Abstract
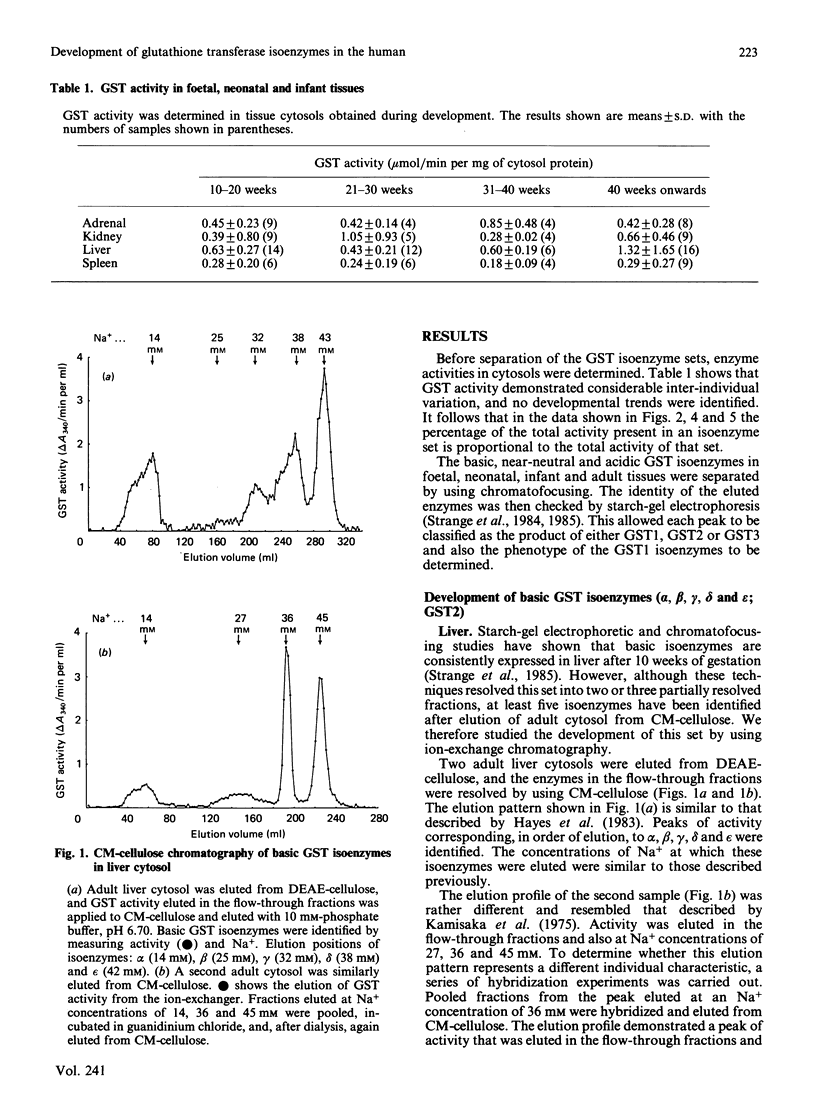
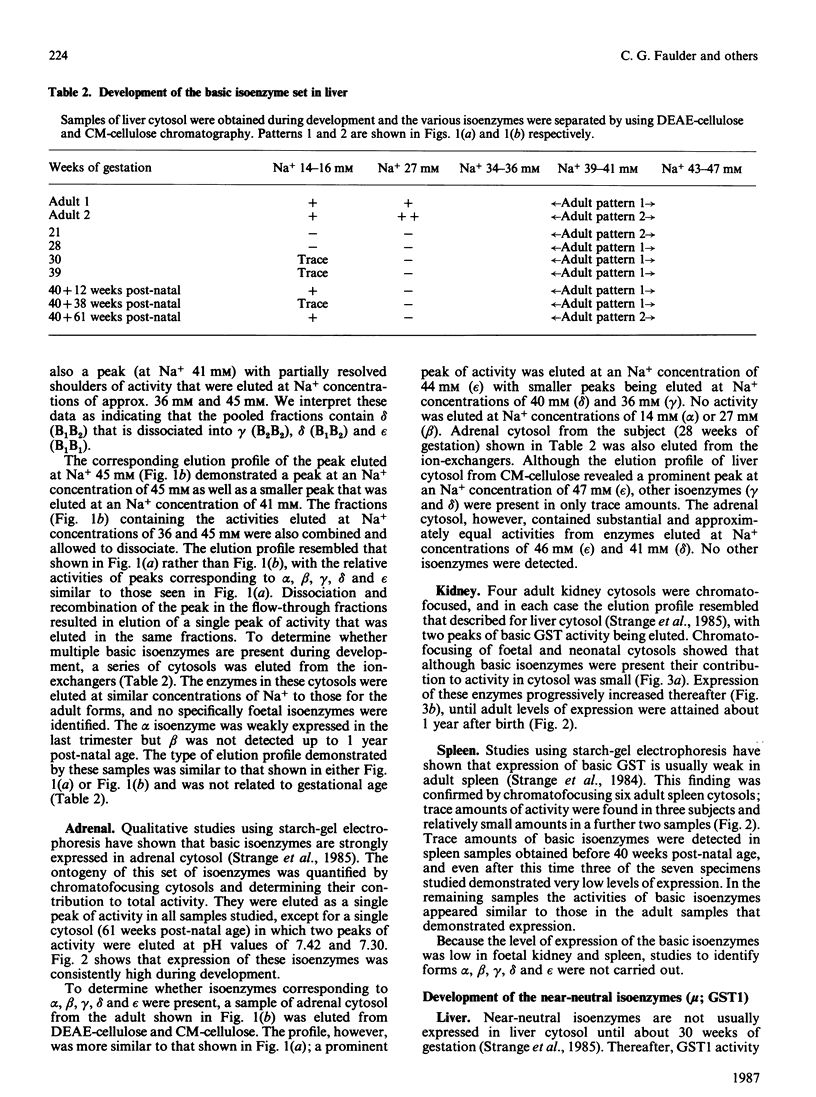
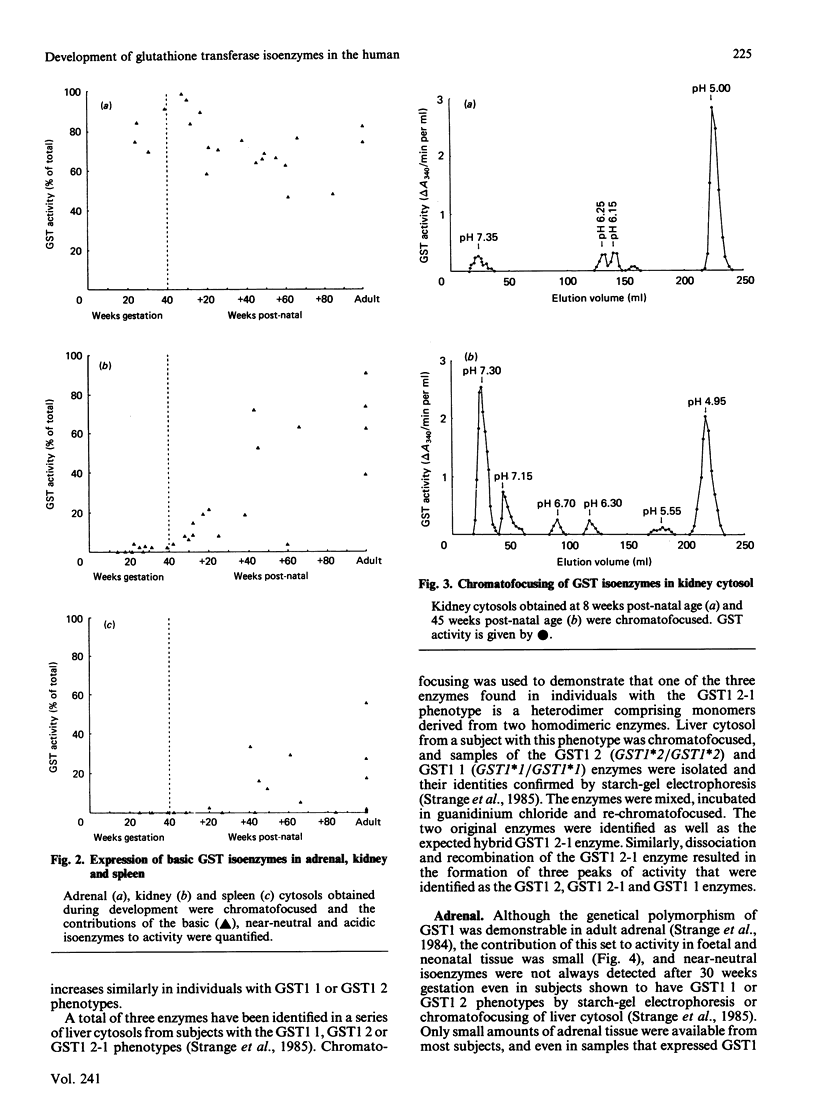
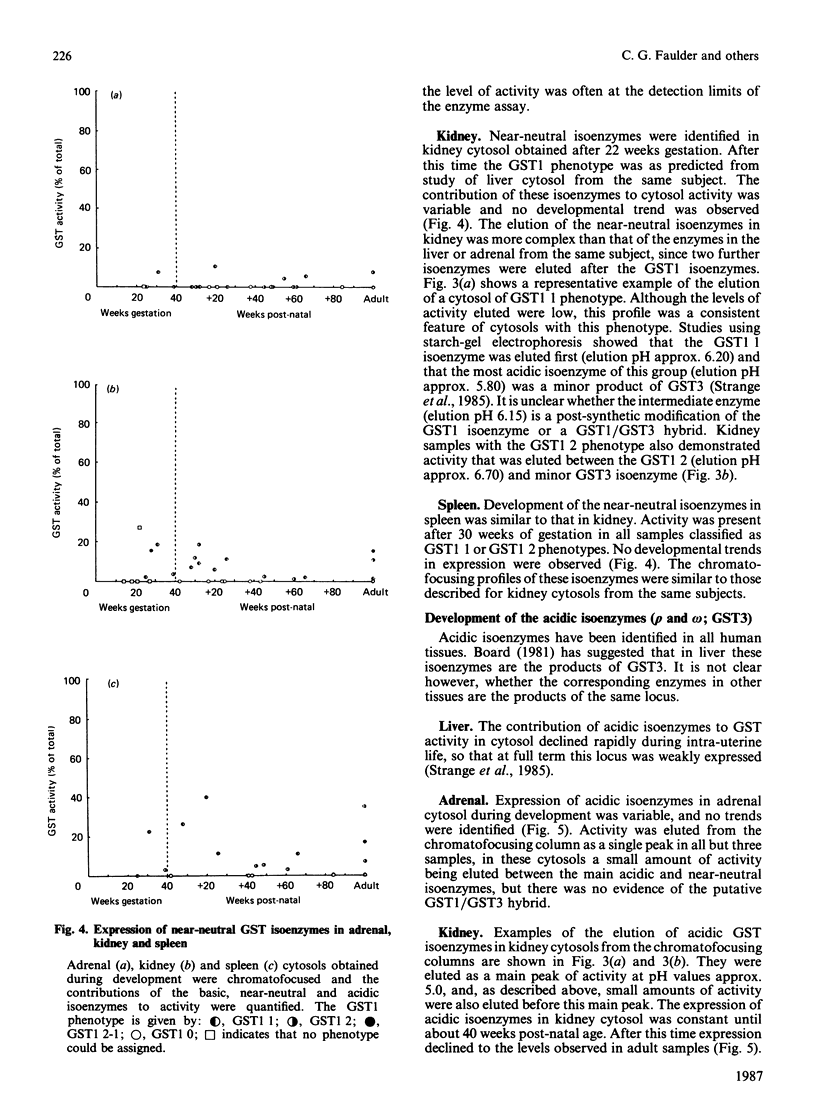
The ontogeny of basic, near-neutral and acidic glutathione S-transferase isoenzymes was studied by using chromatofocusing and ion-exchange chromatography. These isoenzyme sets demonstrated tissue-specific patterns of expression. For example, whereas basic isoenzymes were identified in all liver and adrenal cytosols obtained after 10 weeks gestation, these forms were not detected in kidney until 10 weeks post-natal age and in spleen until about 40 weeks post-natal age. Our data indicate that the basic monomers B1 and B2 are present in liver cytosol at 21 weeks gestation. Expression of the near-neutral isoenzymes was usually weak; for example, they were not generally expressed in liver until 30 weeks gestation, and no developmental patterns in their expression could be identified in adrenal, kidney and spleen. The acidic isoenzymes were usually strongly expressed