protection against lymphocytic choriomeningitis virus mediated by a CD4+ T-cell clone specific for an envelope glycoprotein of Lassa virus. J Virol. 1993;67:3497–3506. doi: 10.1128/jvi.67.6.3497-3506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leifer E, Gocke D J, Bourne H. Lassa fever, a new virus disease of man from West Africa. II. Report of a laboratory-acquired infection treated with plasma from a person recently recovered from the disease. Am J Trop Med Hyg. 1970;19:677–679. doi: 10.4269/ajtmh.1970.19.677. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald G H, Johnston R E. Role of dendritic cell targeting in Venezuelan equine encephalitis virus. J Virol. 2000;74:914–922. doi: 10.1128/jvi.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick J B, Webb P A, Krebs J V, Johnson K M, Smith E S. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155:437–444. doi: 10.1093/infdis/155.3.437. [DOI] [PubMed] [Google Scholar]
- 24.Monath T P, Newhouse V F, Kemp G E, Setzer H W, Cacciapuoti A. Lassa virus isolation from Mastomys natalensis during an epidemic in Sierra Leone. Science. 1974;183:263–265. doi: 10.1126/science.185.4147.263. [DOI] [PubMed] [Google Scholar]
- 25.Morrison H G, Bauer S P, Lange J V, Esposito J J, McCormick J B, Auperin D D. Protection of guinea pigs from Lassa fever by vaccinia virus recombinants expressing the nucleoprotein or the envelope glycoproteins of Lassa virus. Virology. 1989;171:179–188. doi: 10.1016/0042-6822(89)90525-4. [DOI] [PubMed] [Google Scholar]
- 26.Murphy F A, Nathanson N. The emergence of new viral diseases: an overview. Semin Virol. 1994;5:87–102. [Google Scholar]
- 27.Pushko P, Bray M, Ludwig G V, Parker M, Schmaljohn A, Jahrling P B, Smith J F. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine. 2000;19:142–153. doi: 10.1016/s0264-410x(00)00113-4. [DOI] [PubMed] [Google Scholar]
- 28.Pushko P, Parker M, Ludwig G V, Davis N L, Johnston R E, Smith J F. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 29.Redfield R R, Wright D C, James W D, Jones T S, Brown C, Burke D S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer R C, Jr, Bitrick M S., Jr Death after Pichinde virus infection in large and small strain 13 guinea pigs. J Infect Dis. 1993;167:1059–1064. doi: 10.1093/infdis/167.5.1059. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan N J, Sanchez A, Rollin P E, Yang Z, Nabel G J. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 32.Ter Meulen J. Lassa fever: implications of T-cell immunity for vaccine development. J Biotechnol. 1999;73:207–212. doi: 10.1016/s0168-1656(99)00122-4. [DOI] [PubMed] [Google Scholar]
- 33.Vanderzanden L, Bray M, Fuller D, Roberts T, Custer D, Spik K, Jahrling P, Huggins J, Schmaljohn A, Schmaljohn C. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology. 1998;246:134–144. doi: 10.1006/viro.1998.9176. [DOI] [PubMed] [Google Scholar]
- 34.Volchkov V E, Feldmann H, Volchkova V A, Klenk H D. Processing of the Ebola virus glycoprotein by the proprotein convertase. Proc Natl Acad Sci USA. 1998;95:5762–5767. doi: 10.1073/pnas.95.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L, Sanchez A, Yang Z, Zaki S R, Nabel E G, Nichol S T, Nabel G J. Immunization for Ebola virus infection. Nat Med. 1998;4:37–42. doi: 10.1038/nm0198-037. [DOI] [PubMed] [Google Scholar]