in adrenal, kidney and spleen, although there was a decline in the level of expression in kidney after birth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anner B. M. The receptor function of the Na+, K+-activated adenosine triphosphatase system. Biochem J. 1985 Apr 1;227(1):1–11. doi: 10.1042/bj2270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board P. G. Biochemical genetics of glutathione-S-transferase in man. Am J Hum Genet. 1981 Jan;33(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- Fryer A. A., Hume R., Strange R. C. The development of glutathione S-transferase and glutathione peroxidase activities in human lung. Biochim Biophys Acta. 1986 Oct 1;883(3):448–453. doi: 10.1016/0304-4165(86)90283-7. [DOI] [PubMed] [Google Scholar]
- Guthenberg C., Warholm M., Rane A., Mannervik B. Two distinct forms of glutathione transferase from human foetal liver. Purification and comparison with isoenzymes isolated from adult liver and placenta. Biochem J. 1986 May 1;235(3):741–745. doi: 10.1042/bj2350741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Gilligan D., Chapman B. J., Beckett G. J. Purification of human hepatic glutathione S-transferases and the development of a radioimmunoassay for their measurement in plasma. Clin Chim Acta. 1983 Oct 31;134(1-2):107–121. doi: 10.1016/0009-8981(83)90189-4. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Pacifici G. M., Warholm M., Guthenberg C., Mannervik B., Rane A. Organ distribution of glutathione transferase isoenzymes in the human fetus: differences between liver and extrahepatic tissues. Biochem Pharmacol. 1986 May 1;35(9):1616–1619. doi: 10.1016/0006-2952(86)90137-1. [DOI] [PubMed] [Google Scholar]
- Strange R. C., Davis B. A., Faulder C. G., Cotton W., Bain A. D., Hopkinson D. A., Hume R. The human glutathione S-transferases: developmental aspects of the GST1, GST2, and GST3 loci. Biochem Genet. 1985 Dec;23(11-12):1011–1028. doi: 10.1007/BF00499944. [DOI] [PubMed] [Google Scholar]
- Strange R. C., Faulder C. G., Davis B. A., Hume R., Brown J. A., Cotton W., Hopkinson D. A. The human glutathione S-transferases: studies on the tissue distribution and genetic variation of the GST1, GST2 and GST3 isozymes. Ann Hum Genet. 1984 Jan;48(Pt 1):11–20. doi: 10.1111/j.1469-1809.1984.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Strange R. C., Johnson P. H., Lawton A., Moult J. A., Tector M. J., Tyminski R. J., Cotton W. Studies on the variability of glutathione S-transferase from human erythrocytes. Clin Chim Acta. 1982 Apr 8;120(2):251–260. doi: 10.1016/0009-8981(82)90162-0. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Garcia K. B., Royer R. E. Isolation and characterization of the multiple glutathione S-transferases from human liver. Evidence for unique heme-binding sites. J Biol Chem. 1985 Sep 25;260(21):11603–11610. [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B. Molecular and catalytic properties of glutathione transferase mu from human liver: an enzyme efficiently conjugating epoxides. Biochemistry. 1983 Jul 19;22(15):3610–3617. doi: 10.1021/bi00284a011. [DOI] [PubMed] [Google Scholar]


